Delivery of Chemotherapy Agents and Nucleic Acids with pH-Dependent Nanoparticles
Abstract
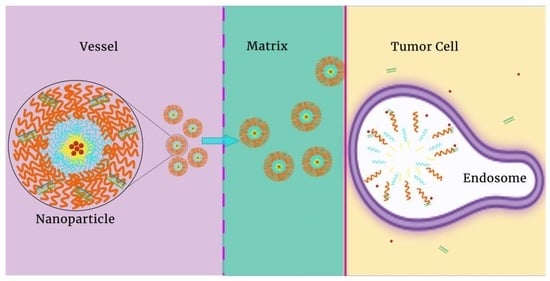
:1. Introduction
2. Acidic Tumor Environments
2.1. The Extracellular pH (pHe) of Tumors
2.2. pH-Regulation within Tumor Endosomes
3. PH-Sensitive Bonds
3.1. Covalent Bonds
3.1.1. Hydrazone Bonds
3.1.2. Imine Bonds
3.1.3. Methylene Bridges
3.1.4. Coordination Bonds
3.2. Non-Covalent Interactions
4. Charge–Charge Repulsion with the Release of Hydrophobic Drugs
4.1. Release of Chemotherapeutic Agents
4.2. Dual Delivery of Chemotherapy and Nucleic Acids
5. Disassembly of Nanoparticles Couple to pH-Sensitive Covalent Linkages
6. PH-Sensitive Coatings
7. Tumor-Penetrating Peptides and Nanoparticles as Potential Chemotherapeutic Carriers
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AB5 | polymer comprising polydimethysiloxane (PDMS) and PDMAEMA |
| ADP | adenosine diphosphate |
| ATP | adenosine triphosphate |
| Afc | aminoferrocene |
| ATRAM | acidity-triggered rational peptide comprising GLAGLAGLLG- LEGLLGLPLGLLEGLWLGLELEGN |
| 4-CB | 4-carboxybenzaldehyde |
| CD | carbon dots |
| Chex50-HA | hyaluronic acid modified by cyclohexyl groups containing a carboxyl group or alkyl esters |
| 4-CB | 4-carboxybenzaldehyde |
| Co-DCL | co-dimethyl maleamidic acid-ε-caprolactone, a copolymer |
| DEAE | diethyl aminoethyl (pH sensitive subunit of polymer) |
| DMAE | dimethyl aminoethyl (pH sensitive subunit of polymer) |
| DPAE | Diisopropyl aminoethyl (pH sensitive subunit of polymer) |
| Dox | doxorubicin |
| Dox-Ma | doxorubicin methacrylamide |
| EGFR | epidermal growth factor receptor |
| EPR | enhanced permeability and retention |
| FA | folic acid |
| HA | hyaluronic acid |
| HIF-1α | hypoxia inducible factor-1 alpha |
| His | histidine |
| H4R4 | histidine-arginine peptide |
| Lys | lysine |
| MCT | monocarboxylate transporter |
| NHE-1-10 | sodium hydrogen exchanger-1 to 10 |
| MAEBA | (methacryloxyethoxy)-benzaldehyde, a component of the copolymer |
| MDR | multidrug resistance |
| MRI/MRS | magnetic resonance imaging/magnetic resonance spectroscopy |
| NAD | nicotinamide adenine dinucleotide |
| NADH | nicotinamide adenine dinucleotide hydrogen |
| NBC | sodium bicarbonate transporter |
| OEI | oligoethylenimine |
| PAA | polyacrylic acid |
| PAsp | poly-L-aspartic acid |
| PAsp(AED) | poly(N-(2,2′-dithiobis(ethylamine)) aspartamide) |
| PBAE | poly(β-amino ester) polymers |
| PCL | poly(ε-caprolactone) |
| (m) PEG | (methyl) polyethylene glycol |
| PDEAEMA | poly(2-(diethylamino)ethyl methacrylate) |
| PDMAEMA | poly(2-(dimethylamino)ethyl methacrylate |
| PDPAEMA (PDPA) | poly(2-(diisopropylamino)ethyl methacrylate |
| PGP | P-glycoprotein transporter |
| pHe | extracellular pH |
| PEI | polyethylenimine |
| PLH | poly-L-histidine |
| PLLA (PLA) | poly-L(L,D)-lactic acid |
| PLGA | poly lactic-co-glycolic acid |
| PMMA | mPEG-b-PDEAEMA |
| PPO | Pluronic F-108 |
| PPEGMA | poly(polyethylene glycol) methyl ether methacrylate |
| PDMS | polydimethylsiloxane |
| PMBC | poly(2-methacryloyloxy-ethyl phosphorylcholine |
| PTX | paclitaxel |
| shRNA | short hairpin RNA expressed by plasmid |
| shTw | short hairpin RNA targeting the Twist transcription factor |
| siBCL-2 | siRNA targeting BCL-2 |
| siPGP | siRNA targeting the P-glycoprotein transporter |
| siRNA | short interfering RNA |
| TCA | tricarboxylic acid |
| TMDP | trimethylene dipiperidine, one potential subunit that is part of PBAE |
| TPGS | D-α-tocopheryl-PEG |
| V-ATPase | vacuolar ATPase transporter |
| ZP | zeta potential, which reflects the surface charge of the particle |
References
