Design, Preparation, and Physicochemical Characterisation of Alginate-Based Honey-Loaded Topical Formulations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Honey Samples
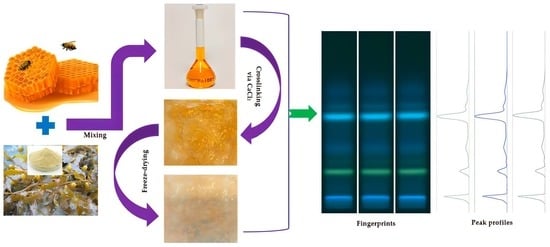
2.3. Preparation of Honey-Based Formulations
2.3.1. Preparation of Pre-Gel Solutions
2.3.2. Preparation of Wet Sheets
2.3.3. Preparation of Dry Sheets
2.4. Physicochemical Evaluation of Honey and Honey-Based Formulations
2.4.1. Determination of pH
2.4.2. Colour Profile
2.4.3. Moisture Content
2.4.4. Spreadability
2.4.5. Rheology
2.4.6. Physical Dimensions
2.4.7. Morphology
2.4.8. Tensile Strength
2.4.9. Swelling Index
2.4.10. Determination of Methylglyoxal (MGO) Content
2.5. Component Analysis
2.6. Stability Study
2.7. Data Analysis
3. Results
3.1. Physicochemical Characterisation
3.1.1. pH
3.1.2. Colour Profile
3.1.3. Moisture Content
3.1.4. Spreadability
3.1.5. Rheology
3.1.6. Thickness and Length
3.1.7. Morphology
3.1.8. Tensile Strength
3.1.9. Swelling Index
3.1.10. MGO Content
3.2. Component Analysis
3.3. Storage Stability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saranraj, P.; Sivasakthi, S.; Feliciano, G. Pharmacology of Honey: A Review. Biol. Res. 2016, 10, 271–289. [Google Scholar]
- Kuropatnicki, A.K.; Kłósek, M.; Kucharzewski, M. Honey as medicine: Historical perspectives. J. Apic. Res. 2018, 57, 113–118. [Google Scholar] [CrossRef]
- Forrest, R.D. Early history of wound treatment. J. R. Soc. Med. 1982, 75, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Foster, K.; Lim, L.Y.; Hammer, K.; Locher, C. A Review of the Phytochemistry and Bioactivity of Clover Honeys (Trifolium spp.). Foods 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Masalha, M.; Abu-Lafi, S.; Abu-Farich, B.; Rayan, M.; Issa, N.; Zeidan, M.; Rayan, A. A New Approach for Indexing Honey for Its Heath/Medicinal Benefits: Visualization of the Concept by Indexing Based on Antioxidant and Antibacterial Activities. Medicines 2018, 5, 135. [Google Scholar] [CrossRef] [PubMed]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef]
- Spilioti, E.; Jaakkola, M.; Tolonen, T.; Lipponen, M.; Virtanen, V.; Chinou, I.; Kassi, E.; Karabournioti, S.; Moutsatsou, P. Phenolic acid composition, antiatherogenic and anticancer potential of honeys derived from various regions in Greece. PLoS ONE 2014, 9, e94860. [Google Scholar] [CrossRef]
- Almasaudi, S.B.; El-Shitany, N.A.; Abbas, A.T.; Abdel-dayem, U.A.; Ali, S.S.; Al Jaouni, S.K.; Harakeh, S. Antioxidant, Anti-inflammatory, and Antiulcer Potential of Manuka Honey against Gastric Ulcer in Rats. Oxid. Med. Cell. Longev. 2016, 2016, 3643824. [Google Scholar] [CrossRef]
- Fedorova, A.A.; Azzami, K.; Ryabchikova, E.I.; Spitsyna, Y.E.; Silnikov, V.N.; Ritter, W.; Gross, H.J.; Tautz, J.; Vlassov, V.V.; Beier, H.; et al. Inactivation of a non-enveloped RNA virus by artificial ribonucleases: Honeybees and acute bee paralysis virus as a new experimental model for in vivo antiviral activity assessment. Antivir. Res. 2011, 91, 267–277. [Google Scholar] [CrossRef]
- Efem, S.E. Clinical observations on the wound healing properties of honey. Br. J. Surg. 1988, 75, 679–681. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. A Review of Commonly Used Methodologies for Assessing the Antibacterial Activity of Honey and Honey Products. Antibiotics 2022, 11, 975. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Honey-Based Medicinal Formulations: A Critical Review. Appl. Sci. 2021, 11, 5159. [Google Scholar] [CrossRef]
