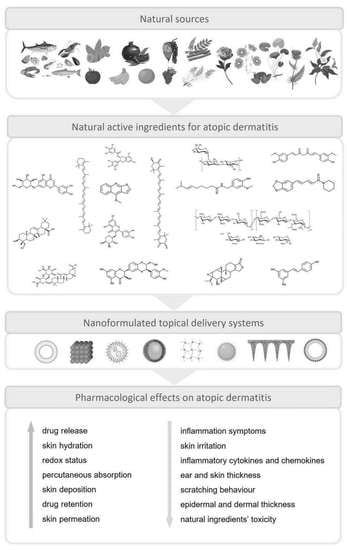
Nanotechnology-Based Topical Delivery of Natural Products for the Management of Atopic Dermatitis
Abstract
:1. Introduction
2. Materials and Methods
3. Pathophysiology and Clinical Treatment Approaches of AD
4. Nanotechnology: Safety Issues, Advantages, and Disadvantages of Application
5. Isolated Natural Compounds Included in Nanotechnology-Based Formulations for the Treatment of AD
5.1. Astaxanthin
5.1.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.1.2. Drug Delivery Systems and Pharmacological Activity
5.2. β-Carotene
5.2.1. Natural Source, physicochemical Features, and Bioactive Properties
5.2.2. Drug Delivery Systems and Pharmacological Activity
5.3. Capsaicin
5.3.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.3.2. Drug Delivery Systems and Pharmacological Activity
5.4. Curcumin
5.4.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.4.2. Drug Delivery Systems and Pharmacological Activity
5.5. Cynaroside
5.5.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.5.2. Drug Delivery Systems and Pharmacological Activity
5.6. Dictamnine
5.6.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.6.2. Drug Delivery Systems and Pharmacological Activity
5.7. Epigallocatechin-3-gallate
5.7.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.7.2. Drug Delivery Systems and Pharmacological Activity
5.8. Glycyrrhizic Acid
5.8.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.8.2. Drug Delivery Systems and Pharmacological Activity
5.9. Guar Gum
5.9.1. General Considerations
5.9.2. Drug Delivery Systems and Pharmacological Activity
5.10. Hederagenin
5.10.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.10.2. Drug Delivery Systems and Pharmacological Activity
5.11. Piperine
5.11.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.11.2. Drug Delivery Systems and Pharmacological Activity
5.12. Quercetin
5.12.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.12.2. Drug Delivery Systems and Pharmacological Activity
5.13. Resveratrol
5.13.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.13.2. Drug Delivery Systems and Pharmacological Activity
5.14. Sacran
5.14.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.14.2. Drug Delivery Systems and Pharmacological Activity
5.15. Silibinin
5.15.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.15.2. Drug Delivery Systems and Pharmacological Activity
5.16. Triptolide
5.16.1. Natural Source, Physicochemical Features, and Bioactive Properties
5.16.2. Drug Delivery Systems and Pharmacological Activity
6. Extracts, Oils, and Plant Mixtures Included in Nanotechnology-Based Formulations for the Treatment of AD
6.1. Centella asiatica (L.) Urban Extract
6.1.1. General Considerations
6.1.2. Drug Delivery Systems and Pharmacological Activity
6.2. Moutan Cortex and PentaHerbs
6.2.1. General Considerations
6.2.2. Drug Delivery Systems and Pharmacological Activity
6.3. Eupatorium japonicum Thunb. Extract
6.3.1. General Considerations
6.3.2. Drug Delivery Systems and Pharmacological Activity
6.4. Houttuynia cordata Thunb. Extract
6.4.1. General Considerations
6.4.2. Drug Delivery Systems and Pharmacological Activity
6.5. Linseed Oil
6.5.1. General Considerations
6.5.2. Drug Delivery Systems and Pharmacological Activity
6.6. Pomegranate Seed Oil
6.6.1. General Considerations
6.6.2. Drug Delivery Systems and Pharmacological Activity
6.7. Rhus verniciflua Stokes Extract
6.7.1. General Considerations
6.7.2. Drug Delivery Systems and Pharmacological Activity
6.8. Tea Tree Oil
6.8.1. General Considerations
6.8.2. Drug Delivery Systems and Pharmacological Activity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marques, M.P.; Mendonça, L.; Neves, B.G.; Varela, C.; Oliveira, P.; Cabral, C. Exploring Iberian Peninsula Lamiaceae as Potential Therapeutic Approaches in Wound Healing. Pharmaceuticals 2023, 16, 347. [Google Scholar] [CrossRef]
- Zaid, N.A.M.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Rani, N.N.I.M.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, 16, 23–66. [Google Scholar] [CrossRef]
- Archer, C.B. Atopic Dermatitis. Medicine 2021, 49, 370–373. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Song, A.; Lee, S.E.; Kim, J.H. Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw. 2022, 22, e7. [Google Scholar] [CrossRef]
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-Communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Gama, M.; Peixoto, D.; Sousa-Oliveira, I.; Ferreira-Faria, I.; Zeinali, M.; Abbaspour-Ravasjani, S.; Mascarenhas-Melo, F.; Hamishehkar, H.; Veiga, F. Nanocarrier-Based Dermopharmaceutical Formulations for the Topical Management of Atopic Dermatitis. Int. J. Pharm. 2022, 618, 121656. [Google Scholar] [CrossRef]
- Sharma, S.; Naura, A.S. Potential of Phytochemicals as Immune-Regulatory Compounds in Atopic Diseases: A Review. Biochem. Pharmacol. 2020, 173, 113790. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A Comprehensive Review of Natural Products against Atopic Dermatitis: Flavonoids, Alkaloids, Terpenes, Glycosides and Other Compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef] [PubMed]
- Qadir, A.; Ullah, S.N.M.N.; Jahan, S.; Ali, A.; Khan, N. Drug Delivery of Natural Products through Nano-carriers for Effective Vitiligo Therapy: A Compendia Review. J. Cosmet. Dermatol. 2022, 21, 5386–5404. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, S.; Huang, H.; Deng, X.; Yue, P.; Lin, J.; Yang, M.; Han, L.; Zhang, D.K. Advances in the Application of Natural Products and the Novel Drug Delivery Systems for Psoriasis. Front. Pharmacol. 2021, 12, 644952. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Yang, C.F.; Li, Q.L.; Tan, Q.; Xi, Y.W.; Liu, W.N.; Zhai, G.X. Development of a Quercetin-Loaded Nanostructured Lipid Carrier Formulation for Topical Delivery. Int. J. Pharm. 2012, 430, 292–298. [Google Scholar] [CrossRef]
- Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. [Google Scholar] [CrossRef] [PubMed]
- Drew, V.J.; Huang, H.; Tsai, Z.-H.; Tsai, H.; Tseng, C.-L. Preparation of Gelatin/Epigallocatechin Gallate Self-Assembly Nanoparticles for Transdermal Drug Delivery. J. Polym. Res. 2017, 24, 188. [Google Scholar] [CrossRef]
- Han, M.; Wang, X.; Wang, J.; Lang, D.; Xia, X.; Jia, Y.; Chen, Y. Ameliorative Effects of Epigallocatechin-3-Gallate Nanoparticles on 2,4-Dinitrochlorobenzene Induced Atopic Dermatitis: A Potential Mechanism of Inflammation-Related Necroptosis Mengguo. Front. Nutr. 2022, 38, 953646. [Google Scholar] [CrossRef]
- Chauhan, S.; Gulati, N.; Nagaich, U. International Journal of Polymeric Materials and Fabrication and Evaluation of Ultra Deformable Vesicles for Atopic Dermatitis as Topical Delivery. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 266–277. [Google Scholar] [CrossRef]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-Inflammatory Effect of Astaxanthin in Phthalic Anhydride-Induced Atopic Dermatitis Animal Model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef]