- Buil-Bruna, N.; López-Picazo, J.-M.; Martín-Algarra, S.; Trocóniz, I.F. Bringing Model-Based Prediction to Oncology Clinical Practice: A Review of Pharmacometrics Principles and Applications. Oncologist 2015, 21, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Sriraman, S.K.; Aryasomayajula, B.; Torchilin, V.P. Barriers to drug delivery in solid tumors. Tissue Barriers 2014, 2, e29528. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Chen, B.; Pan, R.; Askhatova, D. Effective small interfering RNA delivery in vitro via a new stearylated cationic peptide. Int. J. Nanomed. 2015, 10, 3303–3314. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Park, J.W. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. BCR 2002, 4, 95–99. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Nukolova, N.V.; Kabanov, A.V.; Bronich, T.K. Nanocarriers for delivery of platinum anticancer drugs. Adv. Drug Deliv. Rev. 2013, 65, 1667–1685. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Prabu, P.; Chaudhari, A.A.; Dharmaraj, N.; Khil, M.S.; Park, S.Y.; Kim, H.Y. Preparation, characterization, in-vitro drug release and cellular uptake of poly(caprolactone) grafted dextran copolymeric nanoparticles loaded with anticancer drug. J. Biomed. Mater. Res. Part A 2009, 90, 1128–1136. [Google Scholar] [CrossRef]
- Junnuthula, V.; Kolimi, P.; Nyavanandi, D.; Sampathi, S.; Vora, L.K.; Dyawanapelly, S. Polymeric Micelles for Breast Cancer Therapy: Recent Updates, Clinical Translation and Regulatory Considerations. Pharmaceutics 2022, 14, 1860. [Google Scholar] [CrossRef]
- Raza, F.; Zafar, H.; Khan, M.W.; Ullah, A.; Khan, A.U.; Baseer, A.; Fareed, R.; Sohail, M. Recent advances in the targeted delivery of paclitaxel nanomedicine for cancer therapy. Mater. Adv. 2022, 3, 2268–2290. [Google Scholar] [CrossRef]
- Rahman, A.M.; Yusuf, S.W.; Ewer, M.S. Anthracycline-induced cardiotoxicity and the cardiac-sparing effect of liposomal formulation. Int. J. Nanomed. 2007, 2, 567–583. [Google Scholar]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef]
- Ngan, Y.H.; Gupta, M. A comparison between liposomal and nonliposomal formulations of doxorubicin in the treatment of cancer: An updated review. Arch. Pharm. Pract. 2016, 7, 1–13. [Google Scholar]
- Wainberg, Z.A.; Bekaii-Saab, T.S.; Hubner, R.; Macarulla, T.; Paulson, A.S.; Cutsem, E.V.; Maxwell, F.; Moore, Y.; Wang, H.T.; Zhang, B.; et al. NAPOLI-3: An open-label, randomized, phase III study of first-line liposomal irinotecan + 5-fluorouracil/leucovorin + oxaliplatin versus nab-paclitaxel + gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. J. Clin. Oncol. 2020, 38, TPS4661. [Google Scholar] [CrossRef]
- Kieler-Ferguson, H.M.; Chan, D.; Sockolosky, J.; Finney, L.; Maxey, E.; Vogt, S.; Szoka, F.C., Jr. Encapsulation, controlled release, and antitumor efficacy of cisplatin delivered in liposomes composed of sterol-modified phospholipids. Eur. J. Pharm. Sci. 2017, 103, 85–93. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Theek, B.; Oude Blenke, E.; Pieters, E.H.; Fens, M.H.; Ehling, J.; Schiffelers, R.M.; Storm, G.; van Nostrum, C.F.; et al. Complete Regression of Xenograft Tumors upon Targeted Delivery of Paclitaxel via Pi-Pi Stacking Stabilized Polymeric Micelles. ACS Nano 2015, 9, 3740–3752. [Google Scholar] [CrossRef]
- Priwitaningrum, D.L.; Pednekar, K.; Gabriel, A.V.; Varela-Moreira, A.A.; Le Gac, S.; Vellekoop, I.; Storm, G.; Hennink, W.E.; Prakash, J. Evaluation of paclitaxel-loaded polymeric nanoparticles in 3D tumor model: Impact of tumor stroma on penetration and efficacy. Drug Deliv. Transl. Res. 2023, 13, 1470–1483. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Estape Senti, M.; de Jongh, C.A.; Dijkxhoorn, K.; Verhoef, J.J.F.; Szebeni, J.; Storm, G.; Hack, C.E.; Schiffelers, R.M.; Fens, M.H.; Boross, P. Anti-PEG antibodies compromise the integrity of PEGylated lipid-based nanoparticles via complement. J. Control. Release 2022, 341, 475–486. [Google Scholar] [CrossRef]
