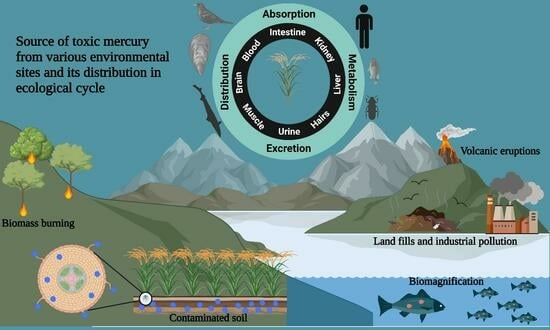
A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects
Abstract
:1. Introduction to Mercury Pollution
1.1. Sources of Mercury Pollution
1.2. Different Routes of Mercury Incorporation
1.3. Patho-Physiological Mode of Action
2. Fate and Transport of Mercury in Terrestrial Ecosystems
2.1. Mercury in Soil and Plant Life
2.2. Mercury in Lacustrine Sediments
2.3. Fate of Mercury in Aquatic Environments
2.3.1. Oxidation of Hg0
2.3.2. Reduction in Hg2+
2.3.3. Methylation of Hg2+
2.3.4. Degradation of MeHg
2.3.5. Demethylation of MeHg
3. Mercury Bioaccumulation and Microbial Community Changes
3.1. Source and Bioconversion of Mercury to Methylmercury
3.2. Bioaccumulation of Mercury in Higher-Order Organisms
3.3. Mercury Toxicity and Its Effect on Microbial Community
3.4. Molecular Mechanism of Mercury Selection
3.4.1. Genes Involved in Mercury Resistance
3.4.2. Horizontal Gene Transfer on Microbial Selection
4. Toxicokinetic and Ecotoxicology of Mercury
4.1. Biomonitoring of Mercury
4.2. Mercury Toxicity to Environment
4.2.1. Mercury Toxicity to Plants and Algae
4.2.2. Mercury Toxicity to Fish
4.3. Mercury Toxicity to Birds and Animals
4.4. Mercury Toxicity to Human Health
5. Advanced Techniques Involved in Mercury Remediation
5.1. Phytoremediation of Mercury
5.2. Microbial Treatment of Mercury
5.3. Algae-Based Mercury Removal
5.4. Biochar-Based Mercury Removal
5.5. Advanced Oxidation of Mercury
5.6. Immobilization of Mercury
6. Challenges and Future Perspectives
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Budnik, L.T.; Casteleyn, L. Mercury pollution in modern times and its socio-medical consequences. Sci. Total Environ. 2019, 654, 720–734. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, Q.; Maavara, T.; Liu, S.; Wang, X.; Raymond, P.A. Rivers as the largest source of mercury to coastal oceans worldwide. Nat. Geosci. 2021, 14, 672–677. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Mulder, J.; Duan, L.; Wu, Q.; Wang, S.; Tack, F.M.; Rinklebe, J. Mercury speciation, transformation, and transportation in soils, atmospheric flux, and implications for risk management: A critical review. Environ. Int. 2019, 126, 747–761. [Google Scholar] [CrossRef]
- Espejo, W.; Celis, J.E.; Chiang, G.; Bahamonde, P. Environment and COVID-19: Pollutants, impacts, dissemination, management and recommendations for facing future epidemic threats. Sci. Total Environ. 2020, 747, 141314. [Google Scholar] [CrossRef] [PubMed]
- Bishop, K.; Shanley, J.B.; Riscassi, A.; de Wit, H.A.; Eklöf, K.; Meng, B.; Mitchell, C.; Osterwalder, S.; Schuster, P.F.; Webster, J.; et al. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 2020, 721, 137647. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Hu, Y.; Pan, Y.; Zhao, Y.; Liu, H. Risk caused by heterogeneity of heavy metals in aquatic products. Asian J. Ecotoxicol. 2021, 16, 80–92. [Google Scholar]
- Dietz, R.; Wilson, S.; Loseto, L.L.; Dommergue, A.; Xie, Z.; Sonne, C.; Chételat, J. Special Issue on the AMAP 2021 Assessment of Mercury in the Arctic; Elsevier: Amsterdam, The Netherlands, 2022; p. 157020. [Google Scholar]
- Liu, S.; Wang, X.; Guo, G.; Yan, Z. Status and environmental management of soil mercury pollution in China: A review. J. Environ. Manag. 2021, 277, 111442. [Google Scholar] [CrossRef]
- Hsu-Kim, H.; Eckley, C.S.; Achá, D.; Feng, X.; Gilmour, C.C.; Jonsson, S.; Mitchell, C.P.J. Challenges and opportunities for managing aquatic mercury pollution in altered landscapes. Ambio 2018, 47, 141–169. [Google Scholar] [CrossRef]
- Cortes, J.; Peralta, J.; Díaz-Navarro, R. Acute respiratory syndrome following accidental inhalation of mercury vapor. Clin. Case Rep. 2018, 6, 1535–1537. [Google Scholar] [CrossRef]
- Dórea, J.G. Neurotoxic effects of combined exposures to aluminum and mercury in early life (infancy). Environ. Res. 2020, 188, 109734. [Google Scholar] [CrossRef]
- Rashid, S.; Shah, I.A.; Tulcan, R.X.S.; Rashid, W.; Sillanpaa, M. Contamination, exposure, and health risk assessment of Hg in Pakistan: A review. Environ. Pollut. 2022, 301, 118995. [Google Scholar] [CrossRef] [PubMed]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Leung, N.L.; Zhang, J.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Coord. Chem. Rev. 2021, 429, 213693. [Google Scholar] [CrossRef]
- Zhang, G.; Li, T.; Zhang, J.; Chen, A. A simple FRET-based turn-on fluorescent aptasensor for 17β-estradiol determination in environmental water, urine and milk samples. Sens. Actuators B Chem. 2018, 273, 1648–1653. [Google Scholar] [CrossRef]
- Obrist, D.; Kirk, J.L.; Zhang, L.; Sunderland, E.M.; Jiskra, M.; Selin, N.E. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 2018, 47, 116–140. [Google Scholar] [CrossRef]
- Zhou, J.; Obrist, D.; Dastoor, A.; Jiskra, M.; Ryjkov, A. Environment, Vegetation uptake of mercury and impacts on global cycling. Nat. Rev. Earth Environ. 2021, 2, 269–284. [Google Scholar] [CrossRef]
- Vogel, N.; Murawski, A.; Schmied-Tobies, M.I.; Rucic, E.; Doyle, U.; Kämpfe, A.; Hoera, C.; Hildebrand, J.; Schaefer, M.; Drexler, H.; et al. Lead, cadmium, mercury, and chromium in urine and blood of children and adolescents in Germany–human biomonitoring results of the German Environmental Survey 2014–2017 (GerES V). Int. J. Hyg. Environ. Health 2021, 237, 113822. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Liu, J.; Zhang, L.; Chen, J.; Feng, X. Stone coal as a potential atmospheric mercury source in Da-Ba-Shan mountain areas, China. Int. J. Coal Geol. 2019, 206, 21–30. [Google Scholar] [CrossRef]
- Schneider, L. When toxic chemicals refuse to die—An examination of the prolonged mercury pesticide use in Australia. Elem. Sci. Anthr. 2021, 9, 53. [Google Scholar]
- Fortuna, L.; Candotto Carniel, F.; Capozzi, F.; Tretiach, M. Congruence Evaluation of Mercury Pollution Patterns Around a Waste Incinerator over a 16-Year-Long Period Using Different Biomonitors. Atmosphere 2019, 10, 183. [Google Scholar] [CrossRef]
- Li, F.; Ma, C.; Zhang, P. Mercury Deposition, Climate Change and Anthropogenic Activities: A Review. Front. Earth Sci. Chin. 2020, 8, 316. [Google Scholar] [CrossRef]
- de Almeida Rodrigues, P.; Ferrari, R.G.; Dos Santos, L.N.; Conte Junior, C.A. Mercury in aquatic fauna contamination: A systematic review on its dynamics and potential health risks. J. Environ. Sci. 2019, 84, 205–218. [Google Scholar] [CrossRef]
- Enrico, M.; Mere, A.; Zhou, H.; Loriau, M.; Tessier, E.; Bouyssiere, B. Methods for Total and Speciation Analysis of Mercury in the Petroleum Industry. Energy Fuels 2020, 34, 13307–13320. [Google Scholar] [CrossRef]
- Jinadasa, B.; Jayasinghe, G.; Pohl, P.; Fowler, S.W. Mitigating the impact of mercury contaminants in fish and other seafood—A review. Mar. Pollut. Bull. 2021, 171, 112710. [Google Scholar] [CrossRef]
- Zhang, L.; Du, M.; Tu, Y.; Liang, D.; Wu, X.; Xie, H. Clinicopathological features and long-term prognosis of glomerular diseases associated with mercury-containing cosmetics. J. Nephrol. 2023, 36, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Joy, A.; Qureshi, A. Mercury in Dental Amalgam, Online Retail, and the Minamata Convention on Mercury. Environ. Sci. Technol. 2020, 54, 14139–14142. [Google Scholar] [CrossRef] [PubMed]
- Chalkidis, A.; Jampaiah, D.; Aryana, A.; Wood, C.D.; Hartley, P.G.; Sabri, Y.M.; Bhargava, S.K. Mercury-bearing wastes: Sources, policies and treatment technologies for mercury recovery and safe disposal. J. Environ. Manag. 2020, 270, 110945. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, Y.; Wu, Y.; Li, Z.; Li, Y.; Liu, M.; Qu, T.; Chen, C. Measurement of mercury with highly selective fluorescent chemoprobe by carbon dots and silver nanoparticles. Chemosphere 2021, 274, 129959. [Google Scholar] [CrossRef]
- Gfeller, L.; Caplette, J.N.; Frossard, A.; Mestrot, A. Organo-mercury species in a polluted agricultural flood plain: Combining speciation methods and polymerase chain reaction to investigate pathways of contamination. Environ. Pollut. 2022, 311, 119854. [Google Scholar] [CrossRef]
- Men, C.; Liu, R.; Xu, L.; Wang, Q.; Guo, L.; Miao, Y.; Shen, Z. Source-specific ecological risk analysis and critical source identification of heavy metals in road dust in Beijing, China. J. Hazard. Mater. 2020, 388, 121763. [Google Scholar] [CrossRef] [PubMed]
- Grasby, S.E.; Them, T.R., II; Chen, Z.; Yin, R.; Ardakani, O.H. Mercury as a proxy for volcanic emissions in the geologic record. Earth-Sci. Rev. 2019, 196, 102880. [Google Scholar] [CrossRef]