- El-Kased, R.F.; Amer, R.I.; Attia, D.; Elmazar, M.M. Honey-based hydrogel: In vitro and comparative in vivo evaluation for burn wound healing. Sci. Rep. 2017, 7, 9692. [Google Scholar] [CrossRef] [PubMed]
- Irish, J.; Blair, S.; Carter, D.A. The Antibacterial Activity of Honey Derived from Australian Flora. PLoS ONE 2011, 6, e18229. [Google Scholar] [CrossRef] [PubMed]
- Saikaly, S.; Khachemoune, A. Honey and Wound Healing: An Update. Am. J. Clin. Dermatol. 2017, 18, 237–251. [Google Scholar] [CrossRef]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Molan, P.C.; Betts, J.A. Clinical usage of honey as a wound dressing: An update. J. Wound Care. 2004, 13, 353–356. [Google Scholar] [CrossRef]
- Yaghoobi, R.; Kazerouni, A.; Kazerouni, O. Evidence for clinical use of honey in wound healing as an anti-bacterial, anti-inflammatory anti-oxidant and anti-viral agent: A review. Jundishapur. J. Nat. Pharm. Prod. 2013, 8, 100–104. [Google Scholar] [CrossRef]
- Simon, A.; Traynor, K.; Santos, K.; Blaser, G.; Bode, U.; Molan, P. Medical honey for wound care—Still the ‘latest resort’? Evid.-Based Complement. Altern. Med. 2009, 6, 165–173. [Google Scholar] [CrossRef]
- Robson, V.V.; Dodd, S.; Thomas, S. Standardized antibacterial honey (MedihoneyTM) with standard therapy in wound care: Randomized clinical trial. J. Adv. Nurs. 2009, 65, 565–575. [Google Scholar] [CrossRef]
- Minden-Birkenmaier, B.A.; Bowlin, G.L. Honey-Based Templates in Wound Healing and Tissue Engineering. Bioengineering 2018, 14, 5–46. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing-Know-how. Int. J. Biol. Macromol. 2021, 1, 656–685. [Google Scholar] [CrossRef]
- Lee, D.S.; Sinno, S.; Khachemoune, A. Honey and Wound Healing. Am. J. Clin. Dermatol. 2011, 12, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Mohd Zohdi, R.; Abu Bakar Zakaria, Z.; Yusof, N.; Mohamed Mustapha, N.; Abdullah, M.N. Gelam (Melaleuca spp.) Honey-Based Hydrogel as Burn Wound Dressing. Evid. Based Complement. Altern. Med. 2012, 2012, 843025. [Google Scholar] [CrossRef]
- Sindi, A.; Chawn, M.V.B.; Hernandez, M.E.; Green, K.; Islam, M.K.; Locher, C.; Hammer, K. Anti-biofilm effects and characterisation of the hydrogen peroxide activity of a range of Western Australian honeys compared to Manuka and multifloral honeys. Sci. Rep. 2019, 9, 17666. [Google Scholar] [CrossRef] [PubMed]
- Roshan, N.; Rippers, T.; Locher, C.; Hammer, K.A. Antibacterial activity and chemical characteristics of several Western Australian honeys compared to manuka honey and pasture honey. Arch. Microbiol. 2017, 199, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Antioxidant HPTLC-DPPH Fingerprinting of Honeys and Tracking of Antioxidant Constituents Upon Thermal Exposure. Foods 2021, 10, 357. [Google Scholar] [CrossRef]
- Green, K.J.; Islam, M.K.; Lawag, I.; Locher, C.; Hammer, K.A. Honeys derived from plants of the coastal sandplains of Western Australia: Antibacterial and antioxidant activity, and other characteristics. J. Apic. Res. 2022, 61, 1–14. [Google Scholar] [CrossRef]
- Nazeri, S.; Ardakani, E.M.; Babavalian, H.; Latifi, A.M. Evaluation of Effectiveness of Honey-Based Alginate Hyrogel on Wound Healing in a Mouse Model of Rat. J. Appl. Biotechnol. Rep. 2015, 2, 293–297. [Google Scholar]
