Research Progress on Stimulus-Responsive Polymer Nanocarriers for Cancer Treatment
Abstract
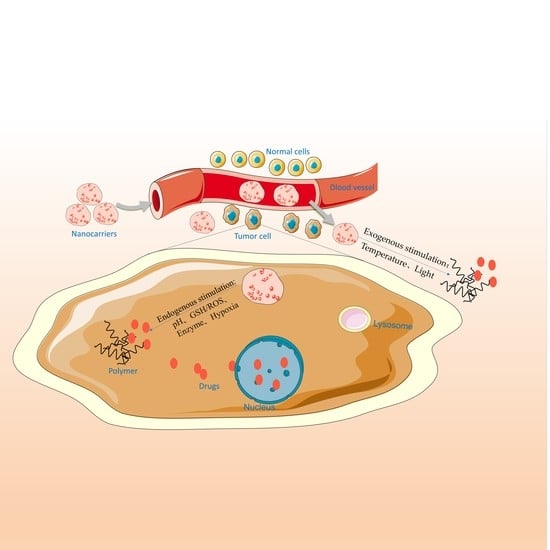
:1. Introduction
2. Single Stimulus-Responsive Polymer Nanocarriers
2.1. Endogenous Stimulus-Responsive Nanocarriers
2.1.1. pH Stimulus-Responsive Polymeric Nanocarriers
2.1.2. Redox Stimulus-Responsive Polymeric Nanocarriers
2.1.3. Enzyme Stimulus-Responsive Polymeric Nanocarriers
2.1.4. Hypoxia Stimulus-Responsive Polymeric Nanocarriers
2.2. Exogenous Stimulus-Responsive Nanocarriers
2.2.1. Temperature Stimulus-Responsive Polymeric Nanocarriers
2.2.2. Light Stimulus-Responsive Polymeric Nanocarriers
2.3. Other Stimulus-Responsive Polymeric Nanocarriers
3. Multiple Stimulation-Responsive Polymeric Nanocarriers
4. Summary and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Zhang, Y.; Zou, Y.; Wang, Y.; Niu, D.; He, Q.; Huang, Z.; Zhu, W.; Tian, H.; Shi, J. Dual intratumoral redox/enzyme-responsive NO-releasing nanomedicine for the specific, high-efficacy, and low-toxic cancer therapy. Adv. Mater. 2018, 30, 1704490. [Google Scholar] [CrossRef]
- Goyal, A.K.; Rath, G.; Faujdar, C.; Malik, B. Application and Perspective of pH-Responsive Nano Drug Delivery Systems. In Applications of Targeted Nano Drugs and Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2019; pp. 15–33. [Google Scholar]
- Seah, I.; Ong, C.; Liu, Z.; Su, X. Polymeric biomaterials in the treatment of posterior segment diseases. Front. Med. 2022, 9, 949543. [Google Scholar] [CrossRef]
- Solanki, R.; Rostamabadi, H.; Patel, S.; Jafari, S.M. Anticancer nano-delivery systems based on bovine serum albumin nanoparticles: A critical review. Int. J. Biol. Macromol. 2021, 193, 528–540. [Google Scholar] [CrossRef]
- Chen, X.; Zou, J.; Zhang, K.; Zhu, J.; Zhang, Y.; Zhu, Z.; Zheng, H.; Li, F.; Piao, J.G. Photothermal/matrix metalloproteinase-2 dual-responsive gelatin nanoparticles for breast cancer treatment. Acta Pharm. Sin. B 2021, 11, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.; El-Refaie, W.M.; Elnaggar, Y.S.R.; El-Mezayen, N.S.; Awaad, A.K.; Abdallah, O.Y. Fucoidan/hyaluronic acid cross-linked zein nanoparticles loaded with fisetin as a novel targeted nanotherapy for oral cancer. Int. J. Biol. Macromol. 2023, 241, 124528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, D.; Guan, S.; Liu, D.; Wang, J.; Xing, G.; Yue, L.; Cai, D. Tumor-targeted delivery of honokiol via polysialic acid modified zein nanoparticles prevents breast cancer progression and metastasis. Int. J. Biol. Macromol. 2022, 203, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control Release 2017, 260, 46–60. [Google Scholar] [CrossRef]
- Feltrin, F.D.S.; Agner, T.; Sayer, C.; Lona, L.M.F. Curcumin encapsulation in functional PLGA nanoparticles: A promising strategy for cancer therapies. Adv. Colloid Interface Sci. 2022, 300, 102582. [Google Scholar] [CrossRef]
- Peres, C.; Matos, A.I.; Conniot, J.; Sainz, V.; Zupančič, E.; Silva, J.M.; Graça, L.; Gaspar, R.S.; Préat, V.; Florindo, H.F. Poly(lactic acid)-based particulate systems are promising tools for immune modulation. Acta Biomater. 2017, 48, 41–57. [Google Scholar] [CrossRef]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaskar, R.; Sinha, J.K.; Paiva-Santos, A.C.; et al. Chitosan Nanoparticles-Based Cancer Drug Delivery: Application and Challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Richards, L.; Bladen, C.L.; Ingham, E.; Fisher, J.; Tipper, J.L. The biological response to nanometre-sized polymer particles. Acta Biomater. 2015, 23, 38–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkert, S.; Schmidt, T.; Gohs, U.; Dorschner, H.; Arndt, K.-F. Cross-linking of poly(N-vinyl pyrrolidone) films by electron beam irradiation. Radiat. Phys. Chem. 2007, 76, 1324–1328. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Williams, D.F.; Zilla, P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 2015, 36, 6–25. [Google Scholar] [CrossRef]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 186–208. [Google Scholar]
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratton, S.E.; Ropp, P.A.; Pohlhaus, P.D.; Luft, J.C.; Madden, V.J.; Napier, M.E.; DeSimone, J.M. The effect of particle design on cellular internalization pathways. Proc. Natl. Acad. Sci. USA 2008, 105, 11613–11618. [Google Scholar] [CrossRef]
- Niu, S.; Williams, G.R.; Wu, J.; Wu, J.; Zhang, X.; Chen, X.; Li, S.; Jiao, J.; Zhu, L.M. A chitosan-based cascade-responsive drug delivery system for triple-negative breast cancer therapy. J. Nanobiotechnol. 2019, 17, 95. [Google Scholar] [CrossRef]