- Gerson, J.R.; Szponar, N.; Zambrano, A.A.; Bergquist, B.; Broadbent, E.; Driscoll, C.T.; Erkenswick, G.; Evers, D.C.; Fernandez, L.E.; Hsu-Kim, H.; et al. Amazon forests capture high levels of atmospheric mercury pollution from artisanal gold mining. Nat. Commun. 2022, 13, 559. [Google Scholar] [CrossRef]
- Jiskra, M.; Heimbürger-Boavida, L.-E.; Desgranges, M.-M.; Petrova, M.V.; Dufour, A.; Ferreira-Araujo, B.; Masbou, J.; Chmeleff, J.; Thyssen, M.; Point, D.J.N. Mercury stable isotopes constrain atmospheric sources to the ocean. Nature 2021, 597, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Jamwal, R.; Mishra, N.; Singh, D.K. Monitoring; Management, Recent developments in environmental mercury bioremediation and its toxicity: A review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100283. [Google Scholar]
- Yan, S.-R.; Foroughi, M.M.; Safaei, M.; Jahani, S.; Ebrahimpour, N.; Borhani, F.; Baravati, N.R.Z.; Aramesh-Boroujeni, Z.; Foong, L.K. A review: Recent advances in ultrasensitive and highly specific recognition aptasensors with various detection strategies. Int. J. Biol. Macromol. 2020, 155, 184–207. [Google Scholar] [CrossRef]
- Barcia, L.G.; Argiro, J.; Babcock, E.A.; Cai, Y.; Shea, S.K.; Chapman, D.D. Mercury and arsenic in processed fins from nine of the most traded shark species in the Hong Kong and China dried seafood markets: The potential health risks of shark fin soup. Mar. Pollut. Bull. 2020, 157, 111281. [Google Scholar] [CrossRef] [PubMed]
- Ajsuvakova, O.P.; Tinkov, A.A.; Aschner, M.; Rocha, J.B.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; et al. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef]
- Cossa, D.; Knoery, J.; Bǎnaru, D.; Harmelin-Vivien, M.; Sonke, J.E.; Hedgecock, I.M.; Bravo, A.G.; Rosati, G.; Canu, D.; Horvat, M.; et al. Technology, Mediterranean Mercury Assessment 2022: An Updated Budget, Health Consequences, and Research Perspectives. Environ. Sci. Technol. 2022, 56, 3840–3862. [Google Scholar] [CrossRef]
- Traina, A.; Ausili, A.; Bonsignore, M.; Fattorini, D.; Gherardi, S.; Gorbi, S.; Quinci, E.; Romano, E.; Manta, D.S.; Tranchida, G.; et al. Organochlorines and Polycyclic Aromatic Hydrocarbons as fingerprint of exposure pathways from marine sediments to biota. Mar. Pollut. Bull. 2021, 170, 112676. [Google Scholar] [CrossRef]
- Koch, W.; Czop, M.; Iłowiecka, K.; Nawrocka, A.; Wiącek, D.J.N. Dietary Intake of Toxic Heavy Metals with Major Groups of Food Products—Results of Analytical Determinations. Nutrients 2022, 14, 1626. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Stegeman, J.J.; Fleming, L.E.; Allemand, D.; Anderson, D.M.; Backer, L.C.; Brucker-Davis, F.; Chevalier, N.; Corra, L.; Czerucka, D.; et al. Human health and ocean pollution. Ann. Glob. Health 2020, 86, 151. [Google Scholar]
- Garrett, R.D.; Cammelli, F.; Ferreira, J.; Levy, S.A.; Valentim, J.; Vieira, I. Resources, Forests and sustainable development in the Brazilian Amazon: History, trends, and future prospects. Annu. Rev. Environ. Resour. 2021, 46, 625–652. [Google Scholar] [CrossRef]
- Laborde, A.; Tomasina, F.; Bianchi, F.; Bruné, M.-N.; Buka, I.; Comba, P.; Corra, L.; Cori, L.; Duffert, C.M.; Harari, R.; et al. Children’s health in Latin America: The influence of environmental exposures. Environ. Health Perspect. 2015, 123, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Bhat, I.; Khanam, Z.; Rak, A.; Yusoff, H.; Akhter, M.S. Phytoremediation: In situ alternative for pollutant removal from contaminated natural media: A brief review. Biointerface Res. Appl. Chem. 2022, 12, 4945–4960. [Google Scholar]
- Sonzogni, L.; Ferlazzo, M.L.; Granzotto, A.; Fervers, B.; Charlet, L.; Foray, N. DNA double-strand breaks induced in human cells by 6 current pesticides: Intercomparisons and influence of the ATM protein. Biomolecules 2022, 12, 250. [Google Scholar] [CrossRef] [PubMed]
- Bhave, P.; Sadhwani, K.; Dhadwad, M. Total mercury in soil and leachate from municipal solid waste dumping grounds in Mumbai, India. Environ. Earth Sci. 2022, 81, 30. [Google Scholar] [CrossRef]
- Streets, D.G.; Horowitz, H.M.; Lu, Z.; Levin, L.; Thackray, C.P.; Sunderland, E.M. Global and regional trends in mercury emissions and concentrations, 2010–2015. Atmos. Environ. 2019, 201, 417–427. [Google Scholar]
- Gaylord, A.; Osborne, G.; Ghassabian, A.; Malits, J.; Attina, T.; Trasande, L. Trends in neurodevelopmental disability burden due to early life chemical exposure in the USA from 2001 to 2016: A population-based disease burden and cost analysis. Mol. Cell. Endocrinol. 2020, 502, 110666. [Google Scholar] [CrossRef] [PubMed]
- Dórea, J.G. Exposure to environmental neurotoxic substances and neurodevelopment in children from Latin America and the Caribbean. Environ. Res. 2021, 192, 110199. [Google Scholar]
- Bjørklund, G.; Antonyak, H.; Polishchuk, A.; Semenova, Y.; Lesiv, M.; Lysiuk, R.; Peana, M. Effect of methylmercury on fetal neurobehavioral development: An overview of the possible mechanisms of toxicity and the neuroprotective effect of phytochemicals. Arch. Toxicol. 2022, 96, 3175–3199. [Google Scholar] [PubMed]
- Fu, Z.; Xi, S. The effects of heavy metals on human metabolism. Toxicol. Mech. Methods 2020, 30, 167–176. [Google Scholar] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K.J.M. Heavy metals and human health: Possible exposure pathways and the competition for protein binding sites. Molecules 2021, 26, 6060. [Google Scholar] [PubMed]
- Bose-O’Reilly, S.; Drasch, G.; Beinhoff, C.; Tesha, A.; Drasch, K.; Roider, G.; Taylor, H.; Appleton, D.; Siebert, U. Health assessment of artisanal gold miners in Tanzania. Sci. Total Environ. 2010, 408, 796–805. [Google Scholar]
- Patel, S.S.; Acharya, A.; Ray, R.; Agrawal, R.; Raghuwanshi, R.; Jain, P. Cellular and molecular mechanisms of curcumin in prevention and treatment of disease. Crit. Rev. Food Sci. Nutr. 2020, 60, 887–939. [Google Scholar]
- Yang, L.; Zhang, Y.; Wang, F.; Luo, Z.; Guo, S.; Strähle, U. Toxicity of mercury: Molecular evidence. Chemosphere 2020, 245, 125586. [Google Scholar]
- Hasan, A.; Nanakali, N.M.Q.; Salihi, A.; Rasti, B.; Sharifi, M.; Attar, F.; Derakhshankhah, H.; Mustafa, I.A.; Abdulqadir, S.Z.; Falahati, M.J.T. Nanozyme-based sensing platforms for detection of toxic mercury ions: An alternative approach to conventional methods. Talanta 2020, 215, 120939. [Google Scholar]
- Wise, J.P., Jr.; Young, J.L.; Cai, J.; Cai, L. Current understanding of hexavalent chromium [Cr (VI)] neurotoxicity and new perspectives. Environ. Int. 2022, 158, 106877. [Google Scholar]
- Wang, H.; Matsushita, M.T. Heavy metals and adult neurogenesis. Curr. Opin. Toxicol. 2021, 26, 14–21. [Google Scholar]
- Lyman, S.N.; Cheng, I.; Gratz, L.E.; Weiss-Penzias, P.; Zhang, L. An updated review of atmospheric mercury. Sci. Total Environ. 2020, 707, 135575. [Google Scholar]
- Evers, D.C.; Sunderland, E.M. Global Mercury Monitoring in Biota; Biodiversity Research Institute: Portland, Maine, 2019; p. 8. [Google Scholar]
- Pereyra, F.X.; Bouza, P. Soils of the Pampean Region. In The soils of ArgentinaThe soils of Argentina; Rubio, G., Lavado, R.S., Pereyra, F.X., Eds.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 101–121. [Google Scholar]
- Gustin, M.S.; Bank, M.S.; Bishop, K.; Bowman, K.; Branfireun, B.; Chételat, J.; Eckley, C.S.; Hammerschmidt, C.R.; Lamborg, C.; Lyman, S.; et al. Mercury biogeochemical cycling: A synthesis of recent scientific advances. Sci. Total Environ. 2020, 737, 139619. [Google Scholar] [PubMed]
- Cooke, C.A.; Martínez-Cortizas, A.; Bindler, R.; Gustin, M.S. Environmental archives of atmospheric Hg deposition—A review. Sci. Total Environ. 2020, 709, 134800. [Google Scholar]
- Bravo, A.G.; Cosio, C. Biotic formation of methylmercury: A bio–physico–chemical conundrum. Limnol. Oceanogr. 2020, 65, 1010–1027. [Google Scholar] [PubMed]
- Duan, P.; Khan, S.; Ali, N.; Shereen, M.A.; Siddique, R.; Ali, B.; Iqbal, H.M.N.; Nabi, G.; Sajjad, W.; Bilal, M. Biotransformation fate and sustainable mitigation of a potentially toxic element of mercury from environmental matrices. Arab. J. Chem. 2020, 13, 6949–6965. [Google Scholar]
- Perry, C.J. Computational Analysis of Various Reactions between hydroxyl Radicals and Organic Mercury Species. 2021. Available online: https://hdl.handle.net/10657.1/2630 (accessed on 11 January 2023).