- Pereira, R.; Carvalho, A.; Vaz, D.C.; Gil, M.H.; Mendes, A.; Bartolo, P. Development of novel alginate based hydrogel films for wound healing applications. Int. J. Biol. Macromol. 2013, 52, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L.; Farrar, D. 8–Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Woodhead Publishing: Sawston, UK, 2011; pp. 186–208. [Google Scholar]
- Gupta, B.S.; Edwards, J.V. Textile materials and structures for wound care products. In Advanced Textiles for Wound Care; Rajendran, S., Ed.; CRC Press: Washington, DC, USA, 2009; pp. 48–96. [Google Scholar]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, J.; Guo, T.; Wang, J.J.; Tang, X.; Nicolosi, V. Multifunctional Ti3C2Tx MXene Composite Hydrogels with Strain Sensitivity toward Absorption-Dominated Electromagnetic- Interference Shielding. ACS Nano. 2021, 15, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Y.; Ding, M.; Fan, X.; Hu, J.; Chen, Y.; Li, J.; Li, Z.; Liu, W. A 4arm-PEG Macromolecule Crosslinked Chitosan Hydrogels as Antibacterial Wound Dressing. Carbohydr. Polym. 2022, 277, 118871. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, K.; Xu, L.; Gao, N.; Zhao, H.; Gong, Z.; Yu, L.; Jiang, J. Oxalic acid cross-linked sodium alginate and carboxymethyl chitosan hydrogel membrane for separation of dye/NaCl at high NaCl concentration. Chin. Chem. Lett. 2022, 33, 1951–1955. [Google Scholar] [CrossRef]
- Bai, T.; Zhao, K.; Lu, Z.; Liu, X.; Lin, Z.; Cheng, M.; Li, Z.; Zhu, D.; Zhang, L. Simple fabrication of Cu2+ doped calcium alginate hydrogel filtration membrane with excellent anti-fouling and antibacterial properties. Chin. Chem. Lett. 2021, 32, 1051–1054. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Seidi, F.; Azarfam, M.Y.; Yazdi, M.K.; Erfani, A.; Barani, M.; Chauhan, N.P.S.; Rabiee, N.; Kuang, T.; Lipka, J.K.; et al. Biopolymer-based composites for tissue engineering applications: A basis for future opportunities. Compos. Part B 2023, 258, 110701. [Google Scholar] [CrossRef]
- Kavanagh, S.; Gunnoo, J.; Marques Passos, T.; Stout, J.C.; White, B. Physicochemical properties and phenolic content of honey from different floral origins and from rural versus urban landscapes. Food Chem. 2019, 272, 66–75. [Google Scholar] [CrossRef]
- Bogdanov, S.; Martin, P.; Lüllmann, C. Harmonised methods of the European Honey Commission. Apidologie 1997, 28, 1–59. [Google Scholar]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef]
- Hervy, M.; Santmarti, A.; Lahtinen, P.; Tammelin, T.; Lee, K.Y. Sample geometry dependency on the measured tensile properties of cellulose nanopapers. Mater. Des. 2017, 121, 421–429. [Google Scholar] [CrossRef]
- Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Monitoring the Release of Methylglyoxal (MGO) from Honey and Honey-Based Formulations. Molecules 2023, 28, 2858. [Google Scholar] [CrossRef] [PubMed]
- Nolan, V.C.; Harrison, J.; Cox, J.A.G. Dissecting the antimicrobial composition of honey. Antibiotics 2019, 8, 251. [Google Scholar] [CrossRef]
- Adams, C.J.; Manley-Harris, M.; Molan, P.C. The origin of methylglyoxal in New Zealand mānuka (Leptospermum scoparium) honey. Carbohydr. Res. 2009, 344, 1050–1053. [Google Scholar] [CrossRef] [PubMed]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Mānuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Williams, S.; King, J.; Revell, M.; Manley-Harris, M.; Balks, M.; Janusch, F.; Dawson, M. Regional, annual, and individual variations in the dihydroxyacetone content of the nectar of maānuka (Leptospermum scoparium) in New Zealand. J. Agric. Food Chem. 2014, 62, 10332–10340. [Google Scholar] [CrossRef]