- Upponi, J.R.; Jerajani, K.; Nagesha, D.K.; Kulkarni, P.; Sridhar, S.; Ferris, C.; Torchilin, V.P. Polymeric micelles: Theranostic co-delivery system for poorly water-soluble drugs and contrast agents. Biomaterials 2018, 170, 26–36. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nanaki, S.; Michailidou, G.; Koumentakou, I.; Lazaridou, M.; Ainali, N.M.; Xanthopoulou, E.; Bikiaris, D.N. Chitosan and its Derivatives for Ocular Delivery Formulations: Recent Advances and Developments. Polymers 2020, 12, 1519. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Zhou, F.; Du, X.; Xu, J.; Gu, X.; Wang, G.; Li, J. Reduction-sensitive mixed micelles for selective intracellular drug delivery to tumor cells and reversal of multidrug resistance. Int. J. Pharm. 2018, 550, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, G.; Su, Y.; Zhou, Y.; Huang, W.; Zhang, R.; Yan, D. Stimuli-responsive nanodrug self-assembled from amphiphilic drug-inhibitor conjugate for overcoming multidrug resistance in cancer treatment. Theranostics 2019, 9, 5755–5768. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, W.; Guo, N.; Huangfu, M.; Liu, H.; Lin, M.; Xu, W.; Chen, J.; Wang, T.; Wei, Q.; et al. Targeting tumor hypoxia with stimulus-responsive nanocarriers in overcoming drug resistance and monitoring anticancer efficacy. Acta Biomater. 2018, 71, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zeng, W.; Huang, P.; Zeng, X.; Mei, L. Smart materials for drug delivery and cancer therapy. View 2020, 2, 20200042. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.J.; Kalhapure, R.S.; Govender, T. Hydrazone linkages in pH responsive drug delivery systems. Eur. J. Pharm. Sci. 2017, 99, 45–65. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef]
- Liu, H.N.; Guo, N.N.; Wang, T.T.; Guo, W.W.; Lin, M.T.; Huang-Fu, M.Y.; Vakili, M.R.; Xu, W.H.; Chen, J.J.; Wei, Q.C.; et al. Mitochondrial Targeted Doxorubicin-Triphenylphosphonium Delivered by Hyaluronic Acid Modified and pH Responsive Nanocarriers to Breast Tumor: In Vitro and in Vivo Studies. Mol. Pharm. 2018, 15, 882–891. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, L.; Li, J.; Song, R.; Wang, Y.; Yu, K.; Hou, X.; Qiao, P.; Zong, L.; Chang, S. A polymeric micelle with an endosomal pH-sensitivity for intracellular delivery and enhanced antitumor efficacy of hydroxycamptothecin. Acta Biomater. 2019, 88, 357–369. [Google Scholar] [CrossRef]
- Liao, S.C.; Ting, C.W.; Chiang, W.H. Functionalized polymeric nanogels with pH-sensitive benzoic-imine cross-linkages designed as vehicles for indocyanine green delivery. J. Colloid Interface Sci. 2020, 561, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.W.; Hsieh, M.H.; Xiao, M.C.; Chou, Y.H.; Wang, T.H.; Chiang, W.H. pH-responsive polymeric micelles self-assembled from benzoic-imine-containing alkyl-modified PEGylated chitosan for delivery of amphiphilic drugs. Int. J. Biol. Macromol. 2020, 163, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Gu, Z. Tumor microenvironment and intracellular signal-activated nanomaterials for anticancer drug delivery. Mater. Today 2016, 19, 274–283. [Google Scholar] [CrossRef]
- Guo, W.; Deng, L.; Yu, J.; Chen, Z.; Woo, Y.; Liu, H.; Li, T.; Lin, T.; Chen, H.; Zhao, M.; et al. Sericin nanomicelles with enhanced cellular uptake and pH-triggered release of doxorubicin reverse cancer drug resistance. Drug Deliv. 2018, 25, 1103–1116. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.; Confeld, M.; Borowicz, P.; Wang, T.; Mallik, S.; Quadir, M. PEG-b-poly (carbonate)-derived nanocarrier platform with pH-responsive properties for pancreatic cancer combination therapy. Colloids Surf. B Biointerfaces 2019, 174, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dai, Z.; Zhou, Y.; Tao, W.; Chen, H.; Zhao, Z.; Liao, X. Acid-sensitive charge-reversal co-assembled polyurethane nanomicelles as drug delivery carriers. Colloids Surf. B Biointerfaces 2022, 209, 112203. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Liu, S.; Ono, R.J.; Yang, C.; Gao, S.; Ming Tan, J.Y.; Hedrick, J.L.; Yang, Y.Y. Dual pH-Responsive Shell-Cleavable Polycarbonate Micellar Nanoparticles for in Vivo Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 19355–19364. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.; Peng, Y.; Ding, J.; Zhou, W. A Smart pH-Sensitive Delivery System for Enhanced Anticancer Efficacy via Paclitaxel Endosomal Escape. Front. Pharmacol. 2019, 10, 10. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Z.; Liu, J.; Deng, X.; Xiong, R.; Cao, X.; Xie, Z.; Lei, X.; Chen, Y.; Tang, G. A pH-sensitive prodrug strategy to co-deliver DOX and TOS in TPGS nanomicelles for tumor therapy. Colloids Surf. B Biointerfaces 2019, 173, 346–355. [Google Scholar] [CrossRef]
- Su, Z.; Liang, Y.; Yao, Y.; Wang, T.; Zhang, N. Polymeric complex micelles based on the double-hydrazone linkage and dual drug-loading strategy for pH-sensitive docetaxel delivery. J. Mater. Chem. B 2016, 4, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Wu, X.; Liu, L.; Yu, H.; Song, S. Hydrazone-Containing Triblock Copolymeric Micelles for pH-Controlled Drug Delivery. Front. Pharmacol. 2018, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezk, A.I.; Obiweluozor, F.O.; Choukrani, G.; Park, C.H.; Kim, C.S. Drug release and kinetic models of anticancer drug (BTZ) from a pH-responsive alginate polydopamine hydrogel: Towards cancer chemotherapy. Int. J. Biol. Macromol. 2019, 141, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiao, Y.; Liu, S.; Yu, J. Camptothecin prodrug nanomicelle based on a boronate ester-linked diblock copolymer as the carrier of doxorubicin with enhanced cellular uptake. J. Biomater. Sci. Polym. Ed. 2018, 29, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zheng, Y.; Xue, Y.; Wu, Y.; Liu, Y.; Cheng, X.; Tang, R. pH-sensitive and tumor-targeting nanogels based on ortho ester-modified PEG for improving the in vivo anti-tumor efficiency of doxorubicin. Colloids Surf. B Biointerfaces 2021, 207, 112024. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, H.; Zhang, Y.; Xu, X.; Yuan, X.; Li, J. pH-Responsive Hyaluronic Acid Nanoparticles for Enhanced Triple Negative Breast Cancer Therapy. Int. J. Nanomed. 2022, 17, 1437–1457. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Zhu, Y.; Wu, X.; Wang, Y.; Liang, F.; Song, S. Hyaluronic-Acid-Based pH-Sensitive Nanogels for Tumor-Targeted Drug Delivery. ACS Biomater. Sci. Eng. 2017, 3, 2410–2419. [Google Scholar] [CrossRef]