- Beda, J.C.A.; Ouattara, J.-M.P.; Messou, A.; Coulibaly, L. Impacts of artisanal and small-scale gold mining on soils in northern regions of Côte d’Ivoire: Cases of Boundiali, Korhogo and Tengrela. Int. J. Biol. Chem. Sci. 2021, 15, 1234–1248. [Google Scholar]
- Santos-Sacramento, L.; Arrifano, G.P.; Lopes-Araújo, A.; Augusto-Oliveira, M.; Albuquerque-Santos, R.; Takeda, P.Y.; Souza-Monteiro, J.R.; Macchi, B.M.; do Nascimento, J.L.M.; Lima, R.R.; et al. Human neurotoxicity of mercury in the Amazon: A scoping review with insights and critical considerations. Ecotoxicol. Environ. Saf. 2021, 208, 111686. [Google Scholar]
- Branfireun, B.A.; Cosio, C.; Poulain, A.J.; Riise, G.; Bravo, A.G. Mercury cycling in freshwater systems—An updated conceptual model. Sci. Total Environ. 2020, 745, 140906. [Google Scholar]
- Al-Ansari, E.M.; Abdel-Moati, M.A.R.; Yigiterhan, O.; Al-Maslamani, I.; Soliman, Y.; Rowe, G.T.; Wade, T.L.; Al-Shaikh, I.M.; Helmi, A.; Kuklyte, L.; et al. Mercury accumulation in Lethrinus nebulosus from the marine waters of the Qatar EEZ. Mar. Pollut. Bull. 2017, 121, 143–153. [Google Scholar]
- Lemaire, J.; Bustamante, P.; Olivier, A.; Lourdais, O.; Michaud, B.; Boissinot, A.; Galán, P.; Brischoux, F. Determinants of mercury contamination in viperine snakes, Natrix maura, in Western Europe. Sci. Total Environ. 2018, 635, 20–25. [Google Scholar] [PubMed]
- Laird, B.; Chan, H.M.; Kannan, K.; Husain, A.; Al-Amiri, H.; Dashti, B.; Sultan, A.; Al-Othman, A.; Al-Mutawa, F. Exposure and risk characterization for dietary methylmercury from seafood consumption in Kuwait. Sci. Total Environ. 2017, 607–608, 375–380. [Google Scholar]
- Yung, L.; Bertheau, C.; Cazaux, D.; Regier, N.; Slaveykova, V.I.; Chalot, M. Insect Life Traits Are Key Factors in Mercury Accumulation and Transfer within the Terrestrial Food Web. Environ. Sci. Technol. 2019, 53, 11122–11132. [Google Scholar] [CrossRef]
- Anual, Z.F.; Maher, W.; Krikowa, F.; Hakim, L.; Ahmad, N.I.; Foster, S. Mercury and risk assessment from consumption of crustaceans, cephalopods and fish from West Peninsular Malaysia. Microchem. J. 2018, 140, 214–221. [Google Scholar] [CrossRef]
- Alizada, N.; Malik, S.; Muzaffar, S.B. Bioaccumulation of heavy metals in tissues of Indian anchovy (Stolephorus indicus) from the UAE coast, Arabian Gulf. Mar. Pollut. Bull. 2020, 154, 111033. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.; Chandran, M.S.S.; Ramasamy, E.V. Mercury contamination at Vembanad Lake and near-shore regions in the southwest coast of India. Reg. Stud. Mar. Sci. 2021, 44, 101754. [Google Scholar] [CrossRef]
- Elsayed, H.; Yigiterhan, O.; Al-Ansari, E.M.A.S.; Al-Ashwel, A.A.; Elezz, A.A.; Al-Maslamani, I.A. Methylmercury bioaccumulation among different food chain levels in the EEZ of Qatar (Arabian Gulf). Reg. Stud. Mar. Sci. 2020, 37, 101334. [Google Scholar] [CrossRef]
- Ramasamy, E.V.; Jayasooryan, K.K.; Chandran, M.S.S.; Mohan, M. Total and methyl mercury in the water, sediment, and fishes of Vembanad, a tropical backwater system in India. Environ. Monit. Assess. 2017, 189, 130. [Google Scholar] [CrossRef] [PubMed]
- Arcagni, M.; Rizzo, A.; Juncos, R.; Pavlin, M.; Campbell, L.M.; Arribére, M.A.; Horvat, M.; Ribeiro Guevara, S. Mercury and selenium in the food web of Lake Nahuel Huapi, Patagonia, Argentina. Chemosphere 2017, 166, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Diéguez, M.d.C.; del Carmen Diéguez, M.; Arcagni, M.; Rizzo, A.; Catán, S.P.; Cárdenas, C.S.; Horvat, M.; Guevara, S.R. Mercury in Aquatic Systems of North Patagonia (Argentina): Sources, Processes, and Trophic Transfer. Nat. Soc. Sci. Patagon. 2022, 163–194. [Google Scholar]
- Lancaster, S.T.; Peniche, G.; Alzahrani, A.; Blanz, M.; Newton, J.; Taggart, M.A.; Corns, W.T.; Krupp, E.M.; Feldmann, J. Mercury speciation in Scottish raptors reveals high proportions of inorganic mercury in Scottish golden eagles (Aquila chrysaetos): Potential occurrence of mercury selenide nanoparticles. Sci. Total Environ. 2022, 829, 154557. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, Z.; Žagar, D. Mercury transport and fate models in aquatic systems: A review and synthesis. Sci. Total Environ. 2018, 639, 538–549. [Google Scholar] [CrossRef]
- Jiang, T.; Bravo, A.G.; Skyllberg, U.; Björn, E.; Wang, D.; Yan, H.; Green, N.W. Influence of dissolved organic matter (DOM) characteristics on dissolved mercury (Hg) species composition in sediment porewater of lakes from southwest China. Water Res. 2018, 146, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Bashir, I.; Lone, F.A.; Bhat, R.A.; Mir, S.A.; Dar, Z.A.; Dar, S.A. Concerns and Threats of Contamination on Aquatic Ecosystems. In Bioremediation and Biotechnology: Sustainable Approaches to Pollution Degradation; Hakeem, K.R., Bhat, R.A., Qadri, H., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–26. [Google Scholar]
- Pavithra, K.G.; SundarRajan, P.; Kumar, P.S.; Rangasamy, G. Mercury sources, contaminations, mercury cycle, detection and treatment techniques: A review. Chemosphere 2022, 312, 137314. [Google Scholar] [CrossRef] [PubMed]
- Moiseenko, T.I.; Gashkina, N.A. Distribution and bioaccumulation of heavy metals (Hg, Cd and Pb) in fish: Influence of the aquatic environment and climate. Environ. Res. Lett. 2020, 15, 115013. [Google Scholar] [CrossRef]
- Tang, W.-L.; Liu, Y.-R.; Guan, W.-Y.; Zhong, H.; Qu, X.-M.; Zhang, T. Understanding mercury methylation in the changing environment: Recent advances in assessing microbial methylators and mercury bioavailability. Sci. Total Environ. 2020, 714, 136827. [Google Scholar] [CrossRef]
- Du, H.; Ma, M.; Igarashi, Y.; Wang, D. Biotic and Abiotic Degradation of Methylmercury in Aquatic Ecosystems: A Review. Bull. Environ. Contam. Toxicol. 2019, 102, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Helmrich, S.; Vlassopoulos, D.; Alpers, C.N.; O’Day, P.A. Critical review of mercury methylation and methylmercury demethylation rate constants in aquatic sediments for biogeochemical modeling. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4353–4378. [Google Scholar] [CrossRef]
- Luo, H.; Cheng, Q.; Pan, X. Photochemical behaviors of mercury (Hg) species in aquatic systems: A systematic review on reaction process, mechanism, and influencing factor. Sci. Total Environ. 2020, 720, 137540. [Google Scholar] [CrossRef]
- Tamayo-Ortiz, M.; Riojas-Rodríguez, H.; Téllez-Rojo, M.M.; Boischio, A.; Mañay, N.; Menezes-Filho, J.A.; Queirolo, E.I.; Cortés, S.; Kordas, K. A Call for Biomonitoring Systems in Latin America and the Caribbean: Considerations for Potentially Toxic Metals/Metalloids. Ann. Glob. Health 2022, 88, 80. [Google Scholar] [CrossRef]
- Herrero Ortega, S.; Catalán, N.; Björn, E.; Gröntoft, H.; Hilmarsson, T.G.; Bertilsson, S.; Wu, P.; Bishop, K.; Levanoni, O.; Bravo, A.G. High methylmercury formation in ponds fueled by fresh humic and algal derived organic matter. Limnol. Oceanogr. 2018, 63 (Suppl. S1), S44–S53. [Google Scholar] [CrossRef]
- Regnell, O.; Watras, C.J. Microbial mercury methylation in aquatic environments: A critical review of published field and laboratory studies. Environ. Sci. Technol. 2018, 53, 4–19. [Google Scholar] [CrossRef]