- Locher, C.; Neumann, J.; Sostaric, T. Authentication of Honeys of Different Floral Origins via High-Performance Thin-Layer Chromatographic Fingerprinting. JPC-J. Planar Chromat. 2017, 30, 57–62. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. A Comprehensive HPTLC-Based Analysis of the Impacts of Temperature on the Chemical Properties and Antioxidant Activity of Honey. Molecules 2022, 27, 8491. [Google Scholar] [CrossRef]
- Fallico, B.; Zappalà, M.; Arena, E.; Verzera, A. Effects of heating process on chemical composition and HMF levels in Sicilian monofloral honeys. Food Chem. 2004, 85, 305–313. [Google Scholar] [CrossRef]
- Julika, W.N.; Ajit, A.; Naila, A.; Sulaiman, A.Z. The effect of storage condition on physicochemical properties of some stingless bee honey collected in malaysia local market. Mater. Today Proc. 2022, 57, 1396–1402. [Google Scholar] [CrossRef]
- Zappala, M.; Fallico, B.; Arena, E.; Verzera, A. Methods for the determination of HMF in honey: A comparison. Food Control 2005, 16, 273–277. [Google Scholar] [CrossRef]
- Samborska, K. Powdered honey–drying methods and parameters, types of carriers and drying aids, physicochemical properties and storage stability. Trends Food Sci. Technol. 2019, 88, 133–142. [Google Scholar] [CrossRef]
- Samborska, K.; Wiktor, A.; Jedlińska, A.; Matwijczuk, A.; Jamróz, W.; Skwarczyńska-Maj, K.; Kiełczewski, D.; Tułodziecki, M.; Błażowski, Ł.; Witrowa-Rajchert, D. Development and characterization of physical properties of honey-rich powder. Food Bioprod. Process. 2019, 115, 78–86. [Google Scholar] [CrossRef]
- Bulut, L.; Kilic, M. Kinetics of hydroxymethylfurfural accumulation and color change in honey during storage in relation to moisture content. J. Food Process. Preserv. 2009, 33, 22–32. [Google Scholar] [CrossRef]
















| Botanical Origin | Supplier, Year |
|---|---|
| WA Manuka Honey 1 (Leptospermum scoparium) | Hive and Wellness, 2019 |
| WA Manuka Honey 2 (Leptospermum scoparium) | Manuka Life, 2019 |
| WA Coastal Peppermint (Agonis flexuosa) | Margaret River Honey Company, 2019 |
| WA Jarrah Honey (Eucalyptus marginata) | Hive and Wellness, 2019 |
| New Zealand Manuka Honey (Leptospermum scoparium) | Hive and Wellness, 2018 |
| Honey | Sample | pH | Colour at Pre- and Post-Filtration * | |
|---|---|---|---|---|
| Pre | Post | |||
| Jarrah | Neat Honey | 4.65 ± 0.01 | 541.00 ± 0.03 | 415.00 ± 0.03 |
| Pre-gel solution | 5.34 ± 0.02 | 451.00 ± 0.03 | 221.00 ± 0.03 | |
| Coastal Peppermint | Neat Honey | 4.75 ± 0.00 | 508.00 ± 0.03 | 320.00 ± 0.02 |
| Pre-gel solution | 5.49 ± 0.01 | 390.00 ± 0.01 | 218.00 ± 0.01 | |
| WA Manuka 1 | Neat Honey | 4.56 ± 0.00 | 855.00 ± 0.03 | 445.00 ± 0.01 |
| Pre-gel solution | 5.35 ± 0.00 | 525.00 ± 0.02 | 263.00 ± 0.03 | |
| WA Manuka 2 | Neat Honey | 4.65 ± 0.01 | 780.00 ± 0.01 | 545.00 ± 0.02 |
| Pre-gel solution | 5.42 ± 0.00 | 523 ± 0.01 | 343 ± 0.00 | |
| NZ Manuka | Neat Honey | 4.60 ± 0.00 | 1146 ± 0.03 | 715 ± 0.01 |
| Pre-gel solution | 5.38 ± 0.00 | 696 ± 0.02 | 361 ± 0.03 | |
| Honey Type | Moisture Content (%) | |
|---|---|---|
| Neat Honey | Pre-Gel Solution | |
| Jarrah Honey | 17.30 ± 0.10 | 49.00 ± 0.53 |
| Coastal Peppermint Honey | 18.00 ± 0.35 | 48.37 ± 0.15 |
| WA Manuka Honey 1 | 18.75 ± 0.26 | 48.57 ± 0.06 |