- Zhou, S.; Fu, S.; Wang, H.; Deng, Y.; Zhou, X.; Sun, W.; Zhai, Y. Acetal-linked polymeric prodrug micelles based on aliphatic polycarbonates for paclitaxel delivery: Preparation, characterization, in vitro release and anti-proliferation effects. J. Biomater. Sci. Polym. Ed. 2020, 31, 2007–2023. [Google Scholar] [CrossRef]
- Wang, T.W.; Yeh, C.W.; Kuan, C.H.; Wang, L.W.; Chen, L.H.; Wu, H.C.; Sun, J.S. Tailored design of multifunctional and programmable pH-responsive self-assembling polypeptides as drug delivery nanocarrier for cancer therapy. Acta Biomater. 2017, 58, 54–66. [Google Scholar] [CrossRef]
- Abbasi, S.; Yousefi, G.; Tamaddon, A.-M. Polyacrylamide–b-copolypeptide hybrid copolymer as pH-responsive carrier for delivery of paclitaxel: Effects of copolymer composition on nanomicelles properties, loading efficiency and hemocompatibility. Colloids Surf. A Physicochem. Eng. Asp. 2018, 537, 217–226. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chou, H.Y.; Tsai, H.C.; Anbazhagan, R.; Yuh, C.H.; Yang, J.M.; Chang, Y.H. Amino acid-modified PAMAM dendritic nanocarriers as effective chemotherapeutic drug vehicles in cancer treatment: A study using zebrafish as a cancer model. RSC Adv. 2020, 10, 20682–20690. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Wu, G.; Su, C.; Dong, Y.; Zhang, K.; Li, J.; Sun, X.; Li, Y.; Chen, X.; Feng, C. pH-sensitive amphiphilic chitosan-quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr. Polym. 2019, 223, 115072. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Jiao, M.; Lin, H.; Tian, C.; Qu, G.; Xue, J.; Xue, L.; Ju, C.; Zhang, C. Anisamide-functionalized pH-responsive amphiphilic chitosan-based paclitaxel micelles for sigma-1 receptor targeted prostate cancer treatment. Carbohydr. Polym. 2020, 229, 115498. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Li, F.; Tang, Y.; Yang, S.D.; Li, J.Z.; Yuan, Z.Q.; Liu, Y.; Zhou, X.F.; Liu, C.; Zhang, X.N. Stepwise pH-responsive nanoparticles for enhanced cellular uptake and on-demand intracellular release of doxorubicin. Int. J. Nanomed. 2017, 12, 4241–4256. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.; Peng, H.; Liu, C.; Li, D.; Yin, Y.; Lu, B.; Zheng, H.; Wang, Q. Dual pH-responsive-charge-reversal micelle platform for enhanced anticancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111527. [Google Scholar] [CrossRef]
- Kanamala, M.; Palmer, B.D.; Ghandehari, H.; Wilson, W.R.; Wu, Z. PEG-Benzaldehyde-Hydrazone-Lipid Based PEG-Sheddable pH-Sensitive Liposomes: Abilities for Endosomal Escape and Long Circulation. Pharm. Res. 2018, 35, 154. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, C.; Xu, Y.; Jiang, Z.; Hu, T.; Li, C.; Cheng, X. Dual-pH responsive chitosan nanoparticles for improving in vivo drugs delivery and chemoresistance in breast cancer. Carbohydr. Polym. 2022, 290, 119518. [Google Scholar] [CrossRef]
- Chen, M.; Liu, D.; Liu, F.; Wu, Y.; Peng, X.; Song, F. Recent advances of redox-responsive nanoplatforms for tumor theranostics. J. Control Release 2021, 332, 269–284. [Google Scholar] [CrossRef]
- Wang, Y.C.; Wang, F.; Sun, T.M.; Wang, J. Redox-responsive nanoparticles from the single disulfide bond-bridged block copolymer as drug carriers for overcoming multidrug resistance in cancer cells. Bioconjug. Chem. 2011, 22, 1939–1945. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Y.; Chen, Y.; Zhao, W.; Zhai, Q.; Rathod, S.; Huang, Y.; Tang, S.; Kwon, Y.T.; Fernandez, C.; et al. Doxorubicin delivered by a redox-responsive dasatinib-containing polymeric prodrug carrier for combination therapy. J. Control Release 2017, 258, 43–55. [Google Scholar] [CrossRef]
- Liping, Y.; Jian, H.; Zhenchao, T.; Yan, Z.; Jing, Y.; Yangyang, Z.; Jing, G.; Liting, Q. GSH-responsive poly-resveratrol based nanoparticles for effective drug delivery and reversing multidrug resistance. Drug. Deliv. 2022, 29, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Jiang, Z.; Li, J.; Wang, M.; Liu, C.; Qi, W.; Su, R.; He, Z. Co-assembly of curcumin and a cystine bridged peptide to construct tumor-responsive nano-micelles for efficient chemotherapy. J. Mater. Chem. B 2020, 8, 1944–1951. [Google Scholar] [CrossRef]
- Qin, B.; Liu, L.; Wu, X.; Liang, F.; Hou, T.; Pan, Y.; Song, S. mPEGylated solanesol micelles as redox-responsive nanocarriers with synergistic anticancer effect. Acta Biomater. 2017, 64, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, W.; Guo, X.; Kong, F.; Wang, Z.; Zhu, C.; Luo, L.; Li, Q.; Yang, J.; Du, Y. Specifically increased paclitaxel release in tumor and synergetic therapy by a hyaluronic acid–tocopherol nanomicelle. ACS Appl. Mater. Interfaces 2017, 9, 20385–20398. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Liu, W.; Yang, H.; Zhang, P.; Xiao, C.; Chen, X. A glutathione-responsive sulfur dioxide polymer prodrug as a nanocarrier for combating drug-resistance in cancer chemotherapy. Biomaterials 2018, 178, 706–719. [Google Scholar] [CrossRef]