- Blanchet, C.C.; Arzel, C.; Davranche, A.; Kahilainen, K.K.; Secondi, J.; Taipale, S.; Lindberg, H.; Loehr, J.; Manninen-Johansen, S.; Sundell, J. Ecology and extent of freshwater browning-What we know and what should be studied next in the context of global change. Sci. Total Environ. 2021, 812, 152420. [Google Scholar] [CrossRef] [PubMed]
- Nurfitriani, S.; Arisoesilaningsih, E.; Nuraini, Y.; Handayanto, E. Bioaccumulation of mercury by bacteria isolated from small scale gold mining tailings in Lombok, Indonesia. J. Ecol. Eng. 2020, 21, 127–136. [Google Scholar] [CrossRef]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Carvalho, C.; Guilhermino, L. Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in Dicentrarchus labrax juveniles. Sci. Rep. 2018, 8, 15655. [Google Scholar] [CrossRef]
- Godfrey, G.L.; Horstmann, L.; Snyder, J.; Trumble, S.J. Toxic and essential trace element concentrations in Pacific walrus (Odobenus rosmarus divergens) skeletal muscle varies by location and reproductive status. Polar Biol. 2022, 45, 1271–1289. [Google Scholar] [CrossRef]
- Grajewska, A.; Falkowska, L.; Saniewska, D.; Pawliczka, I. Changes in total mercury, methylmercury, and selenium blood levels during different life history stages of the Baltic grey seal (Halichoerus grypus grypus). Sci. Total Environ. 2019, 676, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Bazzi, W.; Abou Fayad, A.G.; Nasser, A.; Haraoui, L.-P.; Dewachi, O.; Abou-Sitta, G.; Nguyen, V.-K.; Abara, A.; Karah, N.; Landecker, H. Heavy metal toxicity in armed conflicts potentiates AMR in A. baumannii by selecting for antibiotic and heavy metal co-resistance mechanisms. Front. Microbiol. 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cao, H.; Liu, B.; Zhang, M.; Zhang, C.; Chen, P.; Yang, B. Effects of Mercury Contamination on Microbial Diversity of Different Kinds of Soil. Microorganisms 2022, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Salam, L.B.; Shomope, H.; Ummi, Z.; Bukar, F. Mercury contamination imposes structural shift on the microbial community of an agricultural soil. Bull. Natl. Res. Cent. 2019, 43, 163. [Google Scholar] [CrossRef]
- Hall, J.P.; Harrison, E.; Pärnänen, K.; Virta, M.; Brockhurst, M.A. The impact of mercury selection and conjugative genetic elements on community structure and resistance gene transfer. Front. Microbiol. 2020, 11, 1846. [Google Scholar] [CrossRef]
- Boyd, E.S.; Barkay, T. The mercury resistance operon: From an origin in a geothermal environment to an efficient detoxification machine. Front. Microbiol. 2012, 3, 349. [Google Scholar] [CrossRef]
- Perez-Palacios, P.; Delgado-Valverde, M.; Gual-de-Torrella, A.; Oteo-Iglesias, J.; Pascual, Á.; Fernández-Cuenca, F. Co-transfer of plasmid-encoded bla carbapenemases genes and mercury resistance operon in high-risk clones of Klebsiella pneumoniae. Appl. Microbiol. Biotechnol. 2021, 105, 9231–9242. [Google Scholar] [CrossRef] [PubMed]
- Vats, P.; Kaur, U.J.; Rishi, P. Heavy metal-induced selection and proliferation of antibiotic resistance: A review. J. Appl. Microbiol. 2022, 132, 4058–4076. [Google Scholar] [CrossRef]
- Gworek, B.; Bemowska-Kałabun, O.; Kijeńska, M.; Wrzosek-Jakubowska, J. Mercury in marine and oceanic waters—A review. Water Air Soil Pollut. 2016, 227, 371. [Google Scholar] [CrossRef] [PubMed]
- Clayden, M.G.; Kidd, K.A.; Wyn, B.; Kirk, J.L.; Muir, D.C.; O’Driscoll, N.J. Mercury biomagnification through food webs is affected by physical and chemical characteristics of lakes. Environ. Sci. Technol. 2013, 47, 12047–12053. [Google Scholar] [CrossRef] [PubMed]
- Eagles-Smith, C.A.; Ackerman, J.T.; Willacker, J.J.; Tate, M.T.; Lutz, M.A.; Fleck, J.A.; Stewart, A.R.; Wiener, J.G.; Evers, D.C.; Lepak, J.M. Spatial and temporal patterns of mercury concentrations in freshwater fish across the Western United States and Canada. Sci. Total Environ. 2016, 568, 1171–1184. [Google Scholar] [CrossRef]
- Al-Sulaiti, M.M.; Soubra, L.; Al-Ghouti, M.A. The causes and effects of mercury and methylmercury contamination in the marine environment: A review. Curr. Pollut. Rep. 2022, 8, 249–272. [Google Scholar] [CrossRef]
- Boerleider, R.Z.; Roeleveld, N.; Scheepers, P. Human biological monitoring of mercury for exposure assessment. AIMS Environ. Sci. 2017, 4, 251–276. [Google Scholar] [CrossRef]
- Heine, K.; Eckhardt, A. Limit values and guideline values in regulatory toxicology. Regul. Toxicol. 2020, 875–898. [Google Scholar]
- Arrifano, G.P.; Martín-Doimeadios, R.C.R.; Jiménez-Moreno, M.; Ramírez-Mateos, V.; da Silva, N.F.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.; Macchi, B.M.; do Nascimento, J.L.M. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucuruí Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef]
- Mortensen, M.E.; Caudill, S.P.; Caldwell, K.L.; Ward, C.D.; Jones, R.L. Total and methyl mercury in whole blood measured for the first time in the US population: NHANES 2011–2012. Environ. Res. 2014, 134, 257–264. [Google Scholar] [CrossRef]
- Rice, K.M.; Walker, E.M., Jr.; Wu, M.; Gillette, C.; Blough, E.R. Environmental mercury and its toxic effects. J. Prev. Med. Public Health 2014, 47, 74. [Google Scholar] [CrossRef]
- Shahid, M.; Khalid, S.; Bibi, I.; Bundschuh, J.; Niazi, N.K.; Dumat, C. A critical review of mercury speciation, bioavailability, toxicity and detoxification in soil-plant environment: Ecotoxicology and health risk assessment. Sci. Total Environ. 2020, 711, 134749. [Google Scholar]
- Shekar, C.C.; Sammaiah, D.; Shasthree, T.; Reddy, K.J. Effect of mercury on tomato growth and yield attributes. Int. J. Pharma Bio Sci. 2011, 2, B358–B364. [Google Scholar]
- Azevedo, R.; Rodriguez, E.; Mendes, R.J.; Mariz-Ponte, N.; Sario, S.; Lopes, J.C.; de Oliveira, J.M.P.F.; Santos, C. Inorganic Hg toxicity in plants: A comparison of different genotoxic parameters. Plant Physiol. Biochem. 2018, 125, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.T.; Duarte, B.; Cesário, R.; Mendes, R.; Hintelmann, H.; Eckey, K.; Dimock, B.; Caçador, I.; Canário, J. Mercury mobility and effects in the salt-marsh plant Halimione portulacoides: Uptake, transport, and toxicity and tolerance mechanisms. Sci. Total Environ. 2019, 650, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sahu, G.K.; Upadhyay, S.; Sahoo, B.B. Mercury induced phytotoxicity and oxidative stress in wheat (Triticum aestivum L.) plants. Physiol. Mol. Biol. Plants 2012, 18, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ahammad, S.J.; Sumithra, S.; Senthilkumar, P. Mercury uptake and translocation by indigenous plants. Rasayan J. Chem. 2018, 11, 1–12. [Google Scholar]
- Nicolardi, V.; Cai, G.; Parrotta, L.; Puglia, M.; Bianchi, L.; Bini, L.; Gaggi, C. The adaptive response of lichens to mercury exposure involves changes in the photosynthetic machinery. Environ. Pollut. 2012, 160, 1–10. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Y.; Wang, R.; Wang, S.; Lu, X.; Wang, B. Effect of mercury stress on photosynthetic characteristics of two kinds of warm season turf grass. Int. J. Environ. Monit. Anal. 2015, 3, 293–297. [Google Scholar] [CrossRef]
- Elbaz, A.; Wei, Y.Y.; Meng, Q.; Zheng, Q.; Yang, Z.M. Mercury-induced oxidative stress and impact on antioxidant enzymes in Chlamydomonas reinhardtii. Ecotoxicology 2010, 19, 1285–1293. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Skrobonja, A.; Funk, C.; Garbayo, I.; Vílchez, C. The Role of Microalgae in the Biogeochemical Cycling of Methylmercury (MeHg) in Aquatic Environments. Phycology 2022, 2, 344–362. [Google Scholar] [CrossRef]