| WA Manuka Honey 2 | 19.58 ± 0.32 | 49.00 ± 0.00 |
| NZ Manuka Honey | 20.30 ± 0.10 | 48.63 ± 0.26 |
| Honey Type | Spreadability (g·cm/s) | |
|---|---|---|
| Neat Honey | Pre-Gel Solution | |
| Jarrah Honey | 334.81 ± 0.10 | 425.11 ± 0.10 |
| Coastal Peppermint Honey | 267.51 ± 0.33 | 425.09 ± 0.11 |
| WA Manuka Honey 1 | 368.43 ± 0.46 | 425.11 ± 0.11 |
| WA Manuka Honey 2 | 324.34 ± 0.22 | 424.84 ± 0.11 |
| NZ Manuka Honey | 336.96 ± 0.09 | 424.89 ± 0.11 |
| Honey Type | Neat Honey (mPa·s) | Pre-Gel Solutions (mPa·s) | ||
|---|---|---|---|---|
| Temperature (25 °C) | Temperature (37 °C) | Temperature (25 °C) | Temperature (37 °C) | |
| WA Jarrah Honey | 15,286.73 ± 0.75 | 3509.71 ± 0.79 | 2882.07 ± 0.68 | 2214.39 ± 0.75 |
| WA Coastal Peppermint Honey | 14,516.03 ± 0.67 | 3217.66 ± 0.81 | 2881.07 ± 0.68 | 2214.69 ± 0.73 |
| WA Manuka Honey 1 | 3826.42 ± 0.66 | 2328.04 ± 0.78 | 2880.76 ± 0.59 | 2215.62 ± 0.69 |
| WA Manuka Honey 2 | 6464.73 ± 0.71 | 2016.34 ± 0.77 | 2881.34 ± 0.59 | 2214.88 ± 0.71 |
| NZ Manuka Honey | 5355.28 ± 0.67 | 2561.36 ± 0.81 | 2882.22 ± 0.62 | 2214.79 ± 0.72 |
| Sample | Thickness (mm) | Length (mm) | ||
|---|---|---|---|---|
| Wet Sheet | Dry Sheet | Wet Sheet | Dry Sheet | |
| WA Jarrah Honey | 2.01 ± 0.02 | 1.41 ± 0.02 | 94.24 ± 0.03 | 93.33 ± 0.02 |
| WA Coastal Peppermint Honey | 2.00 ± 0.01 | 1.39 ± 0.02 | 94.22 ± 0.02 | 93.32 ± 0.02 |
| WA Manuka Honey 1 | 2.02 ± 0.01 | 1.40 ± 0.02 | 94.23 ± 0.02 | 93.29 ± 0.02 |
| WA Manuka Honey 2 | 2.01 ± 0.02 | 1.39 ± 0.02 | 94.21 ± 0.02 | 93.29 ± 0.02 |
| NZ Manuka Honey | 2.02 ± 0.01 | 1.41 ± 0.02 | 94.19 ± 0.03 | 93.31 ± 0.02 |
| Honey Type | Tensile Strength (Pa) | |
|---|---|---|
| Wet Sheet | Dry Sheet | |
| Jarrah Honey | 106.15 ± 0.28 | 192.25 ± 0.46 |
| Coastal Peppermint Honey | 100.11 ± 0.25 | 185.28 ± 0.62 |
| WA Manuka Honey 1 | 108.65 ± 0.42 | 190.34 ± 0.56 |
| WA Manuka Honey 2 | 109.31 ± 0.54 | 194.42 ± 0.62 |
| NZ Manuka Honey | 111.55 ± 0.38 | 200.45 ± 0.64 |
| Sample (Dry Sheet) | Swelling Index (%) Measured at Different Time Points | |||
|---|---|---|---|---|
| 10 min | 20 min | 30 min | After 45 min | |
| WA Jarrah Honey | 52.08 ± 0.12 | 57.8 ± 0.17 | 57.8 ± 0.17 | 57.8 ± 0.08 |
| WA Coastal Peppermint Honey | 52.04 ± 0.21 | 58.07 ± 0.22 | 58.06 ± 0.18 | 58.05 ± 0.09 |
| WA Manuka Honey 1 | 52.07 ± 0.18 | 57.79 ± 0.20 | 57.72 ± 0.24 | 57.71 ± 0.07 |
| WA Manuka Honey 2 | 52.06 ± 0.16 | 58.03 ± 0.19 | 58.04 ± 0.16 | 58.04 ± 0.07 |
| NZ Manuka Honey | 51.97 ± 0.18 | 57.86 ± 0.14 | 57.83 ± 0.10 | 57.82 ± 0.08 |
| Honey Type | MGO (mg/kg) | |||
|---|---|---|---|---|
| Neat Honey | Pre-Gel Solution | Wet Sheet | Dry Sheet | |
| WA Manuka Honey 1 | 95.65 ± 1.4 | 94.89 ± 1.3 | 95.27 ± 1.4 | 95.19 ± 1.2 |
| WA Manuka Honey 2 | 180.55 ± 1.2 | 180.65 ± 1.3 | 179.97 ± 1.4 | 180.32 ± 1.3 |
| NZ Manuka Honey | 350.47 ± 1.1 | 349.95 ± 1.4 | 350.07 ± 1.3 | 350.21 ± 1.3 |
| Honey | Weight (g) | Rf of Monitored Compound | Peak Area (AU × 10−3) per Band | Peak Area (AU × 10−3) per Sheet | ||||
|---|---|---|---|---|---|---|---|---|
| Neat Honey | Pre-Gel Solution | Wet Sheet | Dry Sheet | Wet Sheet | Dry Sheet | |||
| WA Jarrah | 1.01 | 0.20 | 5.2 ± 0.01 | 5.2 ± 0.03 | 5.1 ± 0.01 | 14.0 ± 0.04 | 128 ± 0.02 | 126 ± 0.01 |
| 0.53 | 15.2 ± 0.02 | 15.2 ± 0.03 | 14.8 ± 0.05 | 40.8 ± 0.03 | 369 ± 0.01 | 368 ± 0.03 | ||
| WA Coastal Peppermint | 1.02 | 0.20 | 9.4 ± 0.04 | 9.4 ± 0.04 | 9.4 ± 0.04 | 26.1 ± 0.03 | 235 ± 0.03 | 235 ± 0.02 |