- Tao, W.; He, Z. ROS-responsive drug delivery systems for biomedical applications. Asian J. Pharm. Sci. 2018, 13, 101–112. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, S.; Kang, H.C.; Shim, M.S. ROS-responsive thioether-based nanocarriers for efficient pro-oxidant cancer therapy. J. Ind. Eng. Chem. 2019, 75, 238–245. [Google Scholar] [CrossRef]
- Yin, W.; Ke, W.; Chen, W.; Xi, L.; Zhou, Q.; Mukerabigwi, J.F.; Ge, Z. Integrated block copolymer prodrug nanoparticles for combination of tumor oxidative stress amplification and ROS-responsive drug release. Biomaterials 2019, 195, 63–74. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.S.; Woo, J.; Ahn, S.; Lee, E.; Il Choi, W.; Sung, D. Reactive oxygen species (ROS)-responsive ferrocene-polymer-based nanoparticles for controlled release of drugs. J. Mater. Chem. B 2020, 8, 1906–1913. [Google Scholar] [CrossRef]
- Xu, F.; Li, H.; Luo, Y.L.; Tang, W. Redox-Responsive Self-Assembly Micelles from Poly(N-acryloylmorpholine-block-2-acryloyloxyethyl ferrocenecarboxylate) Amphiphilic Block Copolymers as Drug Release Carriers. ACS Appl. Mater. Interfaces 2017, 9, 5181–5192. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Li, J.; Pu, K. Bioenzyme-based nanomedicines for enhanced cancer therapy. Nano Converg. 2022, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Abed, H.F.; Abuwatfa, W.H.; Husseini, G.A. Redox-Responsive Drug Delivery Systems: A Chemical Perspective. Nanomaterials 2022, 12, 3183. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.H.; Almutairi, A. Recent progress of redox-responsive polymeric nanomaterials for controlled release. J. Mater. Chem. B 2021, 9, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Robby, A.I.; Lee, B.C.; Lee, G.; Park, S.Y. Mitochondria-targeted ROS- and GSH-responsive diselenide-crosslinked polymer dots for programmable paclitaxel release. J. Ind. Eng. Chem. 2021, 99, 98–106. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Li, M.; Li, Y.; Xiong, H.; Jiang, D.; Li, L.; Huang, H.; Kang, Y.; Pang, J. Reactive oxygen species and glutathione dual responsive nanoparticles for enhanced prostate cancer therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 111956. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Huang-Fu, M.Y.; Xu, W.H.; Han, M. Stimulus-responsive nanoscale delivery systems triggered by the enzymes in the tumor microenvironment. Eur. J. Pharm. Biopharm. 2019, 137, 122–130. [Google Scholar] [CrossRef]
- Wen, J.; Anderson, S.M.; Du, J.; Yan, M.; Wang, J.; Shen, M.; Lu, Y.; Segura, T. Controlled protein delivery based on enzyme-responsive nanocapsules. Adv. Mater. 2011, 23, 4549–4553. [Google Scholar] [CrossRef] [Green Version]
- Mu, J.; Lin, J.; Huang, P.; Chen, X. Development of endogenous enzyme-responsive nanomaterials for theranostics. Chem. Soc. Rev. 2018, 47, 5554–5573. [Google Scholar] [CrossRef]
- Mi, P. Stimuli-responsive nanocarriers for drug delivery, tumor imaging, therapy and theranostics. Theranostics 2020, 10, 4557–4588. [Google Scholar] [CrossRef]
- Shahriari, M.; Zahiri, M.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Enzyme responsive drug delivery systems in cancer treatment. J. Control Release 2019, 308, 172–189. [Google Scholar] [CrossRef]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.P.; Cho, K.H.; Uthaman, S.; Cho, C.S.; Park, I.K. Stimuli-Regulated Smart Polymeric Systems for Gene Therapy. Polymers 2017, 9, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, W.; Zha, Z.; Mukerabigwi, J.F.; Chen, W.; Wang, Y.; He, C.; Ge, Z. Matrix Metalloproteinase-Responsive Multifunctional Peptide-Linked Amphiphilic Block Copolymers for Intelligent Systemic Anticancer Drug Delivery. Bioconjug. Chem. 2017, 28, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, R.; Piantelli, M.; Falasca, M. Role of phospholipase C in cell invasion and metastasis. Adv. Biol. Regul. 2013, 53, 309–318. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhou, Z.; Piao, Y.; Kalva, N.; Liu, X.; Tang, J.; Shen, Y. Synthesis of enzyme-responsive phosphoramidate dendrimers for cancer drug delivery. Polym. Chem. 2018, 9, 438–449. [Google Scholar] [CrossRef]
- Mort, J.S.; Buttle, D.J. Cathepsin b. Int. J. Biochem. Cell Biol. 1997, 29, 715–720. [Google Scholar] [CrossRef]
- Zhang, C.; Pan, D.; Li, J.; Hu, J.; Bains, A.; Guys, N.; Zhu, H.; Li, X.; Luo, K.; Gong, Q.; et al. Enzyme-responsive peptide dendrimer-gemcitabine conjugate as a controlled-release drug delivery vehicle with enhanced antitumor efficacy. Acta Biomater. 2017, 55, 153–162. [Google Scholar] [CrossRef]
- Turk, V.; Turk, B.; Turk, D. Lysosomal cysteine proteases: Facts and opportunities. EMBO J. 2001, 20, 4629–4633. [Google Scholar] [CrossRef] [Green Version]