- Subhan, M.A.; Yalamarty, S.S.K.; Filipczak, N.; Parveen, F.; Torchilin, V.P. Recent Advances in Tumor Targeting via EPR Effect for Cancer Treatment. J. Pers. Med. 2021, 11, 571. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Leng, Q.; Woodle, M.C.; Mixson, A.J. NRP1 transport of cancer therapeutics mediated by tumor-penetrating peptides. Drugs Future 2017, 42, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.J.; Snider, J.L.; Skalli, O.; Welbourne, T.; Cardelli, J.A. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic 2009, 10, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef]
- Warburg, O.; Posener, K.; Negelein, E. Uber den Stoffwechsel der Carcinomzelle. Bioch. Zeitsch 1924, 152, 309–344. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Payen, V.L.; Porporato, P.E.; Baselet, B.; Sonveaux, P. Metabolic changes associated with tumor metastasis, part 1: Tumor pH, glycolysis and the pentose phosphate pathway. Cell. Mol. Life Sci. 2016, 73, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J. Cancer heterogeneity and metastasis: Life at the edge. Clin. Exp. Metastasis 2021, 39, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Perez-Herrero, E.; Fernandez-Medarde, A. The reversed intra- and extracellular pH in tumors as a unified strategy to chemotherapeutic delivery using targeted nanocarriers. Acta Pharm. Sin. B 2021, 11, 2243–2264. [Google Scholar] [CrossRef] [PubMed]
- Piasentin, N.; Milotti, E.; Chignola, R. The control of acidity in tumor cells: A biophysical model. Sci. Rep. 2020, 10, 13613. [Google Scholar] [CrossRef]
- Martinez-Zaguilan, R.; Seftor, E.A.; Seftor, R.E.; Chu, Y.W.; Gillies, R.J.; Hendrix, M.J. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 1996, 14, 176–186. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Mathiesen, B.; Kindem, K.; Galappathi, K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006, 66, 6699–6707. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef]
- Hjelmeland, A.B.; Wu, Q.; Heddleston, J.M.; Choudhary, G.S.; MacSwords, J.; Lathia, J.D.; McLendon, R.; Lindner, D.; Sloan, A.; Rich, J.N. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011, 18, 829–840. [Google Scholar] [CrossRef]
- Robey, I.F.; Baggett, B.K.; Kirkpatrick, N.D.; Roe, D.J.; Dosescu, J.; Sloane, B.F.; Hashim, A.I.; Morse, D.L.; Raghunand, N.; Gatenby, R.A.; et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res 2009, 69, 2260–2268. [Google Scholar] [CrossRef]
- Volk, T.; Jahde, E.; Fortmeyer, H.P.; Glusenkamp, K.H.; Rajewsky, M.F. pH in human tumour xenografts: Effect of intravenous administration of glucose. Br. J. Cancer 1993, 68, 492–500. [Google Scholar] [CrossRef]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging 2002, 16, 430–450. [Google Scholar] [CrossRef] [PubMed]
- van Sluis, R.; Bhujwalla, Z.M.; Raghunand, N.; Ballesteros, P.; Alvarez, J.; Cerdan, S.; Galons, J.P.; Gillies, R.J. In vivo imaging of extracellular pH using 1H MRSI. Magn. Reson. Med. 1999, 41, 743–750. [Google Scholar] [CrossRef]
- Adams, D.J.; Dewhirst, M.W.; Flowers, J.L.; Gamcsik, M.P.; Colvin, O.M.; Manikumar, G.; Wani, M.C.; Wall, M.E. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother. Pharmacol. 2000, 46, 263–271. [Google Scholar] [CrossRef]
- Fan, F.; Piao, J.-G.; Zhao, Y.; Jin, L.; Li, M.; Wang, Y.; Yang, L. Bioinspired Membrane-Disruptive Macromolecules as Drug-Free Therapeutics. ACS Appl. Bio Mater. 2020, 3, 1267–1275. [Google Scholar] [CrossRef]
- Fan, F.; Jin, L.; Yang, L. pH-Sensitive Nanoparticles Composed Solely of Membrane-Disruptive Macromolecules for Treating Pancreatic Cancer. ACS Appl. Mater. Interfaces 2021, 13, 12824–12835. [Google Scholar] [CrossRef] [PubMed]
- Naeslund, J.; Swenson, K.-E. Investigations on the pH of Malignant Tumours in Mice and Humans after the Administration of Glucose. Acta Obstet. et Gynecol. Scand. 1953, 32, 359–367. [Google Scholar] [CrossRef]