- Beauvais-Flück, R.; Slaveykova, V.I.; Cosio, C. Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii. Sci. Rep. 2017, 7, 8034. [Google Scholar] [CrossRef]
- Ajitha, V.; Sreevidya, C.P.; Sarasan, M.; Park, J.C.; Mohandas, A.; Singh, I.S.B.; Puthumana, J.; Lee, J.-S. Effects of zinc and mercury on ROS-mediated oxidative stress-induced physiological impairments and antioxidant responses in the microalga Chlorella vulgaris. Environ. Sci. Pollut. Res. Int. 2021, 28, 32475–32492. [Google Scholar] [CrossRef]
- Thabet, J.; Elleuch, J.; Martínez, F.; Abdelkafi, S.; Hernández, L.E.; Fendri, I. Characterization of cellular toxicity induced by sub-lethal inorganic mercury in the marine microalgae Chlorococcum dorsiventrale isolated from a metal-polluted coastal site. Chemosphere 2023, 338, 139391. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, Z.; Simansky, S.; Sanabria, A.; Márová, I.; Garbayo, I.; Vílchez, C. Interaction of Naturally Occurring Phytoplankton with the Biogeochemical Cycling of Mercury in Aquatic Environments and Its Effects on Global Hg Pollution and Public Health. Microorganisms 2023, 11, 2034. [Google Scholar] [CrossRef]
- Barst, B.D.; Chételat, J.; Basu, N. Toxicological risk of mercury for fish and invertebrate prey in the Arctic. Sci. Total Environ. 2022, 836, 155702. [Google Scholar] [CrossRef] [PubMed]
- Depew, D.C.; Burgess, N.M.; Anderson, M.R.; Baker, R.; Bhavsar, S.P.; Bodaly, R.; Eckley, C.S.; Evans, M.S.; Gantner, N.; Graydon, J.A. An overview of mercury concentrations in freshwater fish species: A national fish mercury dataset for Canada. Can. J. Fish. Aquat. Sci. 2013, 70, 436–451. [Google Scholar] [CrossRef]
- Monitoring, A. AMAP Assessment 2011: Mercury in the Arctic. Executive Summary and Key Recommendations. 2011. Available online: http://hdl.handle.net/11374/1063 (accessed on 11 January 2023).
- Dietz, R.; Fort, J.; Sonne, C.; Albert, C.; Bustnes, J.O.; Christensen, T.K.; Ciesielski, T.M.; Danielsen, J.; Dastnai, S.; Eens, M. A risk assessment of the effects of mercury on Baltic Sea, Greater North Sea and North Atlantic wildlife, fish and bivalves. Environ. Int. 2021, 146, 106178. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Chen, Z.-H.; Wang, P.; Huang, R.; Huo, W.-I.; Huang, W.-X.; Yang, X.-F.; Peng, J.-W. Health risk assessment for local residents from the South China Sea based on mercury concentrations in marine fish. Bull. Environ. Contam. Toxicol. 2018, 101, 398–402. [Google Scholar] [CrossRef]
- Hakami, M. Risk assessment of heavy metals in fish in Saudi Arabia. Am. J. Environ. Sci. 2016, 12, 341–357. [Google Scholar] [CrossRef]
- Al-Majed, N.B.; Preston, M.R. An assessment of the total and methyl mercury content of zooplankton and fish tissue collected from Kuwait territorial waters. Mar. Pollut. Bull. 2000, 40, 298–307. [Google Scholar] [CrossRef]
- Freije, A.; Awadh, M. Total and methylmercury intake associated with fish consumption in Bahrain. Water Environ. J. 2009, 23, 155–164. [Google Scholar] [CrossRef]
- Walther, E.J.; Arthur, D.E.; Cyr, A.; Fraley, K.M.; Cubbage, T.; Hinkle, E.; McMahon, J.; Westley, P.A. Ecotoxicology of mercury in burbot (Lota lota) from interior Alaska and insights towards human health. Chemosphere 2022, 298, 134279. [Google Scholar] [CrossRef]
- Vieira, H.C.; Rodrigues, A.C.; Soares, A.M.; Abreu, S.; Morgado, F. Mercury accumulation and elimination in different tissues of zebrafish (Danio rerio) exposed to a mercury-supplemented diet. J. Mar. Sci. Eng. 2021, 9, 882. [Google Scholar] [CrossRef]
- Sun, Y.; Li, Y.; Liu, Z.; Chen, Q. Environmentally relevant concentrations of mercury exposure alter thyroid hormone levels and gene expression in the hypothalamic–pituitary–thyroid axis of zebrafish larvae. Fish Physiol. Biochem. 2018, 44, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.N.; Patel, U.D.; Khadayata, A.V.; Vaja, R.K.; Modi, C.M.; Patel, H.B. Long-term exposure of the binary mixture of cadmium and mercury damages the developed ovary of adult zebrafish. Environ. Sci. Pollut. Res. 2022, 29, 44928–44938. [Google Scholar] [CrossRef]
- Scheuhammer, A.; Basu, N.; Burgess, N.; Elliott, J.; Campbell, G.; Wayland, M.; Champoux, L.; Rodrigue, J. Relationships among mercury, selenium, and neurochemical parameters in common loons (Gavia immer) and bald eagles (Haliaeetus leucocephalus). Ecotoxicology 2008, 17, 93–101. [Google Scholar] [CrossRef]
- Henny, C.J.; Hill, E.F.; Grove, R.A.; Chelgren, N.D.; Haggerty, P.K. Mercury and drought along the lower Carson River, Nevada: IV. Snowy egret post-fledging dispersal, timing of migration and survival, 2002–2004. Ecotoxicol. Environ. Saf. 2017, 135, 358–367. [Google Scholar] [CrossRef]
- Wiener, J.; Krabbenhoft, D.; Heinz, G.; Scheuhammer, A. Ecotoxicology of mercury. In Handbook of Ecotoxicology, 2nd ed.; Hoffman, D.J., Rattner, B.A., Burton, G.A., Jr., Cairns, J., Jr., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 409–463. [Google Scholar]
- Whitney, M.C.; Cristol, D.A. Impacts of sublethal mercury exposure on birds: A detailed review. Rev. Environ. Contam. Toxicol. 2017, 244, 113–163. [Google Scholar]
- Fuchsman, P.C.; Brown, L.E.; Henning, M.H.; Bock, M.J.; Magar, V.S. Toxicity reference values for methylmercury effects on avian reproduction: Critical review and analysis. Environ. Toxicol. Chem. 2017, 36, 294–319. [Google Scholar] [CrossRef]
- Martinez, C.S.; Escobar, A.G.; Torres, J.G.D.; Brum, D.S.; Santos, F.W.; Alonso, M.J.; Salaices, M.; Vassallo, D.V.; Peçanha, F.M.; Leivas, F.G. Chronic exposure to low doses of mercury impairs sperm quality and induces oxidative stress in rats. J. Toxicol. Environ. Health Part A 2014, 77, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Shore, R.F.; Pereira, M.G.; Walker, L.A.; Thompson, D.R. Mercury in nonmarine birds and mammals. In Environmental Contaminants in Biota; CRC Press: Boca Raton, FL, USA, 2011; pp. 609–626. [Google Scholar]
- Basu, N.; Scheuhammer, A.M.; Rouvinen-Watt, K.; Grochowina, N.; Evans, R.D.; O’Brien, M.; Chan, H.M. Decreased N-methyl-d-aspartic acid (NMDA) receptor levels are associated with mercury exposure in wild and captive mink. Neurotoxicology 2007, 28, 587–593. [Google Scholar] [CrossRef]
- Kimakova, T.; Kuzmova, L.; Nevolna, Z.; Bencko, V. Fish and fish products as risk factors of mercury exposure. Ann. Agric. Environ. Med. 2018, 25, 488–493. [Google Scholar] [CrossRef]
- Ruggieri, F.; Majorani, C.; Domanico, F.; Alimonti, A. Mercury in children: Current state on exposure through human biomonitoring studies. Int. J. Environ. Res. Public Health 2017, 14, 519. [Google Scholar] [CrossRef] [PubMed]
- Crowe, W.; Allsopp, P.J.; Watson, G.E.; Magee, P.J.; Strain, J.; Armstrong, D.J.; Ball, E.; McSorley, E.M. Mercury as an environmental stimulus in the development of autoimmunity—A systematic review. Autoimmun. Rev. 2017, 16, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Li, M.; Bullard, K. Decadal changes in the edible supply of seafood and methylmercury exposure in the United States. Environ. Health Perspect. 2018, 126, 017006. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Air Quality Guidelines for Europe; World Health Organization; Regional Office for Europe: Geneva, Switzerland, 2000.