- Cervi, V.F.; Saccol, C.P.; Sari, M.H.M.; Martins, C.C.; da Rosa, L.S.; Ilha, B.D.; Soares, F.Z.; Luchese, C.; Wilhelm, E.A.; Cruz, L. Pullulan Film Incorporated with Nanocapsules Improves Pomegranate Seed Oil Anti-Inflammatory and Antioxidant Effects in the Treatment of Atopic Dermatitis in Mice. Int. J. Pharm. 2021, 609, 121144. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, D.K.; Ashawat, M.S. Development of Phospholipids Vesicular Nanocarrier for Topical Delivery of Tea Tree Oil in Management of Atopic Dermatitis Using BALB/c Mice Model. Eur. J. Lipid Sci. Technol. 2021, 123, 2100002. [Google Scholar] [CrossRef]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef]
- Semnani, D.; Nasari, M.; Fakhrali, A. PCL Nanofibers Loaded with Beta-Carotene: A Novel Treatment for Eczema. Polym. Bull. 2018, 75, 2015–2026. [Google Scholar] [CrossRef]
- Saini, K.; Modgill, N.; Singh, K.K. Tetrahydrocurcumin Lipid Nanoparticle Based Gel Promotes Penetration into Deeper Skin Layers and Alleviates Atopic Dermatitis in 2,4-Dinitrochlorobenzene (DNCB) Mouse Model. Nanomaterials 2022, 12, 636. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Tang, C.H.; Luo, F.C.; Yin, S.W.; Yang, X.Q. Topical Application of Zein-Silk Sericin Nanoparticles Loaded with Curcumin for Improved Therapy of Dermatitis. Mater. Today Chem. 2022, 24, 100802. [Google Scholar] [CrossRef]
- Szekalska, M.; Sosnowska, K.; Tomczykowa, M.; Winnicka, K.; Kasacka, I.; Tomczyk, M. In Vivo Anti-Inflammatory and Anti-Allergic Activities of Cynaroside Evaluated by Using Hydrogel Formulations. Biomed. Pharmacother. 2020, 121, 109681. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hsieh, Y.-T.; Chan, L.Y.; Yang, T.-Y.; Maeda, T.; Chang, T.-M.; Huang, H.-C. Dictamnine Delivered by PLGA Nanocarriers Ameliorated Inflammation in an Oxazolone-Induced Dermatitis Mouse Model. J. Control. Release 2021, 329, 731–742. [Google Scholar] [CrossRef]
- Ghosh, N.; Mitra, S.; Banerjee, E.R. Therapeutic Effects of Topically-Administered Guar Gum Nanoparticles in Oxazolone-Induced Atopic Dermatitis in Mice. Biomed. Res. Ther. 2018, 5, 2305–2325. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Abdullah, F.; Mukherjee, A. Fabrication and Fluorescent Labeling of Guar Gum Nanoparticles in a Surfactant Free Aqueous Environment. Mater. Sci. Eng. C 2015, 46, 521–529. [Google Scholar] [CrossRef]
- Lee, K.-J.; Ratih, K.; Kim, G.-J.; Lee, Y.-R.; Shin, J.-S.; Chung, K.-H.; Choi, E.-J.; Kim, E.-K.; An, J.H. Immunomodulatory and Anti-Inflammatory Efficacy of Hederagenin-Coated Maghemite (γ-Fe2O3) Nanoparticles in an Atopic Dermatitis Model. Colloids Surf. B Biointerfaces 2022, 210, 112244. [Google Scholar] [CrossRef]
- Gehrcke, M.; Martins, C.C.; de Bastos Brum, T.; da Rosa, L.S.; Luchese, C.; Wilhelm, E.A.; Soares, F.Z.M.; Cruz, L. Novel Pullulan/Gellan Gum Bilayer Film as a Vehicle for Silibinin-Loaded Nanocapsules in the Topical Treatment of Atopic Dermatitis. Pharmaceutics 2022, 14, 2352. [Google Scholar] [CrossRef]
- Yang, M.; Gu, Y.; Yang, D.; Tang, X.; Liu, J. Development of Triptolide-Nanoemulsion Gels for Percutaneous Administration: Physicochemical, Transport, Pharmacokinetic and Pharmacodynamic Characteristics. J. Nanobiotechnol. 2017, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Chiu, Y.; Wu, Y.; Hung, J.; Chen, M. Epigallocatechin Gallate/L-Ascorbic Acid–Loaded Poly-γ-Glutamate Microneedles with Antioxidant, Anti-Inflammatory, and Immunomodulatory Effects for the Treatment of Atopic Dermatitis. Acta Biomater. 2021, 130, 223–233. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, D.K.; Ashawat, M.S. Topical Creams of Piperine Loaded Lipid Nanocarriers for Management of Atopic Dermatitis: Development, Characterization, and in Vivo Investigation Using BALB/c Mice Model. J. Liposome Res. 2021, 32, 62–73. [Google Scholar] [CrossRef]
- Wathoni, N.; Motoyama, K.; Higashi, T.; Okajima, M.; Kaneko, T.; Arima, H. Physically Crosslinked-Sacran Hydrogel Films for Wound Dressing Application. Int. J. Biol. Macromol. 2016, 89, 465–470. [Google Scholar] [CrossRef]
- Kwon, T.K.; Kim, J.C. In Vitro Skin Permeation and Anti-Atopic Efficacy of Lipid Na- Nocarriers Containing Water Soluble Extracts of Houttuynia Cordata. Drug Dev. Ind. Pharm. 2014, 40, 1350–1357. [Google Scholar] [CrossRef]
- Baboota, S.; Rahman, M.U.; Kumar, A.; Sharma, S.; Sahni, J.; Ali, J. Submicron Size Formulation of Linseed Oil Containing Omega-3 Fatty Acid for Topical Delivery. J. Dispers. Sci. Technol. 2012, 33, 1259–1266. [Google Scholar] [CrossRef]
- Kildaci, I.; Budama-Kilinc, Y.; Kecel-Gunduz, S.; Altuntas, E. Linseed Oil Nanoemulsions for Treatment of Atopic Dermatitis Disease: Formulation, Characterization, in Vitro and in Silico Evaluations. J. Drug Deliv. Sci. Technol. 2021, 64, 102652. [Google Scholar] [CrossRef]
- Jeong, J.H.; Back, S.K.; An, J.H.; Lee, N.; Kim, D.; Na, C.S.; Jeong, Y.; Han, S.Y. Topical Film Prepared with Rhus Verniciflua Extract-Loaded Pullulan Hydrogel for Atopic Dermatitis Treatment. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2325–2334. [Google Scholar] [CrossRef]
- Chatterjee, S.; Hui, P.C.; Wat, E.; Kan, C.; Leung, P.; Wang, W. Drug Delivery System of Dual-Responsive PF127 Hydrogel with Polysaccharide-Based Nano-Conjugate for Textile-Based Transdermal Therapy. Carbohydr. Polym. 2020, 236, 116074. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Moon, S.; Kim, J.; Kim, W.J.; Kim, Y.; Kim, H. Structural Properties and Anti-Dermatitis Effects of Fl Avonoids-Loaded Gold Nanoparticles Prepared by Eupatorium Japonicum. Front. Pharmacol. 2022, 13, 1055378. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, J. Chemical Constituents from Houttuynia Cordata. Planta Med. 2016, 81, S1–S381. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Avila Valle, G.; Barbarot, S.; Bieber, T.; Brough, H.A.; Pinton, P.C.; Christen-Zäch, S.; et al. European Guideline (EuroGuiDerm) on Atopic Eczema: Part I—Systemic Therapy. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1409–1431. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Valle, G.A.; Barbarot, S.; Bieber, T.; Brough, H.A.; Pinton, P.C.; Christen-Zäch, S.; et al. European Guideline (EuroGuiDerm) on Atopic Eczema—Part II: Non-Systemic Treatments and Treatment Recommendations for Special AE Patient Populations. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1904–1926. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel Nanoparticles in Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Jeevanandam, J.; Chan, Y.S.; Danquah, M.K. Nano-Formulations of Drugs: Recent Developments, Impact and Challenges. Biochimie 2016, 128–129, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical Nanotechnology: From the Bench to the Market. Futur. J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Ratemi, E.; Shaik, A.S.; Al Faraj, A.; Halwani, R. Alternative Approaches for the Treatment of Airway Diseases: Focus on Nanoparticle Medicine. Clin. Exp. Allergy 2016, 46, 1033–1042. [Google Scholar] [CrossRef]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious. 2006. Available online: https://health.ec.europa.eu/scientific-committees/former-scientific-committees/scientific-committee-emerging-and-newly-identified-health-risks-scenihr_en (accessed on 4 May 2023).