- Eden, M.; Haines, B.; Kahler, H. The pH of rat tumors measured in vivo. J. Natl. Cancer Inst. 1955, 16, 541–556. [Google Scholar] [PubMed]
- Ashby, B.S. pH studies in human malignant tumours. Lancet 1966, 288, 312–315. [Google Scholar] [CrossRef]
- Kalliomaki, T.; Hill, R.P. Effects of tumour acidification with glucose+MIBG on the spontaneous metastatic potential of two murine cell lines. Br. J. Cancer 2004, 90, 1842–1849. [Google Scholar] [CrossRef]
- Adachi, E.; Tannock, I.F. The effects of vasodilating drugs on pH in tumors. Oncol. Res. 1999, 11, 179–185. [Google Scholar]
- Park, W.; Chen, J.; Cho, S.; Park, S.J.; Larson, A.C.; Na, K.; Kim, D.H. Acidic pH-Triggered Drug-Eluting Nanocomposites for Magnetic Resonance Imaging-Monitored Intra-arterial Drug Delivery to Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2016, 8, 12711–12719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lin, Y.; Gillies, R.J. Tumor pH and its measurement. J. Nucl. Med. 2010, 51, 1167–1170. [Google Scholar] [CrossRef]
- Zhao, Y.; Alakhova, D.Y.; Kim, J.O.; Bronich, T.K.; Kabanov, A.V. A simple way to enhance Doxil® therapy: Drug release from liposomes at the tumor site by amphiphilic block copolymer. J. Control. Release 2013, 168, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.M.P.; Brans, T.; Samal, S.K.; Dubruel, P.; Demeester, J.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Endosomal Size and Membrane Leakiness Influence Proton Sponge-Based Rupture of Endosomal Vesicles. ACS Nano 2018, 12, 2332–2345. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.M.P.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. The proton sponge hypothesis: Fable or fact? Eur. J. Pharm. Biopharm. 2018, 129, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.D.; Bednarski, M.; Frausto, R.; Guccione, S.; Reisfeld, R.A.; Xiang, R.; Cheresh, D.A. Tumor regression by targeted gene delivery to the neovasculature. Science 2002, 296, 2404–2407. [Google Scholar] [CrossRef]
- Wilner, S.E.; Wengerter, B.; Maier, K.; de Lourdes Borba Magalhaes, M.; Del Amo, D.S.; Pai, S.; Opazo, F.; Rizzoli, S.O.; Yan, A.; Levy, M. An RNA alternative to human transferrin: A new tool for targeting human cells. Mol. Ther.-Nucleic Acids 2012, 1, e21. [Google Scholar] [CrossRef]
- Leng, Q.; Woodle, M.C.; Mixson, A.J. Targeted Delivery of siRNA Therapeutics to Malignant Tumors. J. Drug Deliv. 2017, 2017, 6971297. [Google Scholar] [CrossRef]
- Li, J.M.; Wang, Y.Y.; Zhang, W.; Su, H.; Ji, L.N.; Mao, Z.W. Low-weight polyethylenimine cross-linked 2-hydroxypopyl-beta-cyclodextrin and folic acid as an efficient and nontoxic siRNA carrier for gene silencing and tumor inhibition by VEGF siRNA. Int. J. Nanomed. 2013, 8, 2101–2117. [Google Scholar] [CrossRef]
- Ko, M.; Quinones-Hinojosa, A.; Rao, R. Emerging links between endosomal pH and cancer. Cancer Metastasis Rev. 2020, 39, 519–534. [Google Scholar] [CrossRef]
- Heidel, J.D.; Yu, Z.; Liu, J.Y.; Rele, S.M.; Liang, Y.; Zeidan, R.K.; Kornbrust, D.J.; Davis, M.E. Administration in non-human primates of escalating intravenous doses of targeted nanoparticles containing ribonucleotide reductase subunit M2 siRNA. Proc. Natl. Acad. Sci. USA 2007, 104, 5715–5721. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Kim, J.; Herrera, M.; Mukherjee, A.; Kabanov, A.V.; Sahay, G. Brief update on endocytosis of nanomedicines. Adv. Drug Deliv. Rev. 2019, 144, 90–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, J.; Zheng, Y.; Yang, H.; Luo, K.; Liu, Q.; Hu, R.; Tan, Z.; Huang, Q.; Fu, J. Prognostic significance of SLC9A9 in patients with resectable esophageal squamous cell carcinoma. Tumor Biol. 2015, 36, 6797–6803. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.W.; Maher, V.M.; McCormick, J.J.; Schindler, M. Alkalinization of the lysosomes is correlated with ras transformation of murine and human fibroblasts. J. Biol. Chem. 1990, 265, 4775–4777. [Google Scholar] [CrossRef] [PubMed]