- Henriques, M.C.; Loureiro, S.; Fardilha, M.; Herdeiro, M.T. Exposure to mercury and human reproductive health: A systematic review. Reprod. Toxicol. 2019, 85, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Sales, M.V.; da Silva Filho, R.C.; Silva, M.M.; Rocha, J.L.; Freire, R.O.; Tanabe, E.L.d.L.; Silva, E.C.; Fonseca, E.J.S.; Figueiredo, I.M.; Rocha, U. Consequences of thimerosal on human erythrocyte hemoglobin: Assessing functional and structural protein changes induced by an organic mercury compound. J. Trace Elem. Med. Biol. 2022, 71, 126928. [Google Scholar] [CrossRef]
- Zhao, M.M.; Kou, J.-b.; Chen, Y.-p.; Xue, L.-g.; Fan, T.T.; Wang, S.-m. Bioremediation of wastewater containing mercury using three newly isolated bacterial strains. J. Clean. Prod. 2021, 299, 126869. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Zhao, D.; Zhuang, L.; Yang, G.; Gong, Y. Immobilization of mercury by iron sulfide nanoparticles alters mercury speciation and microbial methylation in contaminated groundwater. Chem. Eng. J. 2020, 381, 122664. [Google Scholar] [CrossRef]
- Moharem, M.; Elkhatib, E.; Mesalem, M. Remediation of chromium and mercury polluted calcareous soils using nanoparticles: Sorption–desorption kinetics, speciation and fractionation. Environ. Res. 2019, 170, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Yang, B.; Kou, Y.; Zeng, J.; Wang, R.; Xiao, Y.; Li, F.; Lu, Y.; Mu, Y.; Zhao, C. Assessing the difference of tolerance and phytoremediation potential in mercury contaminated soil of a non-food energy crop, Helianthus tuberosus L. (Jerusalem artichoke). PeerJ 2018, 6, e4325. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Othmani, A.; Malloum, A.; Christ, O.K.; Onyeaka, H.; AlKafaas, S.S.; Nnaji, N.D.; Bornman, C.; Al-Sharify, Z.T.; Ahmadi, S. Removal of mercury from industrial effluents by adsorption and advanced oxidation processes: A comprehensive review. J. Mol. Liq. 2022, 367, 120491. [Google Scholar] [CrossRef]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. J. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Xun, Y.; Feng, L.; Li, Y.; Dong, H. Mercury accumulation plant Cyrtomium macrophyllum and its potential for phytoremediation of mercury polluted sites. Chemosphere 2017, 189, 161–170. [Google Scholar] [CrossRef]
- Su, R.; Ou, Q.; Wang, H.; Dai, X.; Chen, Y.; Luo, Y.; Yao, H.; Ouyang, D.; Li, Z.; Wang, Z. Organic-inorganic composite modifiers enhance restoration potential of Nerium oleander L. to lead-zinc tailing: Application of phytoremediation. Environ. Sci. Pollut. Res. Int. 2023, 30, 56569–56579. [Google Scholar] [CrossRef]
- Marrugo-Negrete, J.; Enamorado-Montes, G.; Durango-Hernández, J.; Pinedo-Hernández, J.; Díez, S. Removal of mercury from gold mine effluents using Limnocharis flava in constructed wetlands. Chemosphere 2017, 167, 188–192. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.-a.; Ding, S.; Xiao, H. Enhancer assisted-phytoremediation of mercury-contaminated soils by Oxalis corniculata L., and rhizosphere microorganism distribution of Oxalis corniculata L. Ecotoxicol. Environ. Saf. 2018, 160, 171–177. [Google Scholar] [CrossRef]
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2017, 17, 1224–1236. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Luo, H.; Zhang, M.; Zhang, D.; Pan, X.; Gadd, G.M. Multiple-pathway remediation of mercury contamination by a versatile selenite-reducing bacterium. Sci. Total Environ. 2018, 615, 615–623. [Google Scholar] [CrossRef]
- McCarthy, D.; Edwards, G.C.; Gustin, M.S.; Care, A.; Miller, M.B.; Sunna, A. An innovative approach to bioremediation of mercury contaminated soils from industrial mining operations. Chemosphere 2017, 184, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Gong, Y.; Hu, Q. Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res. 2018, 29, 142–153. [Google Scholar] [CrossRef]
- Naik, M.M.; Dubey, S. Lead-and mercury-resistant marine bacteria and their application in lead and mercury bioremediation. In Marine Pollution and Microbial Remediation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 29–40. [Google Scholar]
- Nath, S.; Paul, P.; Roy, R.; Bhattacharjee, S.; Deb, B. Isolation and identification of metal-tolerant and antibiotic-resistant bacteria from soil samples of Cachar district of Assam, India. SN Appl. Sci. 2019, 1, 727. [Google Scholar] [CrossRef]
- Saranya, K.; Sundaramanickam, A.; Shekhar, S.; Swaminathan, S.; Balasubramanian, T. Bioremediation of mercury by Vibrio fluvialis screened from industrial effluents. BioMed Res. Int. 2017, 2017, 6509648. [Google Scholar] [CrossRef]
- Mahbub, K.R.; Krishnan, K.; Naidu, R.; Megharaj, M. Mercury remediation potential of a mercury resistant strain Sphingopyxis sp. SE2 isolated from contaminated soil. J. Environ. Sci. 2017, 51, 128–137. [Google Scholar] [CrossRef]
- Bravo, G.; Vega-Celedón, P.; Gentina, J.C.; Seeger, M. Effects of mercury II on Cupriavidus metallidurans strain MSR33 during mercury bioremediation under aerobic and anaerobic conditions. Processes 2020, 8, 893. [Google Scholar] [CrossRef]
- Naguib, M.M.; Khairalla, A.S.; El-Gendy, A.O.; Elkhatib, W.F. Isolation and characterization of mercury-resistant bacteria from wastewater sources in Egypt. Can. J. Microbiol. 2019, 65, 308–321. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Wei, X.; Xu, P.; Xu, W.; Ni, R.; Meng, J. Magnetic solid-phase extraction for the removal of mercury from water with ternary hydrosulphonyl-based deep eutectic solvent modified magnetic graphene oxide. Talanta 2018, 188, 454–462. [Google Scholar] [CrossRef]
- Cheng, S.Y.; Show, P.-L.; Lau, B.F.; Chang, J.-S.; Ling, T.C. New prospects for modified algae in heavy metal adsorption. Trends Biotechnol. 2019, 37, 1255–1268. [Google Scholar] [CrossRef]
- Kumar, M.; Goswami, L.; Singh, A.K.; Sikandar, M. Valorization of coal fired-fly ash for potential heavy metal removal from the single and multi-contaminated system. Heliyon 2019, 5, e02562. [Google Scholar] [CrossRef]
- Fabre, E.; Dias, M.; Costa, M.; Henriques, B.; Vale, C.; Lopes, C.B.; Pinheiro-Torres, J.; Silva, C.M.; Pereira, E. Negligible effect of potentially toxic elements and rare earth elements on mercury removal from contaminated waters by green, brown and red living marine macroalgae. Sci. Total Environ. 2020, 724, 138133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Singh, A.K.; Sikandar, M. Biosorption of Hg (II) from aqueous solution using algal biomass: Kinetics and isotherm studies. Heliyon 2020, 6, e03321. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Rocha, L.S.; Lopes, C.B.; Figueira, P.; Duarte, A.; Vale, C.; Pardal, M.; Pereira, E. A macroalgae-based biotechnology for water remediation: Simultaneous removal of Cd, Pb and Hg by living Ulva lactuca. J. Environ. Manag. 2017, 191, 275–289. [Google Scholar] [CrossRef]
- Lei, P.; Nunes, L.M.; Liu, Y.-R.; Zhong, H.; Pan, K. Mechanisms of algal biomass input enhanced microbial Hg methylation in lake sediments. Environ. Int. 2019, 126, 279–288. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.; Chen, Z.; Zhu, L.; Schnoor, J.L. Insight into multiple and multilevel structures of biochars and their potential environmental applications: A critical review. Environ. Sci. Technol. 2018, 52, 5027–5047. [Google Scholar] [CrossRef]
- Faheem, B.J.; Zheng, H.; Tufail, H.; Irshad, S.; Du, J. Adsorption-assisted decontamination of Hg (II) from aqueous solution by multi-functionalized corncob-derived biochar. RSC Adv. 2018, 8, 38425–38435. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, S.; Lyu, J.; Tang, J. Highly efficient removal of aqueous Hg2+ and CH3Hg+ by selective modification of biochar with 3-mercaptopropyltrimethoxysilane. Chem. Eng. J. 2019, 360, 1646–1655. [Google Scholar] [CrossRef]
- Xu, W.; Adewuyi, Y.G.; Liu, Y.; Wang, Y. Removal of elemental mercury from flue gas using CuOx and CeO2 modified rice straw chars enhanced by ultrasound. Fuel Process. Technol. 2018, 170, 21–31. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Wang, Q.; Pan, J. Removal of elemental mercury from flue gas using wheat straw chars modified by Mn-Ce mixed oxides with ultrasonic-assisted impregnation. Chem. Eng. J. 2017, 326, 169–181. [Google Scholar] [CrossRef]