- Jong, W.; Borm, P. Drug Delivery and Nanoparticles: Applications and Hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef] [Green Version]
- Lotfipour, F.; Shahi, S.; Farjami, A.; Salatin, S.; Mahmoudian, M.; Dizaj, S.M. Safety and Toxicity Issues of Therapeutically Used Nanoparticles from the Oral Route. Biomed Res. Int. 2021, 2021, 9322282. [Google Scholar] [CrossRef]
- Doktorovová, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical Safety of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Current Evidence from In Vitro and In Vivo Evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef] [Green Version]
- Fassett, R.G.; Coombes, J.S. Astaxanthin: A Potential Therapeutic Agent in Cardiovascular Disease. Mar. Drugs 2011, 9, 447–465. [Google Scholar] [CrossRef] [Green Version]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A Mechanistic Review on Its Biological Activities and Health Benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Alugoju, P.; Swamy, V.K.D.K.; Anthikapalli, N.V.A.; Tencomnao, T. Health Benefits of Astaxanthin against Age-Related Diseases of Multiple Organs: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2022, 1–66. [Google Scholar] [CrossRef]
- Hong, L.; Zhou, C.-L.; Chen, F.-P.; Han, D.; Wang, C.-Y.; Li, J.-X.; Chi, Z.; Liu, C.-G. Development of a Carboxymethyl Chitosan Functionalized Nanoemulsion Formulation for Increasing Aqueous Solubility, Stability and Skin Permeability of Astaxanthin Using Low-Energy Method. J. Microencapsul. 2017, 34, 707–721. [Google Scholar] [CrossRef]
- Eren, B.; Tanrıverdi, S.T.; Köse, F.A.; Özer, Ö. Antioxidant Properties Evaluation of Topical Astaxanthin Formulations as Anti-aging Products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef]
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS PharmSciTech 2020, 21, 318. [Google Scholar] [CrossRef]
- Hemrajani, C.; Negi, P.; Parashar, A.; Gupta, G.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; Dua, K. Overcoming Drug Delivery Barriers and Challenges in Topical Therapy of Atopic Dermatitis: A Nanotechnological Perspective. Biomed. Pharmacother. 2022, 147, 112633. [Google Scholar] [CrossRef]
- Choo, W.-T.; Teoh, M.-L.; Phang, S.-M.; Convey, P.; Yap, W.-H.; Goh, B.-H.; Beardall, J. Microalgae as Potential Anti-Inflammatory Natural Product against Human Inflammatory Skin Diseases. Front. Pharmacol. 2020, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Rühl, R.; Taner, C.; Schweigert, F.J.; Wahn, U.; Grüber, C. Serum Carotenoids and Atopy among Children of Different Ethnic Origin Living in Germany. Pediatr. Allergy Immunol. 2010, 21, 1072–1075. [Google Scholar] [CrossRef] [PubMed]
- Kake, T.; Imai, M.; Takahashi, N. Effects of Β-carotene on Oxazolone-induced Atopic Dermatitis in Hairless Mice. Exp. Dermatol. 2019, 28, 1044–1050. [Google Scholar] [CrossRef]
- Takahashi, N.; Kake, T.; Hasegawa, S.; Imai, M. Effects of Post-Administration of β-Carotene on Diet-Induced Atopic Dermatitis in Hairless Mice. J. Oleo Sci. 2019, 68, 793–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, E.; Garip, I.C.; Gulseren, G.; Tekinay, A.B.; Guler, M.O. Bioactive Supramolecular Peptide Nanofibers for Regenerative Medicine. Adv. Healthc. Mater. 2014, 3, 1357–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva-santos, A.C.; Mascarenhas-melo, F.; Coimbra, S.C.; Pawar, K.D.; Peixoto, D.; Chá-chá, R.; Araujo, A.R.T.S.; Pinto, S.; Veiga, F.; Cláudia, A.; et al. Expert Opinion on Drug Delivery Nanotechnology-Based Formulations toward the Improved Topical Delivery of Anti-Acne Active Ingredients. Expert Opin. Drug Deliv. 2021, 18, 1435–1454. [Google Scholar] [CrossRef]
- Basith, S.; Cui, M.; Hong, S.; Choi, S. Harnessing the Therapeutic Potential of Capsaicin and Its Analogues in Pain and Other Diseases. Molecules 2016, 21, 966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Bhatt, V.; Kumar, N. Amides from Plants: Structures and Biological Importance, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 56, ISBN 9780444640581. [Google Scholar]
- Rios, M.Y.; Olivo, H.F. Natural and Synthetic Alkamides: Applications in Pain Therapy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 43, ISBN 9780444634306. [Google Scholar] [CrossRef]
- Ghiasi, Z.; Esmaeli, F.; Aghajani, M.; Ghazi-khansari, M. Enhancing Analgesic and Anti-Inflammatory Effects of Capsaicin When Loaded into Olive Oil Nanoemulsion: An In Vivo Study. Int. J. Pharm. 2019, 559, 341–347. [Google Scholar] [CrossRef]
- Souto, E.B.; Dias-ferreira, J.; Oliveira, J.; Sanchez-lopez, E.; Martins-gomes, C.; Silva, M. Trends in Atopic Dermatitis—From Standard Pharmacotherapy to Novel Drug Delivery Systems. Int. J. Mol. Sci. 2019, 20, 5659. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-R.; Gao, S.-Q.; Niu, X.-Q.; Li, L.-J.; Ying, X.-Y.; Hu, Z.-J.; Gao, J.-Q. Capsaicin-Loaded Nanolipoidal Carriers for Topical Application: Design, Characterization, and In Vitro/In Vivo Evaluation. Int. J. Nanomed. 2017, 12, 3881–3898. [Google Scholar] [CrossRef] [Green Version]
- Raza, K.; Shareef, M.A.; Singal, P.; Sharma, G.; Negi, P.; Prakash, O. Lipid-Based Capsaicin-Loaded Nano-Colloidal Biocompatible Topical Carriers with Enhanced Analgesic Potential and Decreased Dermal Irritation. J. Liposome Res. 2014, 2104, 290–296. [Google Scholar] [CrossRef]
- Ghosalkar, S.; Prabha, M.; Padmini, S. Emerging Topical Drug Delivery Approaches for the Treatment of Atopic Dermatitis. J. Cosmet. Dermatol. 2021, 21, 536–549. [Google Scholar] [CrossRef]
- Vollono, L.; Falconi, M.; Gaziano, R.; Iacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [PubMed] [Green Version]