- Kondapalli, K.C.; Llongueras, J.P.; Capilla-Gonzalez, V.; Prasad, H.; Hack, A.; Smith, C.; Guerrero-Cazares, H.; Quinones-Hinojosa, A.; Rao, R. A leak pathway for luminal protons in endosomes drives oncogenic signalling in glioblastoma. Nat. Commun. 2015, 6, 6289. [Google Scholar] [CrossRef]
- Lucien, F.; Pelletier, P.P.; Lavoie, R.R.; Lacroix, J.M.; Roy, S.; Parent, J.L.; Arsenault, D.; Harper, K.; Dubois, C.M. Hypoxia-induced mobilization of NHE6 to the plasma membrane triggers endosome hyperacidification and chemoresistance. Nat. Commun. 2017, 8, 15884. [Google Scholar] [CrossRef]
- Lucien, F.; Lavoie, R.R.; Dubois, C.M. Targeting endosomal pH for cancer chemotherapy. Mol. Cell. Oncol. 2018, 5, e1435184. [Google Scholar] [CrossRef]
- Fan, S.H.; Numata, Y.; Numata, M. Endosomal Na+/H+ exchanger NHE5 influences MET recycling and cell migration. Mol. Biol. Cell 2016, 27, 702–715. [Google Scholar] [CrossRef]
- Chen, Q.R.; Zhang, L.; Luther, P.W.; Mixson, A.J. Optimal transfection with the HK polymer depends on its degree of branching and the pH of endocytic vesicles. Nucleic Acids Res. 2002, 30, 1338–1345. [Google Scholar] [CrossRef]
- Andrian, T.; Riera, R.; Pujals, S.; Albertazzi, L. Nanoscopy for endosomal escape quantification. Nanoscale Adv. 2021, 3, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.M.P.; Brans, T.; De Smedt, S.C.; Remaut, K.; Braeckmans, K. Methodologies to investigate intracellular barriers for nucleic acid delivery in non-viral gene therapy. Nano Today 2018, 21, 74–90. [Google Scholar] [CrossRef]
- Wong, A.S.; Mann, S.K.; Czuba, E.; Sahut, A.; Liu, H.; Suekama, T.C.; Bickerton, T.; Johnston, A.P.; Such, G.K. Self-assembling dual component nanoparticles with endosomal escape capability. Soft Matter 2015, 11, 2993–3002. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Teo, S.L.Y.; Chen, M.Z.; Smith, S.A.; Pouton, C.W.; Johnston, A.P.R.; Such, G.K. Quantifying the Endosomal Escape of pH-Responsive Nanoparticles Using the Split Luciferase Endosomal Escape Quantification Assay. ACS Appl. Mater. Interfaces 2022, 14, 3653–3661. [Google Scholar] [CrossRef] [PubMed]
- Rehman, Z.U.; Hoekstra, D.; Zuhorn, I.S. Mechanism of Polyplex- and Lipoplex-Mediated Delivery of Nucleic Acids: Real-Time Visualization of Transient Membrane Destabilization without Endosomal Lysis. ACS Nano 2013, 7, 3767–3777. [Google Scholar] [CrossRef]
- Raghunand, N.; He, X.; van Sluis, R.; Mahoney, B.; Baggett, B.; Taylor, C.W.; Paine-Murrieta, G.; Roe, D.; Bhujwalla, Z.M.; Gillies, R.J. Enhancement of chemotherapy by manipulation of tumour pH. Br. J. Cancer 1999, 80, 1005–1011. [Google Scholar] [CrossRef]
- Midoux, P.; Monsigny, M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug. Chem. 1999, 10, 406–411. [Google Scholar] [CrossRef]
- Chen, Q.R.; Zhang, L.; Stass, S.A.; Mixson, A.J. Co-polymer of histidine and lysine markedly enhances transfection efficiency of liposomes. Gene Ther. 2000, 7, 1698–1705. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, e1800917. [Google Scholar] [CrossRef]
- Zhuo, S.; Zhang, F.; Yu, J.; Zhang, X.; Yang, G.; Liu, X. pH-Sensitive Biomaterials for Drug Delivery. Molecules 2020, 25, 5649. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Frechet, J.M.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Iman, M.; Rijcken, C.J.; van Nostrum, C.F.; Hennink, W.E. Targeted core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. J. Control. Release 2010, 148, e121–e122. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Iman, M.; Varkouhi, A.K.; Rijcken, C.J.; Schiffelers, R.M.; Etrych, T.; Ulbrich, K.; van Nostrum, C.F.; Lammers, T.; Storm, G.; et al. Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials 2010, 31, 7797–7804. [Google Scholar] [CrossRef]