- O’Connor, D.; Peng, T.; Li, G.; Wang, S.; Duan, L.; Mulder, J.; Cornelissen, G.; Cheng, Z.; Yang, S.; Hou, D. Sulfur-modified rice husk biochar: A green method for the remediation of mercury contaminated soil. Sci. Total Environ. 2018, 621, 819–826. [Google Scholar] [CrossRef]
- Beckers, F.; Awad, Y.M.; Beiyuan, J.; Abrigata, J.; Mothes, S.; Tsang, D.C.; Ok, Y.S.; Rinklebe, J. Impact of biochar on mobilization, methylation, and ethylation of mercury under dynamic redox conditions in a contaminated floodplain soil. Environ. Int. 2019, 127, 276–290. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Pan, X.; Gadd, G.M. Heteroaggregation of soil particulate organic matter and biogenic selenium nanoparticles for remediation of elemental mercury contamination. Chemosphere 2019, 221, 486–492. [Google Scholar] [CrossRef]
- Liu, P.; Ptacek, C.J.; Blowes, D.W.; Landis, R.C. Mechanisms of mercury removal by biochars produced from different feedstocks determined using X-ray absorption spectroscopy. J. Hazard. Mater. 2016, 308, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Hong, J. Preparation of a novel millet straw biochar-bentonite composite and its adsorption property of Hg2+ in aqueous solution. Materials 2021, 14, 1117. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, J.; Xia, J.; Liu, Z.; Zhang, Y.; Du, Y.; Wei, W. A pilot study on using biochars as sustainable amendments to inhibit rice uptake of Hg from a historically polluted soil in a Karst region of China. Ecotoxicol. Environ. Saf. 2019, 170, 18–24. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Xu, Z.; Wang, M.; Man, Y.B.; Christie, P.; Liang, P.; Shan, S.; Wong, M.H. The role of sewage sludge biochar in methylmercury formation and accumulation in rice. Chemosphere 2019, 218, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Adewuyi, Y.G. A review on removal of elemental mercury from flue gas using advanced oxidation process: Chemistry and process. Chem. Eng. Res. Des. 2016, 112, 199–250. [Google Scholar] [CrossRef]
- Hao, R.; Wang, Z.; Mao, X.; Gong, Y.; Yuan, B.; Zhao, Y.; Tian, B.; Qi, M. Elemental mercury removal by a novel advanced oxidation process of ultraviolet/chlorite-ammonia: Mechanism and kinetics. J. Hazard. Mater. 2019, 374, 120–128. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y. Gaseous elemental mercury removal using VUV and heat coactivation of Oxone/H2O/O2 in a VUV-spraying reactor. Fuel 2019, 243, 352–361. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Liu, Y. Oxidative absorption of elemental mercury from flue gas using a modified Fenton-like wet scrubbing system. Energy Fuels 2019, 33, 3028–3033. [Google Scholar] [CrossRef]
- Chen, W.; Pei, Y.; Huang, W.; Qu, Z.; Hu, X.; Yan, N. Novel effective catalyst for elemental mercury removal from coal-fired flue gas and the mechanism investigation. Environ. Sci. Technol. 2016, 50, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, L.; Chen, X.; Zhu, Q.; Liu, Z.; Xiang, J. Photocatalytic oxidation removal of Hg0 using ternary Ag/AgI-Ag2CO3 hybrids in wet scrubbing process under fluorescent light. Appl. Surf. Sci. 2017, 392, 1107–1116. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, A.; Zhu, Q.; Wang, H.; Zhang, C. Effects of experimental parameters on Hg0 removal over magnetic AgI-BiOI/CoFe2O4 photocatalysts using wet process. J. Fuel Chem. Technol. 2018, 46, 365–374. [Google Scholar] [CrossRef]
- Li, C.; Zhang, A.; Zhang, L.; Song, J.; Su, S.; Sun, Z.; Xiang, J. Enhanced photocatalytic activity and characterization of magnetic Ag/BiOI/ZnFe2O4 composites for Hg0 removal under fluorescent light irradiation. Appl. Surf. Sci. 2018, 433, 914–926. [Google Scholar] [CrossRef]
- Gil-Díaz, M.; Alonso, J.; Rodríguez-Valdés, E.; Gallego, J.; Lobo, M.C. Comparing different commercial zero valent iron nanoparticles to immobilize As and Hg in brownfield soil. Sci. Total Environ. 2017, 584, 1324–1332. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Pan, X.; Lee, D.-J.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M. Aerobic and anaerobic biosynthesis of nano-selenium for remediation of mercury contaminated soil. Chemosphere 2017, 170, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Janeiro-Tato, I.; Lopez-Anton, M.A.; Baragaño, D.; Antuña-Nieto, C.; Rodriguez, E.; Pelaez, A.I.; Gallego, J.; Martinez-Tarazona, M.R. Immobilization of mercury in contaminated soils through the use of new carbon foam amendments. Environ. Sci. Eur. 2021, 33, 127. [Google Scholar] [CrossRef]
- Dai, X.; Zhou, X.; Liu, H.; Wang, T.; Zhang, Y.; Zhang, H.; Sun, B. Molecular-level insights into the immobilization of vapor-phase mercury on Fe/Co/Ni-doped hierarchical molybdenum selenide. J. Hazard. Mater. 2021, 420, 126583. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, R.; Li, T.; Komarneni, S.; Liu, B. Advances in recyclable and superior photocatalytic fibers: Material, construction, application and future perspective. Compos. Part B Eng. 2021, 205, 108512. [Google Scholar] [CrossRef]
- Cui, W.; Liu, G.; Bezerra, M.; Lagos, D.A.; Li, Y.; Cai, Y. Occurrence of Methylmercury in Rice-Based Infant Cereals and Estimation of Daily Dietary Intake of Methylmercury for Infants. J. Agric. Food Chem. 2017, 65, 9569–9578. [Google Scholar] [CrossRef]
- Afonso, C.; Costa, S.; Cardoso, C.; Bandarra, N.M.; Batista, I.; Coelho, I.; Castanheira, I.; Nunes, M.L. Evaluation of the risk/benefit associated to the consumption of raw and cooked farmed meagre based on the bioaccessibility of selenium, eicosapentaenoic acid and docosahexaenoic acid, total mercury, and methylmercury determined by an in vitro digestion model. Food Chem. 2015, 170, 249–256. [Google Scholar]
- Wang, H.-S.; Xu, W.-F.; Chen, Z.-J.; Cheng, Z.; Ge, L.-C.; Man, Y.-B.; Giesy, J.P.; Du, J.; Wong, C.K.C.; Wong, M.-H. In vitro estimation of exposure of Hong Kong residents to mercury and methylmercury via consumption of market fishes. J. Hazard. Mater. 2013, 248–249, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Liberda, E.N.; Tsuji, L.J.S.; Martin, I.D.; Ayotte, P.; Dewailly, E.; Nieboer, E. The complexity of hair/blood mercury concentration ratios and its implications. Environ. Res. 2014, 134, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Zhang, L.; Li, Z.; Liu, J.-m.; Ye, R.; Ren, A. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reprod. Toxicol. 2013, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- García-Esquinas, E.; Pérez-Gómez, B.; Fernández-Navarro, P.; Fernández, M.A.; de Paz, C.; Pérez-Meixeira, A.M.; Gil, E.; Iriso, A.; Sanz, J.C.; Astray, J.; et al. Lead, mercury and cadmium in umbilical cord blood and its association with parental epidemiological variables and birth factors. BMC Public Health 2013, 13, 841. [Google Scholar] [CrossRef]
- Grotto, D.; Valentini, J.; Fillion, M.; Passos, C.J.S.; Garcia, S.C.; Mergler, D.; Barbosa, F. Mercury exposure and oxidative stress in communities of the Brazilian Amazon. Sci. Total Environ. 2010, 408, 806–811. [Google Scholar] [CrossRef]
- Pinheiro, M.; Macchi, B.; Vieira, J.; Oikawa, T.; Amoras, W.; Guimarães, G.; Costa, C.; Crespo-López, M.; Herculano, A.; Silveira, L. Mercury exposure and antioxidant defenses in women: A comparative study in the Amazon. Environ. Res. 2008, 107, 53–59. [Google Scholar] [CrossRef]
- Sharma, B.M.; Sáňka, O.; Kalina, J.; Scheringer, M. An overview of worldwide and regional time trends in total mercury levels in human blood and breast milk from 1966 to 2015 and their associations with health effects. Environ. Int. 2019, 125, 300–319. [Google Scholar] [CrossRef]
- Steenhuisen, F.; Wilson, S.J. Development and application of an updated geospatial distribution model for gridding 2015 global mercury emissions. Atmos. Environ. 2019, 211, 138–150. [Google Scholar] [CrossRef]