- Moon, P.; Jeong, H.; Kim, H. Down-Regulation of Thymic Stromal Lymphopoietin by Curcumin. Pharmacol. Rep. 2013, 65, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Journal, A.I.; Shrotriya, S.; Ranpise, N.; Satpute, P.; Vidhate, B. Skin Targeting of Curcumin Solid Lipid Nanoparticles-Engrossed Topical Gel for the Treatment of Pigmentation and Irritant Contact Dermatitis. Artif. Cells Nanomed. Biotechnol. 2017, 46, 1471–1482. [Google Scholar] [CrossRef] [Green Version]
- Ternullo, S.; Gagnat, E.; Julin, K.; Johannessen, M.; Basnet, P. European Journal of Pharmaceutics and Biopharmaceutics Liposomes Augment Biological Bene Fi Ts of Curcumin for Multitargeted Skin Therapy. Eur. J. Pharm. Biopharm. 2019, 144, 154–164. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, M.; Zhang, T.; Wu, J.; Wang, J.; Liu, K.; Zhan, N. Antioxidant and Anti-Inflammatory Activities of Cynaroside from Elsholtiza bodinieri. Nat. Prod. Commun. 2018, 13, 1934578X1801301122. [Google Scholar] [CrossRef] [Green Version]
- Baskar, A.A.; Ignacimuthu, S.; Michael, G.P.; Al Numair, K. Cancer Chemopreventive Potential of Luteolin-7-O-Glucoside Isolated from Ophiorrhiza mungos Linn. Nutr. Cancer 2010, 63, 130–138. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, L.-M.; Liu, X.-Q.; Li, B.; Wang, Q.; Dong, J.-X. Anti-HBV Active Flavone Glucosides from Euphorbia humifusa Willd. Fitoterapia 2010, 81, 799–802. [Google Scholar] [CrossRef]
- Palombo, R.; Savini, I.; Avigliano, L.; Madonna, S.; Cavani, A.; Albanesi, C.; Mauriello, A.; Melino, G.; Terrinoni, A. Luteolin-7-Glucoside Inhibits IL-22/STAT3 Pathway, Reducing Proliferation, Acanthosis, and Inflammation in Keratinocytes and in Mouse Psoriatic Model. Cell Death Dis. 2016, 7, e2344. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Torres, T.; Lima, S.A.C.; Reis, S. Hydrogels: A Promising Vehicle for the Topical Management of Atopic Dermatitis. Adv. Ther. 2021, 4, 2100028. [Google Scholar] [CrossRef]
- Qing, W.; Wang, Y.; Li, H.; Ma, F.; Zhu, J.; Liu, X. Preparation and Characterization of Copolymer Micelles for the Solubilization and In Vitro Release of Luteolin and Luteoloside. AAPS PharmSciTech 2017, 18, 2095–2101. [Google Scholar] [CrossRef]
- Qing, W.; Wang, Y.; Wang, Y.; Zhao, D.; Liu, X.; Zhu, J. The Modified Nanocrystalline Cellulose for Hydrophobic Drug Delivery. Appl. Surf. Sci. 2016, 366, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Zhao, P.-H.; Hu, J.-F. Chemical Constituents of Plants from the Genus Dictamnus. Chem. Biodivers. 2011, 8, 1234–1244. [Google Scholar] [CrossRef]
- Chang, T.-M.; Yang, T.-Y.; Niu, Y.-L.; Huang, H.-C. The Extract of D. Dasycarpus Ameliorates Oxazolone-Induced Skin Damage in Mice by Anti-Inflammatory and Antioxidant Mechanisms. Antioxidants 2018, 7, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Lee, H.-B.; Kim, S.; Park, Y.C.; Kim, K.; Kim, H. Decoction of Dictamnus Dasycarpus Turcz. Root Bark Ameliorates Skin Lesions and Inhibits Inflammatory Reactions in Mice with Contact Dermatitis. Pharmacogn. Mag. 2017, 13, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, N.; Shao, H.; Deng, J.; Yang, Y.; Tang, Z.; Wu, G.; Liu, Y. Dictamnine Ameliorates Chronic Itch in DNFB-Induced Atopic Dermatitis Mice via Inhibiting MrgprA3. Biochem. Pharmacol. 2023, 208, 115368. [Google Scholar] [CrossRef]
- Mokra, D.; Joskova, M.; Mokry, J. Therapeutic Effects of Green Tea Polyphenol (-)-Epigallocatechin-3-Gallate (EGCG) in Relation to Molecular Pathways Controlling Inflammation, Oxidative Stress, and Apoptosis. Int. J. Mol. Sci. 2023, 24, 340. [Google Scholar] [CrossRef]
- Noh, S.U.; Cho, E.A.; Kim, H.O.; Park, Y.M. Epigallocatechin-3-Gallate Improves Dermatophagoides pteronissinus Extract-Induced Atopic Dermatitis-like Skin Lesions in NC/Nga Mice by Suppressing Macrophage Migration Inhibitory Factor. Int. Immunopharmacol. 2008, 8, 1172–1182. [Google Scholar] [CrossRef]
- Aljuffali, I.A.; Hung, C.; Shih, L.; Yang, C.; Alalaiwe, A.; You, J. Nanoencapsulation of Tea Catechins for Enhancing Skin Absorption and Therapeutic Efficacy. AAPS PharmSciTech 2022, 23, 187. [Google Scholar] [CrossRef]
- Nascimento, M.H.M.D.; de Araújo, D.R. Exploring the Pharmacological Potential of Glycyrrhizic Acid: From Therapeutic Applications to Trends in Nanomedicine. Futur. Pharmacol. 2022, 2, 1–15. [Google Scholar] [CrossRef]
- Kowalska, A. 18 b-Glycyrrhetinic Acid: Its Core Biological Properties and Dermatological Applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Peng, G.; Han, X. International Immunopharmacology Glycyrrhizin Ameliorates Atopic Dermatitis-like Symptoms through Inhibition of HMGB1. Int. Immunopharmacol. 2018, 60, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Barone, A.; Chiara, M.; Cilurzo, F.; Locatelli, M.; Iannotta, D.; Di, L.; Celia, C.; Paolino, D. Ammonium Glycyrrhizate Skin Delivery from Ultradeformable Liposomes: A Novel Use as an Anti-inflammatory Agent in Topical Drug Delivery. Colloids Surf. B Biointerfaces 2020, 193, 111152. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Kong, S.; Ouyang, Q.; Tao, J.; Lu, S.; Huang, Y.; Li, S.; Luo, H. Use of 18β-Glycyrrhetinic Acid Nanocrystals to Enhance Anti-Inflammatory Activity by Improving Topical Delivery. Colloids Surf. B Biointerfaces 2021, 205, 111791. [Google Scholar] [CrossRef]
- Zhang, Y. Improved Stability and Skin Penetration through Glycethosomes Loaded with Glycyrrhetinic Acid. Int. J. Cosmet. Sci. 2022, 44, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, R.; Jacob, P.J.S.; Sahoo, S. Biomedical Applications of Green Biopolymer Guar Gum. J. Pharm. Biomed. Sci. 2013, 35, 1783–1787. [Google Scholar]
- Chemical Book Guar Gum. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB5253559.htm (accessed on 13 February 2023).