- Rejmanova, P.; Kopeček, J.; Duncan, R.; Lloyd, J. Stability in rat plasma and serum of lysosomally degradable oligopeptide sequences in N-(2-hydroxypropyl) methacrylamide copolymers. Biomaterials 1985, 6, 45–48. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Zhang, M.; Tang, Z.; He, M.; Bu, W. Modulating Hypoxia via Nanomaterials Chemistry for Efficient Treatment of Solid Tumors. Acc. Chem. Res. 2018, 51, 2502–2511. [Google Scholar] [CrossRef]
- Sahu, A.; Choi, W.I.; Tae, G. Recent Progress in the Design of Hypoxia-Specific Nano Drug Delivery Systems for Cancer Therapy. Adv. Ther. 2018, 1, 1800026. [Google Scholar] [CrossRef]
- Brahimi-Horn, M.C.; Chiche, J.; Pouyssegur, J. Hypoxia and cancer. J. Mol. Med. 2007, 85, 1301–1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Ontikatze, T.; Rudner, J.; Handrick, R.; Belka, C.; Jendrossek, V. Dihydroartemisinin is a Hypoxia-Active Anti-Cancer Drug in Colorectal Carcinoma Cells. Front. Oncol. 2014, 4, 116. [Google Scholar] [CrossRef] [Green Version]
- Gallez, B. The Role of Imaging Biomarkers to Guide Pharmacological Interventions Targeting Tumor Hypoxia. Front. Pharmacol. 2022, 13, 853568. [Google Scholar] [CrossRef]
- Hao, D.; Meng, Q.; Jiang, B.; Lu, S.; Xiang, X.; Pei, Q.; Yu, H.; Jing, X.; Xie, Z. Hypoxia-Activated PEGylated Paclitaxel Prodrug Nanoparticles for Potentiated Chemotherapy. ACS Nano 2022, 16, 14693–14702. [Google Scholar] [CrossRef]
- Needham, D.; Dewhirst, M.W. The development and testing of a new temperature-sensitive drug delivery system for the treatment of solid tumors. Adv. Drug Deliv. Rev. 2001, 53, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Vanparijs, N.; Nuhn, L.; De Geest, B.G. Transiently thermoresponsive polymers and their applications in biomedicine. Chem. Soc. Rev. 2017, 46, 1193–1239. [Google Scholar] [CrossRef]
- Chang, S.; Wang, S.; Liu, Z.; Wang, X. Advances of Stimulus-Responsive Hydrogels for Bone Defects Repair in Tissue Engineering. Gels 2022, 8, 389. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid. Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef]
- Scarpa, J.S.; Mueller, D.D.; Klotz, I.M. Slow hydrogen-deuterium exchange in a non-alpha-helical polyamide. J. Am. Chem. Soc. 1967, 89, 6024–6030. [Google Scholar] [CrossRef]
- Saad, A.; Mills, R.; Wan, H.; Mottaleb, M.A.; Ormsbee, L.; Bhattacharyya, D. Thermo-responsive adsorption-desorption of perfluoroorganics from water using PNIPAm hydrogels and pore functionalized membranes. J. Memb. Sci. 2020, 599, 117821. [Google Scholar] [CrossRef] [PubMed]
- Karg, M.; Pastoriza-Santos, I.; Rodriguez-Gonzalez, B.; von Klitzing, R.; Wellert, S.; Hellweg, T. Temperature, pH, and ionic strength induced changes of the swelling behavior of PNIPAM− poly (allylacetic acid) copolymer microgels. Langmuir 2008, 24, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Huang, N.; Chen, D.; Zhang, L.; Dong, X.; Sun, Y.; Zhu, X.; Zhang, F.; Gao, J.; Wang, Y.; et al. Successful in vivo hyperthermal therapy toward breast cancer by Chinese medicine shikonin-loaded thermosensitive micelle. Int. J. Nanomed. 2017, 12, 4019–4035. [Google Scholar] [CrossRef] [Green Version]
- Luckanagul, J.A.; Pitakchatwong, C.; Ratnatilaka Na Bhuket, P.; Muangnoi, C.; Rojsitthisak, P.; Chirachanchai, S.; Wang, Q.; Rojsitthisak, P. Chitosan-based polymer hybrids for thermo-responsive nanogel delivery of curcumin. Carbohydr. Polym. 2018, 181, 1119–1127. [Google Scholar] [CrossRef]
- Ghasemi, S.; Ahmadi, L.; Farjadian, F. Thermo-responsive PNIPAAm-b-PLA amphiphilic block copolymer micelle as nanoplatform for docetaxel drug release. J. Mater. Sci. 2022, 57, 17433–17447. [Google Scholar] [CrossRef]
- Kavaliauskaite, M.; Steponaviciute, M.; Kievisaite, J.; Katelnikovas, A.; Klimkevicius, V. Synthesis and Study of Thermoresponsive Amphiphilic Copolymers via RAFT Polymerization. Polymers 2022, 14, 229. [Google Scholar] [CrossRef]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 589–605. [Google Scholar] [CrossRef]
- Borchers, A.; Pieler, T. Programming pluripotent precursor cells derived from Xenopus embryos to generate specific tissues and organs. Genes 2010, 1, 413–426. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Chung, M.; Shim, M.S. Engineered photo-responsive materials for near-infrared-triggered drug delivery. J. Ind. Eng. Chem. 2015, 31, 15–25. [Google Scholar] [CrossRef]
- Fagan, A.; Bartkowski, M.; Giordani, S. Spiropyran-Based Drug Delivery Systems. Front. Chem. 2021, 9, 720087. [Google Scholar] [CrossRef]
- Pham, S.H.; Choi, Y.; Choi, J. Stimuli-Responsive Nanomaterials for Application in Antitumor Therapy and Drug Delivery. Pharmaceutics 2020, 12, 630. [Google Scholar] [CrossRef]
- Chen, J.; Qian, C.; Ren, P.; Yu, H.; Kong, X.; Huang, C.; Luo, H.; Chen, G. Light-Responsive Micelles Loaded With Doxorubicin for Osteosarcoma Suppression. Front. Pharmacol. 2021, 12, 679610. [Google Scholar] [CrossRef]
- Kim, K.N.; Oh, K.S.; Shim, J.; Schlaepfer, I.R.; Karam, S.D.; Lee, J.J. Light-Responsive Polymeric Micellar Nanoparticles with Enhanced Formulation Stability. Polymer 2021, 13, 377. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Shi, G.; Song, H.; Shi, S.; Zhang, X.; Huang, P.; Wang, Z.; Wang, W.; Wang, C.; et al. A Light Responsive Nanoparticle-Based Delivery System Using Pheophorbide A Graft Polyethylenimine for Dendritic Cell-Based Cancer Immunotherapy. Mol. Pharm. 2017, 14, 1760–1770. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, P.; Liu, Y.; Lu, C.; Qiu, Y.; Mu, H.; Duan, J. A photo-controlled hyaluronan-based drug delivery nanosystem for cancer therapy. Carbohydr. Polym. 2019, 206, 309–318. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Ohulchanskyy, T.Y.; Chen, G. Lanthanide-Doped Near-Infrared Nanoparticles for Biophotonics. Adv. Mater. 2021, 33, e2000678. [Google Scholar] [CrossRef]