| 0.53 | 22.9 ± 0.01 | 22.8 ± 0.02 | 22.7 ± 0.05 | 63.2 ± 0.04 | 570 ± 0.02 | 569 ± 0.04 | ||
| WA Manuka 1 | 1.01 | 0.38 | 26.8 ± 0.02 | 26.7 ± 0.02 | 26.7 ± 0.06 | 74.0 ± 0.02 | 668 ± 0.03 | 667 ± 0.03 |
| 0.53 | 6.8 ± 0.06 | 6.7 ± 0.06 | 6.7 ± 0.07 | 18.5 ± 0.03 | 168 ± 0.02 | 167 ± 0.02 | ||
| WA Manuka 2 | 1.01 | 0.20 | 17.5 ± 0.05 | 17.2 ± 0.07 | 17.2 ± 0.04 | 47.6 ± 0.04 | 430 ± 0.03 | 429 ± 0.02 |
| 0.38 | 23.6 ± 0.04 | 23.4 ± 0.01 | 23.4 ± 0.04 | 65.0 ± 0.02 | 586 ± 0.02 | 585 ± 0.03 | ||
| NZ Manuka | 1.02 | 0.32 | 13.7 ± 0.06 | 13.7 ± 0.04 | 13.3 ± 0.06 | 36.8 ± 0.01 | 332 ± 0.04 | 331 ± 0.02 |
| 0.39 | 25.3 ± 0.02 | 25.0 ± 0.05 | 24.7 ± 0.07 | 67.8 ± 0.02 | 617 ± 0.02 | 617 ± 0.04 | ||
| Honey | Storage Temperature (°C) | Sample | pH | Moisture Content (%) | Spreadability (g·cm/s) | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | Baseline | 6 Months | |||
| Jarrah | 5 | Neat Honey | 4.62 ± 0.02 | 4.62 ± 0.03 | 17.88 ± 0.3 | 17.87 ± 0.2 | 334.77 ± 0.2 | 334.75 ± 0.2 |
| Pre-gel | 5.30 ± 0.03 | 5.31 ± 0.03 | 48.98 ± 0.2 | 48.95 ± 0.3 | 425.15 ± 0.1 | 425.14 ± 0.2 | ||
| 30 | Neat Honey | 4.61 ± 0.03 | 4.62 ± 0.02 | 17.87 ± 0.3 | 17.87 ± 0.2 | 336.77 ± 0.2 | 337.75 ± 0.2 | |
| Pre-gel | 5.29 ± 0.02 | 5.30 ± 0.04 | 48.89 ± 0.2 | 48.91 ± 0.3 | 427.15 ± 0.1 | 428.14 ± 0.3 | ||
| 40 | Neat Honey | 4.61 ± 0.02 | 4.63 ± 0.03 | 17.89 ± 0.3 | 17.88 ± 0.2 | 338.77 ± 0.2 | 337.75 ± 0.2 | |
| Pre-gel | 5.29 ± 0.04 | 5.31 ± 0.03 | 48.86 ± 0.2 | 48.85 ± 0.3 | 426.15 ± 0.1 | 427.14 ± 0.3 | ||
| WA Manuka 2 | 5 | Neat Honey | 4.63 ± 0.01 | 4.62 ± 0.02 | 19.45 ± 0.2 | 19.44 ± 0.2 | 325.47 ± 0.2 | 325.45 ± 0.2 |
| Pre-gel | 5.40 ± 0.02 | 5.38 ± 0.02 | 49.08 ± 0.2 | 49.09 ± 0.3 | 424.93 ± 0.2 | 424.94 ± 0.1 | ||
| 30 | Neat Honey | 4.61 ± 0.02 | 4.61 ± 0.03 | 19.44 ± 0.2 | 19.41 ± 0.2 | 324.47 ± 0.2 | 324.45 ± 0.2 | |
| Pre-gel | 5.38 ± 0.02 | 5.39 ± 0.03 | 49.08 ± 0.2 | 49.08 ± 0.3 | 424.93 ± 0.2 | 426.94 ± 0.1 | ||
| 40 | Neat Honey | 4.60 ± 0.02 | 4.62 ± 0.03 | 19.43 ± 0.2 | 19.43 ± 0.2 | 325.47 ± 0.2 | 324.45 ± 0.3 | |
| Pre-gel | 5.38 ± 0.02 | 5.29 ± 0.02 | 49.08 ± 0.2 | 49.10 ± 0.3 | 424.93 ± 0.2 | 423.94 ± 0.2 | ||
| Honey | Storage Temperature (°C) | Sample | Thickness (mm) | Length (mm) | Tensile Strength (Pa) | |||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | Baseline | 6 Months | |||
| Jarrah | 5 | Wet | 2.01 ± 0.02 | 2.02 ± 0.02 | 94.29 ± 0.02 | 94.27 ± 0.02 | 107.18 ± 0.31 | 106.89 ± 0.31 |
| Dry | 1.41 ± 0.02 | 1.41 ± 0.03 | 93.32 ± 0.02 | 93.34 ± 0.02 | 193.33 ± 0.32 | 193.36 ± 0.32 | ||
| 30 | Wet | 2.02 ± 0.02 | 2.00 ± 0.02 | 94.28 ± 0.02 | 94.27 ± 0.03 | 107.38 ± 0.21 | 107.89 ± 0.21 | |
| Dry | 1.41 ± 0.02 | 1.39 ± 0.03 | 93.31 ± 0.01 | 93.34 ± 0.02 | 193.83 ± 0.22 | 193.86 ± 0.22 | ||
| 40 | Wet | 2.02 ± 0.02 | 2.00 ± 0.02 | 94.28 ± 0.02 | 94.26 ± 0.02 | 107.78 ± 0.21 | 108.07 ± 0.21 | |
| Dry | 1.40 ± 0.02 | 1.39 ± 0.03 | 93.30 ± 0.02 | 93.32 ± 0.02 | 194.33 ± 0.22 | 194.66 ± 0.26 | ||
| WA Manuka 2 | 5 | Wet | 2.01 ± 0.02 | 2.01 ± 0.02 | 94.25 ± 0.02 | 94.21 ± 0.02 | 108.33 ± 0.32 | 108.31 ± 0.34 |
| Dry | 1.39 ± 0.02 | 1.39 ± 0.02 | 93.28 ±0.02 | 93.27 ±0.03 | 194.65 ± 0.32 | 194.64 ± 0.32 | ||
| 30 | Wet | 2.02 ± 0.01 | 2.01 ± 0.02 | 94.26 ± 0.02 | 94.23 ± 0.02 | 108.64 ± 0.22 | 108.66 ± 0.24 | |
| Dry | 1.39 ± 0.01 | 1.39 ± 0.02 | 93.27 ± 0.02 | 93.24 ± 0.03 | 194.85 ± 0.22 | 194.97 ± 0.22 | ||