- Rodríguez-Hernández, D.; Demuner, A.J.; Barbosa, L.C.A.; Csuk, R.; Heller, L. Hederagenin as a Triterpene Template for the Development of New Antitumor Compounds. Eur. J. Med. Chem. 2015, 105, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.H.; Oh, T.-W.; Nguyen, U.T.; Choi, M.-J.; Yang, I.-J.; Shin, H.-M. A Natural Compound Mixture Containing Arctigenin, Hederagenin, and Baicalein Alleviates Atopic Dermatitis in Mice by Regulating HPA Axis and Immune Activity. Evid.-Based Complement. Altern. Med. 2020, 2020, 1970349. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, D.; Barbosa, L.C.A.; Demuner, A.J.; Nain-Perez, A.; Ferreira, S.R.; Fujiwara, R.T.; de Almeida, R.M.; Heller, L.; Csuk, R. Leishmanicidal and Cytotoxic Activity of Hederagenin-Bistriazolyl Derivatives. Eur. J. Med. Chem. 2017, 140, 624–635. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, J.; Yang, B.; Ma, S.; Zhang, C.; Zhao, G. Therapeutic Effect of Tetrapanax Papyriferus and Hederagenin on Chronic Neuropathic Pain of Chronic Constriction Injury of Sciatic Nerve Rats Based on KEGG Pathway Prediction and Experimental Verification. Evid.-Based Complement. Altern. Med. 2020, 2020, 2545806. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Piperine—The Bioactive Compound of Black Pepper: From Isolation to Medicinal Formulations. Compr. Food Sci. Food Saf. 2017, 16, 124–140. [Google Scholar] [CrossRef]
- Jung, S.K.; Choi, D.W.; Jung, C.H.; Kim, Y.; Jung, S.Y. Piper Nigrum Fruit Extract Prevents TMA-Induced Allergic Contact Dermatitis by Regulating Th2 Cytokine Production. J. Agric. Sci. 2015, 7, 135–146. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.W.; Jung, S.Y.; Shon, D.-H.; Shin, H.S. Piperine Ameliorates Trimellitic Anhydride-Induced Atopic Dermatitis-Like Symptoms by Suppressing Th2-Mediated Immune Responses via Inhibition of STAT6 Phosphorylation. Molecules 2020, 25, 2186. [Google Scholar] [CrossRef]
- Politi, S.; Carvalho, S.G.; Rodero, C.F.; Pini, K.; Meneguin, B.; Sorrechia, R.; Chiavacci, L.A.; Chorilli, M. International Journal of Biological Macromolecules Piperine-Loaded Nanoparticles Incorporated into Hyaluronic Acid/Sodium Alginate-Based Membranes for the Treatment of Inflammatory Skin Diseases. Int. J. Biol. Macromol. 2023, 227, 736–748. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxid. Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Wadhwa, K.; Kadian, V.; Puri, V.; Bhardwaj, B.Y.; Sharma, A.; Pahwa, R.; Rao, R.; Gupta, M.; Singh, I. New Insights into Quercetin Nanoformulations for Topical Delivery. Phytomed. Plus 2022, 2, 100257. [Google Scholar] [CrossRef]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Sreedhar, R.; Giridharan, V.V.; Watanabe, K. Molecular Targets of Quercetin with Anti-Inflammatory Properties in Atopic Dermatitis. Drug Discov. Today 2016, 21, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.N.; Shin, S.A.; Choo, G.S.; Kim, H.J.; Park, Y.S.; Kim, B.S.; Kim, S.K.; Cho, S.D.; Nam, J.S.; Choi, C.S.; et al. Anti-Inflammatory Effect of Quercetin and Galangin in LPS-Stimulated RAW264.7 Macrophages and DNCB-Induced Atopic Dermatitis Animal Models. Int. J. Mol. Med. 2018, 41, 888–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Sharopov, F.; Tumer, T.B.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, S.; Zhang, J.; Yang, B.; Elias, P.M.; Man, M.Q. Role of Resveratrol in Regulating Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2020, 2020, 2416837. [Google Scholar] [CrossRef] [Green Version]
- Ratz-Łyko, A.; Arct, J. Resveratrol as an Active Ingredient for Cosmetic and Dermatological Applications: A Review. J. Cosmet. Laser Ther. 2019, 21, 84–90. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.C.; Cho, K.; Lee, J.H.; Subedi, L.; Yumnam, S.; Kim, S.Y. Effect of Resveratrol-Enriched Rice on Skin Inflammation and Pruritus in the NC/Nga Mouse Model of Atopic Dermatitis. Int. J. Mol. Sci. 2019, 20, 1428. [Google Scholar] [CrossRef] [Green Version]
- Karuppagounder, V.; Arumugam, S.; Thandavarayan, R.A.; Pitchaimani, V.; Sreedhar, R.; Afrin, R.; Harima, M.; Suzuki, H.; Nomoto, M.; Miyashita, S.; et al. Resveratrol Attenuates HMGB1 Signaling and Inflammation in House Dust Mite-Induced Atopic Dermatitis in Mice. Int. Immunopharmacol. 2014, 23, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Sozmen, S.C.; Karaman, M.; Micili, S.C.; Isik, S.; Ayyildiz, Z.A.; Bagriyanik, A.; Uzuner, N.; Karaman, O. Resveratrol Ameliorates 2,4-Dinitrofluorobenzene-Induced Atopic Dermatitis-like Lesions through Effects on the Epithelium. PeerJ 2016, 2016, e1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soldati, P.P.; Polonini, H.C.; Paes, C.Q.; Restrepob, J.A.S.; Creczynksi-Pasa, T.B.; Chaves, M.G.A.M.; Brandão, M.A.F.; Pittella, F.; Raposo, N.R.B. Controlled Release of Resveratrol from Lipid Nanoparticles Improves Antioxidant Effect. IFAC-PapersOnLine 2018, 51, 16–21. [Google Scholar] [CrossRef]
- Sun, R.; Zhao, G.; Ni, S.; Xia, Q. Lipid Based Nanocarriers with Different Lipid Compositions for Topical Delivery of Resveratrol: Comparative Analysis of Characteristics and Performance. J. Drug Deliv. Sci. Technol. 2014, 24, 591–600. [Google Scholar] [CrossRef]
- Shrotriya, S.N.; Ranpise, N.S.; Vidhate, B.V. Skin Targeting of Resveratrol Utilizing Solid Lipid Nanoparticle-Engrossed Gel for Chemically Induced Irritant Contact Dermatitis. Drug Deliv. Transl. Res. 2017, 7, 37–52. [Google Scholar] [CrossRef]
- Ren, S.; Gao, Y.; Wang, L.; Qiu, C.; Yang, L.; Li, L.; Xiao, Y.; Xiao, N.; Liao, L.; Zuo, Z.; et al. Sacran Polysaccharide Improves Atopic Dermatitis through Inhibiting Th2 Type Immune Response. Life Sci. 2022, 288, 120205. [Google Scholar] [CrossRef]