- Liu, C.; Liu, F.; Feng, L.; Li, M.; Zhang, J.; Zhang, N. The targeted co-delivery of DNA and doxorubicin to tumor cells via multifunctional PEI-PEG based nanoparticles. Biomaterials 2013, 34, 2547–2564. [Google Scholar] [CrossRef] [PubMed]
- Wuang, S.C.; Neoh, K.G.; Kang, E.T.; Leckband, D.E.; Pack, D.W. Acid-Sensitive Magnetic Nanoparticles as Potential Drug Depots. AIChE J. 2011, 57, 1638–1645. [Google Scholar] [CrossRef]
- Etrych, T.; Jelinkova, M.; Rihova, B.; Ulbrich, K. New HPMA copolymers containing doxorubicin bound via pH-sensitive linkage: Synthesis and preliminary in vitro and in vivo biological properties. J. Control. Release 2001, 73, 89–102. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Song, J.; Li, L.; Ye, Q.; Zuo, S.; Liu, T.; Dong, F.; Liu, X.; He, Z.; et al. Structure–Activity Relationship of pH-Sensitive Doxorubicin-Fatty Acid Prodrug Albumin Nanoparticles. Nano Lett. 2023, 23, 1530–1538. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, C.; Wang, W.; Liu, J.; Liu, Q.; Huang, F.; Chu, L.; Gao, H.; Li, C.; Kong, D.; et al. Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer. Sci. Rep. 2016, 6, 21225. [Google Scholar] [CrossRef]
- Zhang, P.; Ye, J.; Liu, E.; Sun, L.; Zhang, J.; Lee, S.-J.; Gong, J.; He, H.; Yang, V.C. Aptamer-coded DNA nanoparticles for targeted doxorubicin delivery using pH-sensitive spacer. Front. Chem. Sci. Eng. 2017, 11, 529–536. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, V.; Bhat, C.; Vahermo, M.; Makila, E.; Kemell, M.; Fontana, F.; Janoniene, A.; Petrikaite, V.; Salonen, J.; et al. Quercetin-Based Modified Porous Silicon Nanoparticles for Enhanced Inhibition of Doxorubicin-Resistant Cancer Cells. Adv. Healthc. Mater. 2017, 6, 1601009. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-N.; Guo, N.-N.; Wang, T.-T.; Guo, W.-W.; Lin, M.-T.; Huang-Fu, M.-Y.; Vakili, M.R.; Xu, W.-H.; Chen, J.-J.; Wei, Q.-C.; et al. Mitochondrial Targeted Doxorubicin-Triphenylphosphonium Delivered by Hyaluronic Acid Modified and pH Responsive Nanocarriers to Breast Tumor: In Vitro and in Vivo Studies. Mol. Pharm. 2018, 15, 882–891. [Google Scholar] [CrossRef]
- Lei, M.; Chen, G.; Zhang, M.; Lei, J.; Li, T.; Li, D.; Zheng, H. A pH-sensitive drug delivery system based on hyaluronic acid co-deliver doxorubicin and aminoferrocene for the combined application of chemotherapy and chemodynamic therapy. Colloids Surf. B Biointerfaces 2021, 203, 111750. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Teves, S.S.; Kemp, C.J.; Henikoff, S. Doxorubicin, DNA torsion, and chromatin dynamics. Biochim. Biophys Acta 2014, 1845, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Boyacioglu, O.; Stuart, C.H.; Kulik, G.; Gmeiner, W.H. Dimeric DNA Aptamer Complexes for High-capacity-targeted Drug Delivery Using pH-sensitive Covalent Linkages. Mol. Ther. Nucleic Acids 2013, 2, e107. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.A.; Lee, S.-W. pH-triggered degradation and release of doxorubicin from zeolitic imidazolate framework-8 (ZIF8) decorated with polyacrylic acid. RSC Adv. 2021, 11, 9222–9234. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Hu, Y.; Yang, Z.; Li, J.; Liu, X.; Deng, L.; Wang, Y.; Zhang, X.; Jiang, T. Targeting PD-1 and Tim-3 pathways to reverse CD8 T-cell exhaustion and enhance ex vivo T-cell responses to autologous dendritic/tumor vaccines. J. Immunother. 2016, 39, 171–180. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Kasprzak, A.; Poplawska, M.; Ruzycka, M.; Grudzinski, I.P.; Nowicka, A.M. Controlled Drug Release and Cytotoxicity Studies of Beta-Lapachone and Doxorubicin Loaded into Cyclodextrins Attached to a Polyethyleneimine Matrix. Int. J. Mol. Sci. 2020, 21, 5832. [Google Scholar] [CrossRef]
- Hailing, Y.; Xiufang, L.; Lili, W.; Baoqiang, L.; Kaichen, H.; Yongquan, H.; Qianqian, Z.; Chaoming, M.; Xiaoshuai, R.; Rui, Z.; et al. Doxorubicin-loaded fluorescent carbon dots with PEI passivation as a drug delivery system for cancer therapy. Nanoscale 2020, 12, 17222–17237. [Google Scholar] [CrossRef]