- Hou, W.; Liu, R.; Bi, S.; He, Q.; Wang, H.; Gu, J. Photo-Responsive Polymersomes as Drug Delivery System for Potential Medical Applications. Molecules 2020, 25, 5147. [Google Scholar] [CrossRef]
- Hakomori, S.-i. Tumor malignancy defined by aberrant glycosylation and sphingo (glyco) lipid metabolism. Cancer Res. 1996, 56, 5309–5318. [Google Scholar]
- Han, X.; Zhou, S.J.; Tan, Y.Z.; Wu, X.; Gao, F.; Liao, Z.J.; Huang, R.B.; Feng, Y.Q.; Lu, X.; Xie, S.Y. Crystal structures of saturn-like C50Cl10 and pineapple-shaped C64Cl4: Geometric implications of double-and triple-pentagon-fused chlorofullerenes. Angew. Chem. Int. Ed. 2008, 47, 5340–5343. [Google Scholar] [CrossRef]
- Djanashvili, K.; Frullano, L.; Peters, J.A. Molecular recognition of sialic acid end groups by phenylboronates. Chem.-A Eur. J. 2005, 11, 4010–4018. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, S.; Cabral, H.; Ishii, T.; Miura, Y.; Kobayashi, S.; Yamashita, T.; Matsumoto, A.; Miyahara, Y.; Nishiyama, N.; Kataoka, K. Phenylboronic Acid-Installed Polymeric Micelles for Targeting Sialylated Epitopes in Solid Tumors. J. Am. Chem. Soc. 2013, 135, 15501–15507. [Google Scholar] [CrossRef]
- Luo, D.; Yao, Z.; Wu, J. Polymers Based on Phenyl Boric Acid in Tumor-Targeted Therapy. Anti Cancer Agents Med. Chem. 2021, 21, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.Y.; Chin, W.; Ke, X.; Gao, S.; Liu, S.; Cheng, W.; Hedrick, J.L.; Yang, Y.Y. pH and redox dual-responsive biodegradable polymeric micelles with high drug loading for effective anticancer drug delivery. Nanomedicine 2017, 13, 431–442. [Google Scholar] [CrossRef]
- Leong, J.; Chin, W.; Ke, X.; Gao, S.; Kong, H.; Hedrick, J.L.; Yang, Y.Y. Disease-directed design of biodegradable polymers: Reactive oxygen species and pH-responsive micellar nanoparticles for anticancer drug delivery. Nanomedicine 2018, 14, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hou, H.; Zhang, P.; Zhang, Z. Co-delivery of doxorubicin and paclitaxel by reduction/pH dual responsive nanocarriers for osteosarcoma therapy. Drug Deliv. 2020, 27, 1044–1053. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Lu, Q.; Wei, H.; Zhang, S. Phosphorylcholine zwitterionic shell-detachable mixed micelles for enhanced cancerous cellular uptakes and increased DOX release. J. Mater. Chem. B 2022, 10, 5624–5632. [Google Scholar] [CrossRef]
- Hiruta, Y.; Kanda, Y.; Katsuyama, N.; Kanazawa, H. Dual temperature- and pH-responsive polymeric micelle for selective and efficient two-step doxorubicin delivery. RSC Adv. 2017, 7, 29540–29549. [Google Scholar] [CrossRef] [Green Version]
- Pantshwa, J.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Penny, C.; Pillay, V. Synthesis of novel amphiphilic poly(N-isopropylacrylamide)-b-poly(aspartic acid) nanomicelles for potential targeted chemotherapy in ovarian cancer. J. Drug Deliv. Sci. Technol. 2017, 39, 308–323. [Google Scholar] [CrossRef]
- Niu, S.; Bremner, D.H.; Wu, J.; Wu, J.; Wang, H.; Li, H.; Qian, Q.; Zheng, H.; Zhu, L. l-Peptide functionalized dual-responsive nanoparticles for controlled paclitaxel release and enhanced apoptosis in breast cancer cells. Drug Deliv. 2018, 25, 1275–1288. [Google Scholar] [CrossRef] [Green Version]
- Abedi, F.; Davaran, S.; Hekmati, M.; Akbarzadeh, A.; Baradaran, B.; Moghaddam, S.V. An improved method in fabrication of smart dual-responsive nanogels for controlled release of doxorubicin and curcumin in HT-29 colon cancer cells. J. Nanobiotechnol. 2021, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Kalva, N.; Uthaman, S.; Augustine, R.; Jeon, S.H.; Huh, K.M.; Park, I.K.; Kim, I. Photo- and pH-Responsive Polycarbonate Block Copolymer Prodrug Nanomicelles for Controlled Release of Doxorubicin. Macromol. Biosci. 2020, 20, e2000118. [Google Scholar] [CrossRef]
- Cao, Z.; Li, D.; Wang, J.; Xiong, M.; Yang, X. Direct Nucleus-Targeted Drug Delivery Using Cascade pH(e)/Photo Dual-Sensitive Polymeric Nanocarrier for Cancer Therapy. Small 2019, 15, e1902022. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, X.; Nan, Y.; Jia, L.; Sun, J.; Zhang, L.; Wang, Y. Hyaluronidase and pH Dual-Responsive Nanoparticles for Targeted Breast Cancer Stem Cells. Front. Oncol. 2021, 11, 760423. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Liang, Z.; Jiang, X.; Gong, P.; Zhou, L.; Sun, Z.; Xiang, J.; Xu, Z.; Peng, X.; Li, S.; et al. Enzyme and pH dual-responsive hyaluronic acid nanoparticles mediated combination of photodynamic therapy and chemotherapy. Int. J. Biol. Macromol. 2019, 130, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Zhao, T.; Zhou, P.; Chen, S.; Gao, Y.; Liang, L.; Wang, X.; Wang, L. “Click” functionalization of dual stimuli-responsive polymer nanocapsules for drug delivery systems. Polym. Chem. 2017, 8, 3056–3065. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Kumar, D.V.; Kim, H.J.; Seok, Y.J.; Han, S.S. Dual Responsive poly(vinyl caprolactam)-Based Nanogels for Tunable Intracellular Doxorubicin Delivery in Cancer Cells. Pharmaceutics 2022, 14, 852. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Zhao, Y.; Liu, H.; Zhao, Y.; Li, X.; Lin, Q. Biodegradable Micelles for NIR/GSH-Triggered Chemophototherapy of Cancer. Nanomaterials 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Li, J.; Lin, X.; Wei, Z.; Zhang, D.; Zhao, B.; Liu, X.; Liu, J. Reduction/photo dual-responsive polymeric prodrug nanoparticles for programmed siRNA and doxorubicin delivery. Biomater. Sci. 2018, 6, 1457–1468. [Google Scholar] [CrossRef]