| 40 | Wet | 2.01 ± 0.02 | 2.01 ± 0.02 | 94.26 ± 0.02 | 94.22 ± 0.02 | 108.93 ± 0.22 | 108.41 ± 0.24 | |
| Dry | 1.40 ± 0.02 | 1.39 ± 0.02 | 93.26 ± 0.02 | 93.27 ± 0.01 | 194.95 ± 0.22 | 194.88 ± 0.22 | ||
| Duration | Time (min) | 5 °C | 30 °C | 40 °C | |||
|---|---|---|---|---|---|---|---|
| WA Jarrah Honey | WA Manuka Honey 2 | WA Jarrah Honey | WA Manuka Honey 2 | WA Jarrah Honey | WA Manuka Honey 2 | ||
| 1 month | 10 | 52.12 ± 0.15 | 54.52 ± 0.16 | 52.22 ± 0.17 | 54.32 ± 0.18 | 52.21 ± 0.17 | 54.22 ± 0.18 |
| 20 | 55.57 ± 0.17 | 58.60 ± 0.19 | 55.55 ± 0.15 | 58.57 ± 0.19 | 55.56 ± 0.15 | 58.56 ± 0.19 | |
| 30 | 55.61 ± 0.15 | 58.63 ± 0.16 | 55.60 ± 0.15 | 58.60 ± 0.17 | 55.60 ± 0.15 | 58.59 ± 0.17 | |
| 6 months | 10 | 52.11 ± 0.17 | 54.51 ± 0.18 | 52.13 ± 0.17 | 54.31 ± 0.18 | 52.14 ± 0.17 | 54.21 ± 0.18 |
| 20 | 55.56 ± 0.17 | 58.59 ± 0.19 | 55.57 ± 0.17 | 58.57 ± 0.19 | 55.57 ± 0.17 | 58.57 ± 0.19 | |
| 30 | 55.59 ± 0.17 | 58.61 ± 0.21 | 55.60 ± 0.17 | 58.61 ± 0.21 | 55.60 ± 0.17 | 58.60 ± 0.21 | |
| Honey | Storage Temperature (°C) | Sample | Weight (g) | Rf of Monitored Compound | Peak Area (AU × 10−3) per Band (±SD) | Peak Area (AU × 10−3) per Sheet (±SD) | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | Baseline | 6 Months | |||||
| WA Jarrah | 5 | Neat honey | 1.01 | 0.20 | 5.3 ± 0.01 | 5.3 ± 0.01 | N/A | N/A |
| 0.53 | 15.2 ± 0.02 | 15.2 ± 0.02 | N/A | N/A | ||||
| Pre-gel | 1.02 | 0.20 | 5.3 ± 0.03 | 5.3 ± 0.03 | N/A | N/A | ||
| 0.53 | 15.2 ± 0.02 | 15.2 ± 0.02 | N/A | N/A | ||||
| Wet sheet | 1.01 | 0.20 | 5.2 ± 0.01 | 5.2 ± 0.01 | 130 ± 0.02 | 130 ± 0.02 | ||
| 0.53 | 14.8 ± 0.03 | 14.8 ± 0.03 | 370 ± 0.02 | 370 ± 0.02 | ||||
| Dry sheet | 1.02 | 0.20 | 14.0 ± 0.04 | 14.0 ± 0.02 | 126 ± 0.01 | 126 ± 0.01 | ||
| 0.53 | 40.8 ± 0.03 | 40.8 ± 0.02 | 367 ± 0.03 | 367 ± 0.03 | ||||
| 30 | Neat honey | 1.02 | 0.20 | 5.2 ± 0.02 | 5.2 ± 0.03 | N/A | N/A | |
| 0.53 | 15.2 ± 0.02 | 15.2 ± 0.02 | N/A | N/A | ||||
| Pre-gel | 1.01 | 0.20 | 5.2 ± 0.02 | 5.2 ± 0.02 | N/A | N/A | ||
| 0.53 | 15.2 ± 0.02 | 15.2 ± 0.02 | N/A | N/A | ||||
| Wet sheet | 1.02 | 0.20 | 5.1 ± 0.01 | 5.1 ± 0.02 | 128 ± 0.02 | 128 ± 0.02 | ||
| 0.53 | 14.8 ± 0.03 | 14.8 ± 0.03 | 370 ± 0.03 | 370 ± 0.03 | ||||
| Dry sheet | 1.02 | 0.20 | 14.1 ± 0.03 | 14.1 ± 0.03 | 127 ± 0.03 | 127 ± 0.03 | ||
| 0.53 | 40.8 ± 0.03 | 40.8 ± 0.02 | 367 ± 0.02 | 367 ± 0.02 | ||||
| 40 | Neat honey | 1.01 | 0.20 | 5.2 ± 0.01 | 5.2 ± 0.01 | N/A | N/A | |
| 0.53 | 15.2 ± 0.01 | 15.2 ± 0.01 | N/A | N/A | ||||
| Pre-gel | 1.02 | 0.20 | 5.2 ± 0.03 | 5.2 ± 0.02 | N/A | N/A | ||
| 0.53 | 15.2 ± 0.02 | 15.2 ± 0.02 | N/A | N/A | ||||
| Wet sheet | 1.01 | 0.20 | 5.1 ± 0.02 | 5.1 ± 0.02 | 128 ± 0.02 | 128 ± 0.02 | ||
| 0.53 | 14.8 ± 0.02 | 14.8 ± 0.03 | 369 ± 0.02 | 369 ± 0.02 | ||||
| Dry sheet | 1.01 | 0.20 | 14.0 ± 0.03 | 14.0 ± 0.02 | 126 ± 0.03 | 126 ± 0.03 | ||
| 0.53 | 40.8 ± 0.02 | 40.8 ± 0.02 | 367 ± 0.02 | 367 ± 0.02 | ||||
| WA Manuka 2 | 5 | Neat honey | 1.01 | 0.20 | 17.4 ± 0.03 | 17.4 ± 0.03 | N/A | N/A |
| 0.38 | 23.5 ± 0.03 | 23.5 ± 0.02 | N/A | N/A | ||||
| Pre-gel | 1.02 | 0.20 | 17.3 ± 0.03 | 17.3 ± 0.03 | N/A | N/A | ||
| 0.38 | 23.4 ± 0.02 | 23.4 ± 0.03 | N/A | N/A | ||||
| Wet sheet | 1.02 | 0.20 | 17.1 ± 0.03 | 17.1 ± 0.04 | 427 ± 0.02 | 427 ± 0.02 | ||
| 0.38 | 23.3 ± 0.02 | 23.3 ± 0.03 | 582 ± 0.03 | 582 ± 0.03 | ||||
| Dry sheet | 1.01 | 0.20 | 47.5 ± 0.03 | 47.5 ± 0.03 | 428 ± 0.03 | 428 ± 0.03 | ||