- Charbgoo, F.; Alibolandi, M.; Taghdisi, S.M.; Abnous, K.; Soltani, F.; Ramezani, M. MUC1 aptamer-targeted DNA micelles for dual tumor therapy using doxorubicin and KLA peptide. Nanomedicine 2018, 14, 685–697. [Google Scholar] [CrossRef]
- Chou, S.T.; Hom, K.; Zhang, D.; Leng, Q.; Tricoli, L.J.; Hustedt, J.M.; Lee, A.; Shapiro, M.J.; Seog, J.; Kahn, J.D.; et al. Enhanced silencing and stabilization of siRNA polyplexes by histidine-mediated hydrogen bonds. Biomaterials 2014, 35, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Traeger, A.; Sungur, P.; Hoeppener, S.; Kellner, C.; Yildirim, I.; Pretzel, D.; Schubert, S.; Schubert, U.S. Polymersomes with Endosomal pH-Induced Vesicle-to-Micelle Morphology Transition and a Potential Application for Controlled Doxorubicin Delivery. Biomacromolecules 2017, 18, 3280–3290. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Richardson, J.J.; Ejima, H.; Such, G.K.; Cui, J.; Caruso, F. Peptide-tunable drug cytotoxicity via one-step assembled polymer nanoparticles. Adv. Mater. 2014, 26, 2398–2402. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xue, Z.; Liu, Y.; Xiao, J.; Chen, J.; Zhang, L.; Guo, J.; Lin, W. Delivery of anticancer drug using pH-sensitive micelles from triblock copolymer MPEG-b-PBAE-b-PLA. Mater. Sci. Eng. C 2018, 84, 254–262. [Google Scholar] [CrossRef]
- Lee, E.S.; Shin, H.J.; Na, K.; Bae, Y.H. Poly(L-histidine)-PEG block copolymer micelles and pH-induced destabilization. J. Control. Release 2003, 90, 363–374. [Google Scholar] [CrossRef]
- Samiei Foroushani, M.; Niroumand, N.; Karimi Shervedani, R.; Yaghoobi, F.; Kefayat, A.; Torabi, M. A theranostic system based on nanocomposites of manganese oxide nanoparticles and a pH sensitive polymer: Preparation, and physicochemical characterization. Bioelectrochemistry 2019, 130, 107347. [Google Scholar] [CrossRef]
- Patchornik, A.; Berger, A.; Katchalski, E. Poly-L-histidine. J. Am. Chem. Soc. 1957, 79, 5227–5236. [Google Scholar] [CrossRef]
- Curtis, K.A.; Miller, D.; Millard, P.; Basu, S.; Horkay, F.; Chandran, P.L. Unusual Salt and pH Induced Changes in Polyethylenimine Solutions. PLoS ONE 2016, 11, e0158147. [Google Scholar] [CrossRef]
- Ziebarth, J.D.; Wang, Y. Understanding the protonation behavior of linear polyethylenimine in solutions through Monte Carlo simulations. Biomacromolecules 2010, 11, 29–38. [Google Scholar] [CrossRef]
- Zhu, L.; Powell, S.; Boyes, S.G. Synthesis of tertiary amine-based pH-responsive polymers by RAFT Polymerization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1010–1022. [Google Scholar] [CrossRef]
- von Harpe, A.; Petersen, H.; Li, Y.; Kissel, T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J. Control. Release 2000, 69, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, C.; Gu, X.; Wang, G. Self-assembly, pH-responsibility and controlled release of doxorubicin of PDEAEMA-PEG-PDEAEMA triblock copolymers: Effects of PEG length. J. Polym. Res. 2021, 28, 175. [Google Scholar] [CrossRef]
- Putnam, D.; Zelikin, A.N.; Izumrudov, V.A.; Langer, R. Polyhistidine-PEG:DNA nanocomposites for gene delivery. Biomaterials 2003, 24, 4425–4433. [Google Scholar] [CrossRef] [PubMed]
- Lynn, D.M.; Langer, R. Degradable Poly(β-amino esters): Synthesis, Characterization, and Self-Assembly with Plasmid DNA. J. Am. Chem. Soc. 2000, 122, 10761–10768. [Google Scholar] [CrossRef]
- Kim, M.S.; Hwang, S.J.; Han, J.K.; Choi, E.K.; Park, H.J.; Kim, J.S.; Lee, D.S. pH-Responsive PEG-Poly(β-amino ester) Block Copolymer Micelles with a Sharp Transition. Macromol. Rapid Commun. 2006, 27, 447–451. [Google Scholar] [CrossRef]
- Zhao, S.; Tan, S.; Guo, Y.; Huang, J.; Chu, M.; Liu, H.; Zhang, Z. pH-sensitive docetaxel-loaded D-alpha-tocopheryl polyethylene glycol succinate-poly(beta-amino ester) copolymer nanoparticles for overcoming multidrug resistance. Biomacromolecules 2013, 14, 2636–2646. [Google Scholar] [CrossRef]