- Goto, M.; Azuma, K.; Arima, H.; Kaneko, S.; Higashi, T.; Motoyama, K.; Michihara, A.; Shimizu, T.; Kadowaki, D.; Maruyama, T.; et al. Sacran, a Sulfated Polysaccharide, Suppresses the Absorption of Lipids and Modulates the Intestinal Flora in Non-Alcoholic Steatohepatitis Model Rats. Life Sci. 2021, 268, 118991. [Google Scholar] [CrossRef]
- Fukushima, S.; Motoyama, K.; Tanida, Y.; Higashi, T.; Ishitsuka, Y.; Kondo, Y.; Irie, T.; Tanaka, T.; Ihn, H.; Arima, H. Clinical Evaluation of Novel Natural Polysaccharides Sacran as a Skincare Material for Atopic Dermatitis Patients. J. Cosmet. Dermatol. Sci. Appl. 2016, 6, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybum Marianum L. Gaernt.)—Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigon, C.; Marchiori, M.C.L.; da Silva Jardim, F.; Pegoraro, N.S.; Chaves, P.D.S.; Velho, M.C.; Beck, R.C.R.; Ourique, A.F.; Sari, M.H.M.; de Oliveira, S.M.; et al. Hydrogel Containing Silibinin Nanocapsules Presents Effective Anti-Inflammatory Action in a Model of Irritant Contact Dermatitis in Mice. Eur. J. Pharm. Sci. 2019, 137, 104969. [Google Scholar] [CrossRef] [PubMed]
- Shrotriya, S.N.; Vidhate, B.V.; Shukla, M.S. Formulation and Development of Silybin Loaded Solid Lipid Nanoparticle Enriched Gel for Irritant Contact Dermatitis. J. Drug Deliv. Sci. Technol. 2017, 41, 164–173. [Google Scholar] [CrossRef]
- Mady, F.M.; Essa, H.; El-Ammawi, T.; Abdelkader, H.; Hussein, A.K. Formulation and Clinical Evaluation of Silymarin Pluronic-Lecithin Organogels for Treatment of Atopic Dermatitis. Drug Des. Dev. Ther. 2016, 10, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Zhang, Y.; Liu, X.; Wu, X.; Huang, L.; Gao, W. Triptolide: Pharmacological Spectrum, Biosynthesis, Chemical Synthesis and Derivatives. Theranostics 2021, 11, 7199–7221. [Google Scholar] [CrossRef]
- Park, K.S. Pharmacological Effects of Centella asiatica on Skin Diseases: Evidence and Possible Mechanisms. Evid.-Based Complement. Altern. Med. 2021, 2021, 5462633. [Google Scholar] [CrossRef]
- Susilawati, Y.; Chaerunisa, A.Y.; Purwaningsih, H. Phytosome Drug Delivery System for Natural Cosmeceutical Compounds: Whitening Agent and Skin Antioxidant Agent. J. Adv. Pharm. Technol. Res. 2021, 12, 327–334. [Google Scholar] [CrossRef]
- Ho, P.J.; Sung, J.J.; Cheon, K.K.; Tae, H.J. Anti-Inflammatory Effect of Centella asiatica Phytosome in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis. Phytomedicine 2018, 43, 110–119. [Google Scholar] [CrossRef]
- He, C.; Xiao, P. Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia suffruticosa Andrew): A Systematic Review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef] [Green Version]
- Chung, B.; Chan, L.; Lun, K.; Hon, E.; Chung, P.; Wing, S.; Pui, K.; Yuk, M.; Lee, H.; Yung, H.; et al. Traditional Chinese Medicine for Atopic Eczema: PentaHerbs Formula Suppresses Inflammatory Mediators Release from Mast Cells. J. Ethnopharmacol. 2008, 120, 85–91. [Google Scholar] [CrossRef]
- Tsang, M.S.M.; Jiao, D.; Chan, B.C.L.; Hon, K.; Leung, P.C.; Lau, C.B.S.; Wong, E.C.W.; Cheng, L.; Chan, C.K.M.; Lam, C.W.K.; et al. Atopic Dermatitis-Like Skin Inflammation. Molecules 2016, 21, 21. [Google Scholar] [CrossRef] [Green Version]
- Phan, M.G.; Do, T.T.; Nguyen, T.N.; Viet, T.; Do, H.; Dong, N.P.; Vu, M.T. Chemical Constituents of Eupatorium Japonicum and Anti-Inflammatory, Cytotoxic, and Apoptotic Activities of Eupatoriopicrin on Cancer Stem Cells. Hindawi Evid.-Based Complement. Altern. Med. Hoechst 2021, 2021, 6610347. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Jeon, Y.; Lee, S.; Lee, Y.G.; Kim, J.B.; Kwon, H.C.; Kim, S.H.; Kim, I.; Lee, K.; Han, Y.S. Apoptotic and Anti-Inflammatory Effects of Eupatorium Japonicum Thunb. in Rheumatoid Arthritis Fibroblast-Like Synoviocytes. BioMed Res. Int. 2018, 2018, 1383697. [Google Scholar] [CrossRef] [Green Version]
- Damiani, G.; Eggenhöffner, R.; Pigatto, P.D.M.; Bragazzi, N.L. Nanotechnology Meets Atopic Dermatitis: Current Solutions, Challenges and Future Prospects. Insights and Implications from a Systematic Review of the Literature. Bioact. Mater. 2019, 4, 380–386. [Google Scholar] [CrossRef]
- Touré, A.; Xueming, X. Flaxseed Lignans: Source, Biosynthesis, Metabolism, Antioxidant Activity, Bio-Active Components, and Health Benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [Google Scholar] [CrossRef]
- Hashempur, M.H.; Homayouni, K.; Ashraf, A.; Salehi, A.; Taghizadeh, M.; Heydari, M. Effect of Linum Usitatissimum L. (Linseed) Oil on Mild and Moderate Carpal Tunnel Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. DARU J. Pharm. Sci. 2014, 22, 43. [Google Scholar] [CrossRef] [Green Version]
- James, M.J.; Gibson, R.A.; Cleland, L.G. Dietary Polyunsaturated Fatty Acids and Inflammatory Mediator Production. Am. J. Clin. Nutr. 2000, 71, 343S–348S. [Google Scholar] [CrossRef] [Green Version]
- Takic, M.; Pokimica, B.; Petrovic-Oggiano, G.; Popovic, T. Effects of Dietary α-Linolenic Acid Treatment and the Efficiency of Its Conversion to Eicosapentaenoic and Docosahexaenoic Acids in Obesity and Related Diseases. Molecules 2022, 27, 4471. [Google Scholar] [CrossRef]
- Thakur, N.; Garg, G.; Sharma, P.K.; Kumar, N. Nanoemulsions: A Review on Various Pharmaceutical Application. Glob. J. Pharmacol. 2012, 6, 222–225. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Mohammed, A.S.; Abu-Serie, M.M.; Maher, A.M.; Habashy, N.H. Inhibition of Oxidative Stress, IL-13, and WNT/β-Catenin in Ovalbumin-Sensitized Rats by a Novel Organogel of Punica granatum Seed Oil Saponifiable Fraction. Biomed. Pharmacother. 2022, 154, 113667. [Google Scholar] [CrossRef] [PubMed]