- Nakamura, M.; Hachiya, N.; Murata, K.-y.; Nakanishi, I.; Kondo, T.; Yasutake, A.; Miyamoto, K.-i.; Ser, P.H.; Omi, S.; Furusawa, H. Methylmercury exposure and neurological outcomes in Taiji residents accustomed to consuming whale meat. Environ. Int. 2014, 68, 25–32. [Google Scholar] [CrossRef]
- Tang, W.; Dang, F.; Evans, D.; Zhong, H.; Xiao, L. Understanding reduced inorganic mercury accumulation in rice following selenium application: Selenium application routes, speciation and doses. Chemosphere 2017, 169, 369–376. [Google Scholar] [CrossRef]
- Fillion, M.; Lemire, M.; Philibert, A.; Frenette, B.; Weiler, H.A.; Deguire, J.R.; Guimarães, J.R.D.; Larribe, F.; Barbosa, F., Jr.; Mergler, D. Toxic risks and nutritional benefits of traditional diet on near visual contrast sensitivity and color vision in the Brazilian Amazon. Neurotoxicology 2013, 37, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, S.E.; Keiser, S.; Ajami, N.J.; Wong, M.C.; Gesell, J.; Petrosino, J.F.; Johs, A. The role of gut microbiota in fetal methylmercury exposure: Insights from a pilot study. Toxicol. Lett. 2016, 242, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.S.; Blum, J.D.; Franzblau, A.; Basu, N. New insight into biomarkers of human mercury exposure using naturally occurring mercury stable isotopes. Environ. Sci. Technol. 2013, 47, 3403–3409. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-L.; Kwon, S.Y.; Poulin, B.A.; Tsui, M.T.-K.; Motta, L.C.; Cho, M. Internal Dynamics and Metabolism of Mercury in Biota: A Review of Insights from Mercury Stable Isotopes. Environ. Sci. Technol. 2022, 56, 9182–9195. [Google Scholar] [CrossRef]
- Ewald, J.D.; Kirk, J.L.; Li, M.; Sunderland, E.M. Organ-specific differences in mercury speciation and accumulation across ringed seal (Phoca hispida) life stages. Sci. Total Environ. 2019, 650, 2013–2020. [Google Scholar] [CrossRef]
- Dietz, R.; Sonne, C.; Basu, N.; Braune, B.; O’Hara, T.; Letcher, R.J.; Scheuhammer, T.; Andersen, M.; Andreasen, C.; Andriashek, D.; et al. What are the toxicological effects of mercury in Arctic biota? Sci. Total Environ. 2013, 443, 775–790. [Google Scholar] [CrossRef]



| Exposure Groups | Sources | Reference |
|---|---|---|
| Atmospheric exposure | Burning of fossil fuels and coal | [20] |
| Metal mining | [1] | |
| Fertilizers, pesticides, and other chemical manufacturing | [21] | |
| Incineration of domestic and industrial waste | [22] | |
| Volcanic eruption, first fire | [23] | |
| Hydrosphere exposure | Entry of Hg to water bodies through effluents | [24] |
| Discharges from oil refineries | [25] | |
| Food products | Consumption of food products contaminated with Hg | [26] |
| Cosmetic products | Skin lightening creams, toothpaste, and soap | [27] |
| Medical products | Dental amalgam, antiseptics and some Ayurvedic medicines. | [28] |
| Consumer goods | Plastic, paint, batteries, lamps, switches, thermometers, bulbs | [29] |
| Organism | Mercuric Concentration (ppm) | Geographical Locations | Reference |
|---|---|---|---|
| Lethrinus nebulosus | 0.771 | Qatar | [72] |
| Natrix maura | 0.194 | Europe | [73] |
| Epinephelus coioides | 0.55 | Kuwait | [74] |
| Epinephelus coioides | 0.045 | China | [16] |
| Urtica dioica | 21.4 | France | [75] |
| Elateridae sp. | 3.6798 | France | [75] |
| Rastrelliger brachysoma | 0.025 | Malaysia | [76] |
| Stolephorus indicus | 0.04–0.18 | UAE | [77] |
| Scylla serrata | 4.11 | India | [78] |
| Penaeus monodon | 2.25 | India | [78] |
| Gerres oyena | 0.0283 | Qatar | [79] |
| Chiloscyllium arabicum | 0.1662 | Qatar | [79] |
| Rhizoprionodon oligolinx | 0.7942 | Qatar | [79] |
| Chaetobranchus semifasciatus | 2.85 | India | [80] |
| Liza macrolepis | 1.860 | India | [80] |
| Gazza minuta | 1.97 | India | [80] |
| Chilina sp. (snails) | 0.564 | Argentina | [81] |
| Samastacus spinifrons (crayfsh) | 0.561 | Argentina | [82] |
| Aquila chrysaetos (Scottish golden eagles) | 0.0348 | Scotland | [83] |
| Arius arius | 0.977 | India | [78] |
| Caranx affinis | 3 | India | [78] |
| Trophic Region | Fish | Concentration of Mercury (ppm) | Portion | Reference |
|---|---|---|---|---|
| Omnivores | Siganus fuscessens | 0.018 | Muscle | [135] |
| Herbivore | Siganus rivulatus | 0.02 | Muscle | [136] |
| Carnivores | Epinephelus coioides | 4.65 | Liver | [137] |
| Herbivore | Siganus canaliculatus | 0.032 | Muscle | [138] |
| Carnivores | Lethrinus nebulosus | 0.773 | Liver | [72] |
| Carnivores | Lethrinus nebulosus | 0.522 | Muscle | [138] |
| Carnivores | Gerres oyena | 0.028 | Muscle | [79] |
| Planktivores | Sardinella albella | 0.028 | Muscle | [138] |
| Human System | Health Effects |
|---|---|
| Intestinal system | Effects on the gastrointestinal system include nausea, cramping, diarrhoea, and corrosiveness, disorders of the digestive tract. |
| Urogenital system | Short-term proteinuria with renal dysfunction, renal failure |
| Central nervous system | Erethism, amnesia, sleeplessness, impaired nerve sensation, and motor skills are all symptoms of a breakdown in the nervous system. Acrodynia, convulsions, vision and hearing loss, language difficulties, memory loss, apathy, paraesthesia, and limb and facial numbness are all symptoms of this condition. Defective brain function and stunted growth in children and unborn babies. Discomfort in the nerves, a lightening of the cerebellum and brain, erratic arm motions, difficulty swallowing, and so on. |
| Skin and eye irritation, Increased blood pressure: a problem for the cardiovascular system, pain in the chest, shortness of breath, and diminished lung capacity | |
| Genotoxicity | Abnormalities and diseases of the chromosomes, lymphocytes with chromosomal abnormalities. |
| Sr. No. | Name of the Technique | Positives | Negatives | References |
|---|---|---|---|---|
| Biological remediation | ||||
| 1. | Phytoremediation | More affordable; eco-friendly; easy to usage | Time-consuming; root depth and Hg concentration limit this process | [163,164,166,167,168] |
| 2. | Microbial treatment | High effectivity; economic; and environmentally benign | Less studied; not always appropriate for the other ecosystems | [66,174] |
| 3. | Algae-based treatment | Cosmopolitans in nature; low-cost adsorbents; environmentally friendly; no secondary hazardous products formation | Time-consuming, less effective, some controversial where hazardous MeHg production takes place | [181,182] |
| 4. | Biochar-based method | Excellent removal efficiency; cost-effective | Time- consuming; involve the use of caustic acids and bases and other hazardous chemical reagents | [186,194] |
| Physicochemical remediation | ||||
| 5. | Advance oxidation processes (AOPs) | Strong oxidation capability and low energy usage | Use of expensive chemicals; generation of poisonous sludge, and other associated by-products | [198] |
| 6. | Immobilization method | High and fast mercury sorption rate; good selectivity for Hg; affordable and accessible | Environmental unfriendly; generation of toxic secondary products | [208] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.; Umesh, M.; Shanmugam, M.K.; Chakraborty, P.; Duhan, L.; Gummadi, S.N.; Pasrija, R.; Jayaraj, I.; Dasarahally Huligowda, L.K. A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects. Sustainability 2023, 15, 13292. https://doi.org/10.3390/su151813292
Kumar V, Umesh M, Shanmugam MK, Chakraborty P, Duhan L, Gummadi SN, Pasrija R, Jayaraj I, Dasarahally Huligowda LK. A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects. Sustainability. 2023; 15(18):13292. https://doi.org/10.3390/su151813292
Chicago/Turabian StyleKumar, Vinay, Mridul Umesh, Manoj Kumar Shanmugam, Pritha Chakraborty, Lucky Duhan, Sathyanarayana N. Gummadi, Ritu Pasrija, Iyyappan Jayaraj, and Lohith Kumar Dasarahally Huligowda. 2023. "A Retrospection on Mercury Contamination, Bioaccumulation, and Toxicity in Diverse Environments: Current Insights and Future Prospects" Sustainability 15, no. 18: 13292. https://doi.org/10.3390/su151813292