- Xie, J.; Tian, S.; Zhang, H.; Feng, C.; Han, Y.; Dai, H.; Yan, L. A Novel NQO1 Enzyme-Responsive Polyurethane Nanocarrier for Redox-Triggered Intracellular Drug Release. Biomacromolecules 2023, 24, 2225–2236. [Google Scholar] [CrossRef]
- Teng, J.; Yue, L.; Li, B.; Yang, J.; Yang, C.; Yang, T.; Zhi, X.; Liu, X.; Zhao, Y.; Zhang, J. Synthesis of cyclodextrin-based temperature/enzyme-responsive nanoparticles and application in antitumor drug delivery. J. Mol. Struct. 2023, 1274, 134596. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Ma, X.; Lei, L.; Li, Y.; Yang, H.; Lei, Z. Temperature, pH, and reduction triple-stimuli-responsive inner-layer crosslinked micelles as nanocarriers for controlled release. J. Appl. Polym. Sci. 2018, 135, 46714. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Tang, D.; An, X.; Guo, Y.; Zhao, Y. Multi-responsive graft copolymer micelles comprising acetal and disulfide linkages for stimuli-triggered drug delivery. J. Mater. Chem. B 2015, 3, 3959–3971. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ni, R.; Ma, X.; Huang, R.; Tang, Z.; Xu, J.; Han, Y.; Guo, Y.; Gu, Z. Hyaluronic acid-based dual-responsive nanomicelles mediated mutually synergistic photothermal and molecular targeting therapies. Nano Res. 2022, 15, 6361–6371. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, J.G.; Chen, Q.; Luo, Y.L.; Xu, F.; Chen, Y.S. Temperature and Photo Dual-Stimuli Responsive Block Copolymer Self-Assembly Micelles for Cellular Controlled Drug Release. Macromol. Biosci. 2021, 21, e2000291. [Google Scholar] [CrossRef]
- Tang, M.; Hu, P.; Zheng, Q.; Tirelli, N.; Yang, X.; Wang, Z.; Wang, Y.; Tang, Q.; He, Y. Polymeric micelles with dual thermal and reactive oxygen species (ROS)-responsiveness for inflammatory cancer cell delivery. J. Nanobiotechnol. 2017, 15, 39. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Lv, F.; Huang, J.; Tang, X.; Zhao, Z.; Chen, J. A multiple environment-sensitive prodrug nanomicelle strategy based on chitosan graftomer for enhanced tumor therapy of gambogic acid. Carbohydr. Polym. 2021, 267, 118229. [Google Scholar] [CrossRef]
- Yang, L.; Hou, X.; Zhang, Y.; Wang, D.; Liu, J.; Huang, F.; Liu, J. NIR-activated self-sensitized polymeric micelles for enhanced cancer chemo-photothermal therapy. J. Control Release 2021, 339, 114–129. [Google Scholar] [CrossRef]






| Stimulus-Responsive | Chemical Bonds or Group | Nanocarriers | Drugs | Refs. |
|---|---|---|---|---|
| Single pH | Hydrazone bond | PTX-loaded poly(ethyleneglycol)-phenylhydrazone-dilaurate (PEG-BHyd-dC12) micelles (PEG-Bhyd-dC12/PTX). | PTX | [41] |
| TOS-DOX prodrugs (TOS-H-DOX) | DOX | [42] | ||
| Pluronic P123-double (d)–hydrazone bond (hyd)–docetaxel (DTX) conjugates (P123-d–hyd–DTX) | DTX | [43] | ||
| PEG-DiHyd-PLA | PTX | [44] | ||
| Boronate ester bond | Alginate-dopamine–bortezomib (AlgPD-BTZ hydrogel) | BTZ | [45] | |
| mPEG-BCPGluCPT | DOX | [46] | ||
| Ester bond | APBA and OEAM were copolymerised to obtain nanogel PEGOE-OMAP | DOX | [47] | |
| HDO-NPs | Dasatinib (DAS) and Olaparib (OLA) | [48] | ||
| Acetal | Ketal containing HA nanogels | DOX | [49] | |
| (mPEG-b-MTC-EGVE-graft-PTX) | PTX | [50] | ||
| Imidazole groups | Poly(ethyleneglycol)3400-aconityllinkage-poly(L-glutamicacid)15-poly(L-histidine)10-poly(L-leucine)10 | DOX | [51] | |
| Poly N-(2-hydroxypropyl) methacry-lamid–b-poly histidine/poly leucine (pHPMA-pHis/pLeu) | PTX | [52] | ||
| Amino acid-modified PAMAM dendritic nanocarriers | Amino acid-modified PAMAM dendritic nanocarriers | DOX | [53] | |
| Amide bond | Nanomicelle (QT-CA-CS) based on chitosan, quercetin and citraconic | DOX | [54] | |
| Maleic acid group | Anisamide-conjugated N-octyl-N,O-maleoyl-O-phosphoryl chitosan (a-OMPC) | PTX | [55] | |
| Polymer dimethylmaleic acid-chitosan-UA (DA-CS-UA) | DOX | [56] | ||
| Dual pH | Hydrazone and PDEA | mPEG-g-PDPP-g-hyd-DOX | DOX | [57] |
| Hydrazone and Ester bonds | PEG-benzaldehyde-hydrazone-cholesteryl hemisuccinate (PEGB-Hz-CHEMS) | Gemcitabine | [58] | |
| β-carboxylic amide and Schiff | DCCA/DOX-NPs | DOX | [59] |
| Stimulus-Responsive | Chemical Bond | Nanocarriers | Drugs | Refs. |
|---|---|---|---|---|
| GSH | Disulphide bonds | A cystine-bridged peptide (CBP) was designed to co-assembly with a hydrophobic anti-tumour drug curcumin (CCM) (CCM-CBP) | Curcumin (CCM) | [64] |
| solanesyl poly(ethylene glycol) dithiodipropionate (SPDP) | DOX | [65] | ||
| HA conjugated with D-α-Tocopherol Succinate (TOS) using a disulphide bond as the linker (HA-SS-TOS, HSST) | PTX | [66] | ||