- Shaban, N.Z.; Talaat, I.M.; Elrashidy, F.H.; Hegazy, A.Y.; Sultan, A.S. Therapeutic Role of Punica granatum (Pomegranate) Seed Oil Extract on Bone Turnover and Resorption Induced in Ovariectomized Rats. J. Nutr. Health Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef]
- Park, D.K.; Lee, Y.G.; Park, H.J. Extract of Rhus Verniciflua Bark Sup- Presses 2,4-Dinitrofluorobenzene-Induced Allergic Contact Dermati-Tis. Evid.-Based Complement. Altern. Med. 2013, 2013, 879696. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Sun, H. Sulfuretin Alleviates Atopic Dermatitis-like Symptoms in Mice via Suppressing Th2 Cell Activity. Immunol. Res. 2018, 66, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.S.; Long, X.; Su, X.Z.; Lu, F. Melaleuca Alternifolia (Tea Tree) Oil and Its Monoterpene Constituents in Treating Protozoan and Helminthic Infections. Biomed. Pharmacother. 2020, 130, 110624. [Google Scholar] [CrossRef] [PubMed]


| Natural Isolated Compound | Nanotechnology-Based Formulation | Preparation Approach | EE (%) | PS (nm) | ZP (mV) | PDI | References |
|---|---|---|---|---|---|---|---|
| Astaxanthin | LIPs | Mixing with high-pressure homogenizer | NA | 64.5 ± 2.8 | NA | NA | [20] |
| β-carotene | NFs | Electrospinning | NA | 400–800 | NA | NA | [21] |
| Capsaicin | SLNs | Microelmusion method | 99 | 277.4 ± 12.0 | NA | 0.192 ± 0.095 | [13] |
| Curcumin | SLNs | Microelmusion method | 62 | 493.6 ± 183.90 | NA | 263 ± 0.043 | [13] |
| SLN-based gel | Microelmusification with high-speed homogenization method | 83.10 ± 2.29 | 109.2 | NA | NA | [22] | |
| Zein–silk sericin NPs | Antisolvent method | NA | 330 to 400 | −22 to −25 | 0.29 to 0.49 | [23] | |
| Cynaroside | Hydrogels | Mixing | NA | 22,000–26,000 | NA | NA | [24] |
| Dictamnine | Nanocarrier-encapsulated | Using U-SiM bioreactor (ultrasound composite stream-impinging mixer) | 93.70 | 186 ± 30 | NA | 0.146 ± 0.072 | [25] |
| Epigallocatechin-3-gallate | Gelatin NPs | Self-assembly method | NA | 112.5 ± 19.09 | 23.2 ± 0.5 | 0.3 ± 0.05 | [14] |
| Polyethylene glycol-poly lactic-co-glycolic acid -epigallocatechin-3-gallate nanoparticles (PEG-PLGA-EGCG-NPs) | Double emulsion method | 86 | 176.2 | −33.3 | 0.044 | [15] | |
| Glycyrrhizic acid | TRAs | Thin film hydration method | 66.23 ± 0.61 to 93.10 ± 0.3 | 56.94 to 270.40 | −4.76 | 0.13 to 1.00 | [16] |
| Guar gum | NPs | Acid hydrolysis from guar gum dispersed in water | NA | 30–80 | −30 ± 5 | 0.259 | [26,27] |
| Hederagenin | NPs | Emulsion method | NA | 10.9 | NA | NA | [28] |
| Piperine | ETO-based cream | Cold method | 74.30 ± 3.88 | 318.1 | −32.6 | NA | [19] |
| Quercetin | NLCs | Emulsion evaporation–solidification method | 89.95 ± 0.16 | 215.2 | −20.10 ± 1.22 | NA | [12] |
| Resveratrol | SLNs | Microelmusion method | 85 | 271.8 ± 4.0 | NA | 0.005 | [13] |
| Silibinin | NC-based bilayer film | NCs were prepared by interfacial deposition of preformed polymer method. Films were prepared by two-step solvent-casting method | 99 | 115 ± 3 | −10 | <0.2 | [29] |
| Triptolide | NE-based gel | High-energy emulsification method | 85 | 62.1 ± 9.9 | NA | 0.19 ± 0.023 | [30] |
| Natural Isolated Compound | Major Natural Source | Nanotechnology-Based Formulation | Pharmacological Effects | Reference |
|---|---|---|---|---|
| Astaxanthin | Microalgae, crustaceans, seafood, yeast, fungi, complex plants, birds’ feathers | LIPs | In vivo: STAT3 and NF-κB inhibition | [20] |
| β-carotene | Plants, marine algae, fungi, and bacteria | NFs | In vitro: very slow degradability rate and gradual release of beta-carotene | [21] |
| Curcumin | Curcuma longa L. | SLNs | In vitro: ↓ IL-6. No cytotoxic effects for NCTC 2544 and THP-1 monocytes differentiated into M2 macrophages | [13] |
| SLN-based gel | In vivo: ↓ TNF-α and IL-6. ↑ healing lesions and skin hydration. Improved redox status (↑ GSH and catalase; MDA ↓). Ex vivo: ↑ deep penetration into the dermis. | [22] | ||
| Zein–silk sericin NPs | Ex vivo: ↑ deep penetration and skin permeability. In vitro: ↓ NF-κBp65, inflammatory cytokines, and chemokines in HaCaT keratinocytes | [23] | ||
| Cynaroside | Bidens tripartita L., Verbascum lychnitis L., Elsholtiza bodinieri Vaniot | Hydrogels | In vivo: ↓ skin and tissue inflammation and inflammatory infiltrates; ↓ number of T and mast cells and histiocytes; hindered the overexpression of cytokines and IgE levels | [24] |
| Dictamnine | Dictamnus dasycarpus Turcz. | Nanocarrier-encapsulated | In vivo: ↓ thymic stromal lymphopoietin (TSLP), IL-1β and TNF-α expression; improvement of skin inflammation | [25] |
| Epigallocatechin-3-gallate | Vitis vinifera L. | Gelatin NPs | In vivo: ↑ skin absorbance and no side effects. In vitro: ↓ IL-6 and IL-8 in LPS-inflamed WS1 dermal fibroblasts | [14] |
| Polyethylene glycol-poly lactic-co-glycolic acid -epigallocatechin-3-gallate nanoparticles (PEG-PLGA-EGCG-NPs) | In vivo: ↓ ear and skin thickness, dermatitis score, and scratching behavior. Restoration of redox status (↑ SOD, GSH, and T-AOC). ↓ Th1 (IFN-g and TNF-α), Th2 (IL-4 and IL-6), and Th17 (IL-17A) cytokines. Supression of RIP1, RIP3, MLKL, p-p38, ERK1, and ERK2 | [15] | ||
| Epigallocatechin gallate/L-ascorbic acid-loaded poly-γ-glutamate microneedles (EGCG/AA-loaded-γ-PGA MNs) | In vivo: ↓ dermatitis score, mast cell infiltration, IFN-γ expression, Th2 cytokine secretion, IgE, and histamine | [31] | ||
| Glycyrrhizic acid | Glycyrrhiza glabra L. | TRAs | In vivo: ↓ erythema signs and scratching behavior. Ex vivo: ↓ permeation and ↑ skin deposition | [16] |
| Guar gum | Cyamopsis tetragonoloba (L.) Taub. | NPs | In vitro: successful wound-healing effect; In vivo: ↓ AD symptoms, such as redness and epidermal thickness; ↓ serum IgE levels, total counts for blood cells, skin cells, eosinophils, macrophages, and neutrophils | [26] |