| 0.38 | 64.9 ± 0.02 | 64.9 ± 0.02 | 584 ± 0.03 | 584 ± 0.03 | ||||
| 30 | Neat honey | 1.02 | 0.20 | 17.4 ± 0.03 | 17.4 ± 0.03 | N/A | N/A | |
| 0.38 | 23.5 ± 0.03 | 23.5 ± 0.02 | N/A | N/A | ||||
| Pre-gel | 1.01 | 0.20 | 17.3 ± 0.03 | 17.3 ± 0.03 | N/A | N/A | ||
| 0.38 | 23.4 ± 0.02 | 23.4 ± 0.02 | N/A | N/A | ||||
| Wet sheet | 1.02 | 0.20 | 17.2 ± 0.03 | 17.2 ± 0.04 | 428 ± 0.02 | 428 ± 0.02 | ||
| 0.38 | 23.3 ± 0.02 | 23.3 ± 0.03 | 581 ± 0.03 | 581 ± 0.03 | ||||
| Dry sheet | 1.02 | 0.20 | 47.6 ± 0.03 | 47.6 ± 0.03 | 427 ± 0.03 | 427 ± 0.03 | ||
| 0.38 | 65.0 ± 0.02 | 65.0 ± 0.03 | 585 ± 0.02 | 585 ± 0.02 | ||||
| 40 | Neat honey | 1.01 | 0.20 | 17.4 ± 0.03 | 17.4 ± 0.03 | N/A | N/A | |
| 0.38 | 23.5 ± 0.02 | 23.5 ± 0.02 | N/A | N/A | ||||
| Pre-gel | 1.02 | 0.20 | 17.3 ± 0.03 | 17.3 ± 0.03 | N/A | N/A | ||
| 0.38 | 23.4 ± 0.03 | 23.4 ± 0.03 | N/A | N/A | ||||
| Wet sheet | 1.02 | 0.20 | 17.1 ± 0.03 | 17.1 ± 0.02 | 427 ± 0.03 | 427 ± 0.03 | ||
| 0.38 | 23.3 ± 0.02 | 23.3 ± 0.03 | 582 ± 0.02 | 582 ± 0.02 | ||||
| Dry sheet | 1.02 | 0.20 | 47.5 ± 0.03 | 47.5 ± 0.03 | 427 ± 0.02 | 427 ± 0.02 | ||
| 0.38 | 64.9 ± 0.03 | 64.9 ± 0.02 | 584 ± 0.02 | 584 ± 0.02 | ||||
| Sample Type | Storage Temperature (°C) | MGO (mg/kg) | |||||
|---|---|---|---|---|---|---|---|
| 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | ||
| Neat honey | 5 | 181.34 ± 1.2 | 181.65 ± 1.3 | 182.47 ± 1.2 | 181.82 ± 1.3 | 182.55 ± 1.2 | 182.55 ± 1.3 |
| 30 | 180.12 ± 1.1 | 179.25 ± 1.2 | 178.54 ± 1.3 | 176.82 ± 1.3 | 175.55 ± 1.2 | 174.55 ± 1.2 | |
| 40 | 176.12 ± 1.2 | 174.45 ± 1.2 | 173.78 ± 1.2 | 171.88 ± 1.3 | 169.75 ± 1.2 | 168.65 ± 1.3 | |
| Pre-gel solution | 5 | 182.34 ± 1.3 | 181.65 ± 1.3 | 182.47 ± 1.2 | 181.62 ± 1.3 | 182.65 ± 1.2 | 181.35 ± 1.3 |
| 30 | 180.34 ± 1.2 | 179.85 ± 1.3 | 178.44 ± 1.2 | 177.73 ± 1.3 | 175.15 ± 1.2 | 174.35 ± 1.2 | |
| 40 | 177.14 ± 1.2 | 176.35 ± 1.3 | 173.34 ± 1.2 | 172.37 ± 1.3 | 169.19 ± 1.2 | 167.32 ± 1.2 | |
| Wet sheet | 5 | 181.51 ± 1.1 | 181.55 ± 1.3 | 181.24 ± 1.2 | 181.42 ± 1.2 | 181.55 ± 1.2 | 181.54 ± 1.3 |
| 30 | 180.75 ± 1.2 | 180.12 ± 1.3 | 179.42 ± 1.3 | 178.28 ± 1.3 | 177.72 ± 1.1 | 175.55 ± 1.1 | |
| 40 | 175.29 ± 1.2 | 174.53 ± 1.2 | 172.41 ± 1.2 | 171.22 ± 1.1 | 169.45 ± 1.2 | 168.33 ± 1.2 | |
| Dry sheet | 5 | 181.67 ± 1.1 | 181.35 ± 1.3 | 181.34 ± 1.1 | 181.27 ± 1.2 | 181.22 ± 1.2 | 181.34 ± 1.2 |
| 30 | 180.14 ± 1.2 | 179.25 ± 1.3 | 178.11 ± 1.2 | 177.32 ± 1.3 | 175.81 ± 1.1 | 174.55 ± 1.3 | |
| 40 | 175.61 ± 1.2 | 174.44 ± 1.3 | 173.33 ± 1.1 | 171.28 ± 1.2 | 169.27 ± 1.3 | 167.23 ± 1.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, M.L.; Lim, L.Y.; Hammer, K.; Hettiarachchi, D.; Locher, C. Design, Preparation, and Physicochemical Characterisation of Alginate-Based Honey-Loaded Topical Formulations. Pharmaceutics 2023, 15, 1483. https://doi.org/10.3390/pharmaceutics15051483
Hossain ML, Lim LY, Hammer K, Hettiarachchi D, Locher C. Design, Preparation, and Physicochemical Characterisation of Alginate-Based Honey-Loaded Topical Formulations. Pharmaceutics. 2023; 15(5):1483. https://doi.org/10.3390/pharmaceutics15051483
Chicago/Turabian StyleHossain, Md Lokman, Lee Yong Lim, Katherine Hammer, Dhanushka Hettiarachchi, and Cornelia Locher. 2023. "Design, Preparation, and Physicochemical Characterisation of Alginate-Based Honey-Loaded Topical Formulations" Pharmaceutics 15, no. 5: 1483. https://doi.org/10.3390/pharmaceutics15051483