| mPEG-PLG(DNs) | DOX | [67] | ||
| A mixed micelle system (THSP) was prepared by combining reduction-sensitive HA-poly(lactide) (HA-ss-PLA) conjugates and D-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) | PTX | [15] |
| Stimulus-Responsive | Chemical Bonds or Group | Nanocarriers | Drugs | Refs. |
|---|---|---|---|---|
| pH and redox | Disulphide bond and carboxylic acid groups | Synthesis of PEG and polycarbonate diblock copolymers containing disulphide bonds and carboxylic acid groups (PEG-SS-COOH) | DOX | [126] |
| Carboxylic acid groups and thioethers | Thioether-containing carboxylic acid- and phenylurea-functionalised PEG-block-polycarbonates were synthesised to form mixed micelles | DOX | [127] | |
| Amine group, carboxyl groups and disulphide bonds | The amphiphilic PEGylated poly(a-lipoic acid) co-polymer (mPEG-PaLA) | PTX and DOX | [128] | |
| Tertiary amine groups and disulphide bonds | Fabricated a series of mixed micelles with different mass ratios using two amphiphilic copolymers P(DMAEMA-co-MaPCL) and PCL-SS-PMPC | DOX | [129] | |
| pH and temperature | (Diisopropyla–mino) ethyl groups and poly(N-isopropylacrylamide | Composed of a temperature-responsive corona segment with poly(N-isopropylacrylamide-co-dimethylacrylamide) and a pH-responsive core segment with poly[2-(diisopropylamino)ethyl methacrylate] (PDPA) | DOX | [130] |
| Carboxyl group and poly(N-isopropylacrylamide) | Poly(N-isopropylacrylamide) (PNIPAm-NH2) with L-Aspartic acid-N-carboxyanhydride (L-Asp-NCA) to synthesise an amphiphilic PNIPAm-b-Pasp co-polymer | Methotrexate (MTX) | [131] | |
| Amino group and poly(N-isopropylacrylamide) | L-peptide functionalised dual-responsive nanoparticles (L-CS-g-PNIPAM-PTX) | PTX | [132] | |
| Amine groups and poly(N-isopropylacrylamide) | A non-toxic pH/thermo-responsive hydrogel P(NIPAAm-co-DMAEMA) | Curcumin and DOX | [133] | |
| pH and photo | Schiff-base and o-nitrobenzyl ester group | Combining the ROP of the newly designed carbonate monomer, PEGylation, azide-alkyne Huisgen cycloaddition and Schiff-base reaction, two amphiphilic diblock polycarbonates covalently conjugated on the side chains were prepared | DOX | [134] |
| Thioketal and Ce6 | Thioketal cross-linked polyphosphoester-based NPs with a tumour acidity (pHe)-sensitive transactivator of transcription (TAT) peptide (DA-masked TAT-decorating reactive oxygen species (ROS)-sensitive Ce6/DOX-loaded hyperbranched NPs (DTRCD)) | DOX | [135] | |
| pH and enzymes | Poly(β-amino ester) and hyaluronidase | HA-coated self-assembly PEG-poly(β-amino ester) (PEG-PBAE) micelles | Thioridazine | [136] |
| hydrazone bond and hyaluronidase | HA-Ce6 (DOX) NPs combined HA and chlorin e6 (Ce6) using adipic dihydrazide (ADH) as a linker | DOX | [137] | |
| Redox and temperature | Disulphide linkages and poly(NIPAAm) | P(NIPAAm-co-PMA) nanocapsules | DOX | [138] |
| Disulphide linkages and PVCL chains | DOX-encapsulated poly(vinyl caprolactam) (PVCL)break// | DOX | [139] | |
| Redox and photo | Disulphide bond and o-nitrobenzyl ester derivative | Loading o-nitrobenzyl ester derivative caged DOX (DOC) into the inner poly(lactic-co-glycolic acid) (PLGA) core and adsorbing siRNA of P-gp protein onto the cationic polymeric shell derived from a disulphide-containing alkyl modified polyethylenimine (C16-S-S-PEI) | DOX | [140] |
| Disulphide bond and Cypate | Proportionally mixing polycaprolactone-disulphide bond-biodegradable photoluminescent polymer (PCL-SS-BPLP) and biotin-polyethylene glycol-cypate (biotin-PEG-cypate) | DOX | [141] | |
| Redox and enzymes | quinone oxidoreductase 1 (NQO1) and monomer (TMBQ) | polyurethane (PEG-PTU-PEG) block copolymer was designed and synthesised by co-polymerising diisocyanate, a reduction-sensitive monomer (TMBQ) and poly(ethylene glycol) | DOX | [142] |
| Enzymes and temperature | Acetylcholinesterase (AchE) and the long carbon chains of LCC | Prepared based on seven-[6-(diaminodipropylamine)-6-deoxy]–β-CD (SA- β-CD) and lauroyl choline chloride (LCC) | Celastrol (CSL) | [143] |
| Temperature, pH and reduction | poly(N-isopropylacrylamide), carboxylic acid groups and tertiary amine groups, disulphide bonds | tetrablock co-polymer poly(polyethylene glycol methacrylate)–poly [2-(dimethylamino) ethylmethacrylate]–poly(N-isopropylacrylamide)–poly(methylacrylic acid) (PPEGMA-PDMAEMA-PNIPAM-PMAA) | DOX | [144] |
| PNIPAM, disulphide linkage, acetal-bridged | A comb-like copolymer (G3) comprising one disulphide linkage and PEG, PCL and acetal-bridged PCL-b-PNIPAM grafts | DOX | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Lv, Z.; Yang, Q.; Chang, R.; Wu, J. Research Progress on Stimulus-Responsive Polymer Nanocarriers for Cancer Treatment. Pharmaceutics 2023, 15, 1928. https://doi.org/10.3390/pharmaceutics15071928
Luo S, Lv Z, Yang Q, Chang R, Wu J. Research Progress on Stimulus-Responsive Polymer Nanocarriers for Cancer Treatment. Pharmaceutics. 2023; 15(7):1928. https://doi.org/10.3390/pharmaceutics15071928
Chicago/Turabian StyleLuo, Shicui, Zhuo Lv, Qiuqiong Yang, Renjie Chang, and Junzi Wu. 2023. "Research Progress on Stimulus-Responsive Polymer Nanocarriers for Cancer Treatment" Pharmaceutics 15, no. 7: 1928. https://doi.org/10.3390/pharmaceutics15071928