| Hederagenin | Sapindus saponaria L., Lonicera japonica Thunb. | NPs | In vivo: Dose-dependent inhibition of IFN-γ, TNF-α, IL-4, IL-6, IL-17, and thymic stromal lymphopoietin (TSLP); ↓ mast cell infiltration, epidermal and dermal thickness of mouse skin; relieved lumping lymph nodes | [28] |
| Piperine | Piper nigrum L. | ETO-based cream | In vivo: ↓ ear and skin thickness, skin severity, white blood cells, granulocytes, and IgE. Ex vivo: penetration and deposition. In vitro: no cytotoxic effects in HaCaT keratinocytes | [32] |
| Quercetin | Present in several food products such as fruits and vegetables | NLCs | In vivo: ↓ inflammation symptoms. In vitro: ↑ percutaneous permeabilization and retention at the dermis and epidermis | [12] |
| Resveratrol | Vitis vinifera L. | SLNs | In vitro: ↓ IL-6 and MCP-1. No cytotoxic effects for NCTC 2544 and THP-1 monocytes differentiated into M2 macrophages | [13] |
| Sacran | Aphanothece sacrum (Sur.) Okada | Hydrogel films | In vivo: ↑ moisture content | [33] |
| Silibinin | Silybum marianum L. | NC-based bilayer film | In vivo: ↓ oxidative and inflammatory markers, ↓ scratching behavior and ear edema, ↑ skin hydration. Ex vivo: controlled dug release, ↑ drug retention. In vitro: ↑ antioxidant potential | [29] |
| Triptolide | Tripterygium wilfordii Hook. F. | NE-based gel | In vivo: ↓ ear edema. ↓ IFN-γ and IL-4. Ex vivo: ↑ deep penetration and percutaneous delivery | [30] |
| Extract/Oil/Mixture | Nanotechnology-Based Formulation | Preparation Approach | EE (%) | PS (nm) | ZP (mV) | PDI | Reference |
|---|---|---|---|---|---|---|---|
| Houttuynia cordata Thunb. | CUBs | Sonication | NA | 231.7 | NA | NA | [34] |
| LIP suspensions | Film hydration method | NA | 273.3 | NA | NA | [34] | |
| Linseed oil | Microemulsion | NA | NA | 186 | NA | NA | [35] |
| NEs | Ultrasonication method | NA | 99.02 ± 1.06 | −8.79 ± 0.034 | 0.14 ± 0.020 | [36] | |
| Pomegranate seed oil | NC-based film | Solvent-casting method to prepare the pullulan films and the interfacial precipitation of preformed polymer methodology to produce NCs | NA | 181 ± 6 | 43.13 ± 0.7 | <0.2 | [18] |
| Rhus verniciflua Stokes | Hydrogel | Mixture stirred for complete solubilization at RT and then cast onto glass plates of 4 mm thickness | 0.95 | NA | NA | NA | [37] |
| Tea tree oil | ETO-based cream | ETOs were obtained by mixing of reagents and subsequent sonication. Creams were obtained by phase inversion method | 76.19 ± 3.26 | 333.6 | −35.3 | NA | [19] |
| Extract/Oil/Mixture | Major Compounds | Nanotechnology-Based Formulation | Pharmacological Effects | References |
|---|---|---|---|---|
| Centella asiatica (L.) Urban | Triterpenes, namely asiaticoside and madecassoside, and their aglycones | Phytosome | In vivo: ↓ hyperkeratosis, proliferation of mast cells and infiltration of inflammatory cells. ↓ expression of iNOS, COX-2, NF-κB, TNF-α, IL-1β, and IgE. In vitro: ↓ NO, iNOS, and COX-2 in LPS-stimulated RAW 264.7 macrophages. ↓ LPS-induced DNA-binding activities of NF-κB | [17] |
| Cortex Moutan | Plant mixture. Gallic acid | PF127/HA-Ala-Chito(oligo)-based hydrogel | In vitro: cell viability of >80.0% in HaCaT keratinocytes, considering concentrations ranging between 0.0 and 20.0 μg/mL | [38] |
| Eupatorium japonicum Thunb. | Flavonoids, namely melilotoside, rutin, hyperoside, nictoflorin, cymaroside, and rhamnetin | AuNPs | In vitro: suppression of MAPK and nuclear factor-κB signaling pathways. ↓ RANTES, TARC, CTACK, IL-6, and IL-8. ↓ production of ROS | [39] |
| Houttuynia cordata Thunb. | Harmala alkaloids, phenolic acids, chlorogenic acid derivatives, phenolic glycosides, phenylpropanoid derivatives, and flavonoids | CUB and LIP suspensions | In vivo: ↑ skin permeation of the extract; ↓ IgE production and IL-4 expression; ↑ IFN-γ expression | [34,40] |
| Linseed oil | Omega-3 fatty acid, such as α-linolenic acid and short chain polyunsaturated fatty acids (PUFAs) | Microemulsion | In vitro: ↑ linseed permeation | [35] |
| Omega-3 fatty acid, such as α-linolenic acid and short chain polyunsaturated fatty acids (PUFAs) | NEs | In vitro: adequate linseed permeation | [36] | |
| Pomegranate seed oil | Complex mixture rich in punicic acid | NC-based film | In vivo: ↓ AD-like skin injury, ↑ oxidative defenses, ↓ hypernocipetive behavior. In vitro: absence of irritation | [18] |
| Rhus verniciflua Stokes | Fustin, gallic acid, fisetin, resorcinol, garbanzol, butein, and sulfuretin | Hydrogel | In vivo: ↓ mast cell lesions | [37] |
| Tea tree oil | Terpinen-4-ol is the major monoterpene in this essential oil | ETO-based cream | In vivo: ↓ severity of clinical score, infiltration of white blood cells, eosinophils, and IgE antibodies. Ex vivo: ↑ Drug permeation and retention. In vitro: absence of cytotoxic effects in HaCaT keratinocytes | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, M.P.; Varela, C.; Mendonça, L.; Cabral, C. Nanotechnology-Based Topical Delivery of Natural Products for the Management of Atopic Dermatitis. Pharmaceutics 2023, 15, 1724. https://doi.org/10.3390/pharmaceutics15061724
Marques MP, Varela C, Mendonça L, Cabral C. Nanotechnology-Based Topical Delivery of Natural Products for the Management of Atopic Dermatitis. Pharmaceutics. 2023; 15(6):1724. https://doi.org/10.3390/pharmaceutics15061724
Chicago/Turabian StyleMarques, Mário Pedro, Carla Varela, Laura Mendonça, and Célia Cabral. 2023. "Nanotechnology-Based Topical Delivery of Natural Products for the Management of Atopic Dermatitis" Pharmaceutics 15, no. 6: 1724. https://doi.org/10.3390/pharmaceutics15061724