- Shenoy, D.; Little, S.; Langer, R.; Amiji, M. Poly(ethylene oxide)-modified poly(beta-amino ester) nanoparticles as a pH-sensitive system for tumor-targeted delivery of hydrophobic drugs. 1. In vitro evaluations. Mol. Pharm. 2005, 2, 357–366. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Feng, Z.; Wang, M.; Cai, C.; Wang, J.; Zhang, L. Poly(2-(diethylamino)ethyl methacrylate)-based, pH-responsive, copolymeric mixed micelles for targeting anticancer drug control release. Int. J. Nanomed. 2017, 12, 6857–6870. [Google Scholar] [CrossRef]
- Feng, J.; Wen, W.; Jia, Y.-G.; Liu, S.; Guo, J. pH-Responsive Micelles Assembled by Three-Armed Degradable Block Copolymers with a Cholic Acid Core for Drug Controlled-Release. Polymers 2019, 11, 511. [Google Scholar] [CrossRef]
- Kongkatigumjorn, N.; Smith, S.A.; Chen, M.; Fang, K.; Yang, S.; Gillies, E.R.; Johnston, A.P.R.; Such, G.K. Controlling Endosomal Escape Using pH-Responsive Nanoparticles with Tunable Disassembly. ACS Appl. Nano Mater. 2018, 1, 3164–3173. [Google Scholar] [CrossRef]
- Car, A.; Baumann, P.; Duskey, J.T.; Chami, M.; Bruns, N.; Meier, W. pH-responsive PDMS-b-PDMAEMA micelles for intracellular anticancer drug delivery. Biomacromolecules 2014, 15, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, Y.; Nan, S.; Zhang, L.; Huang, H.; Wang, J. Synthesis and characterization of pH-sensitive poly(itaconic acid)-poly(ethylene glycol)-folate-poly(l-histidine) micelles for enhancing tumor therapy and tunable drug release. J. Colloid Interface Sci. 2015, 458, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Swetha, K.L.; Maravajjala, K.S.; Sharma, S.; Chowdhury, R.; Roy, A. Development of a tumor extracellular pH-responsive nanocarrier by terminal histidine conjugation in a star shaped poly(lactic-co-glycolic acid). Eur. Polym. J. 2021, 147, 110337. [Google Scholar] [CrossRef]
- Davoodi, P.; Srinivasan, M.P.; Wang, C.H. Synthesis of intracellular reduction-sensitive amphiphilic polyethyleneimine and poly(epsilon-caprolactone) graft copolymer for on-demand release of doxorubicin and p53 plasmid DNA. Acta Biomater. 2016, 39, 79–93. [Google Scholar] [CrossRef]
- Zhang, C.G.; Zhu, W.J.; Liu, Y.; Yuan, Z.Q.; Yang, S.D.; Chen, W.L.; Li, J.Z.; Zhou, X.F.; Liu, C.; Zhang, X.N. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci. Rep. 2016, 6, 23859. [Google Scholar] [CrossRef]
- Shen, J.; Yin, Q.; Chen, L.; Zhang, Z.; Li, Y. Co-delivery of paclitaxel and survivin shRNA by pluronic P85-PEI/TPGS complex nanoparticles to overcome drug resistance in lung cancer. Biomaterials 2012, 33, 8613–8624. [Google Scholar] [CrossRef]
- Shen, J.; Sun, H.; Xu, P.; Yin, Q.; Zhang, Z.; Wang, S.; Yu, H.; Li, Y. Simultaneous inhibition of metastasis and growth of breast cancer by co-delivery of twist shRNA and paclitaxel using pluronic P85-PEI/TPGS complex nanoparticles. Biomaterials 2013, 34, 1581–1590. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, Y.; Cheng, D.; Chen, J.; Wang, L.; Shuai, X. Co-delivery of doxorubicin and siRNA with reduction and pH dually sensitive nanocarrier for synergistic cancer therapy. Small 2014, 10, 2678–2687. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, L.; Wang, Q.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. pH/Redox Dual-Responsive Polyplex with Effective Endosomal Escape for Codelivery of siRNA and Doxorubicin against Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, H. Notch-1 siRNA and Methotrexate towards a Multifunctional Approach in Rhematoid Arthritis Management: A Nanomedicine Approach. Pharm. Res. 2018, 35, 123. [Google Scholar] [CrossRef]
- Diaz, I.L.; Sierra, C.A.; Jérôme, V.; Freitag, R.; Perez, L.D. Target grafting of poly(2-(dimethylamino)ethyl methacrylate) to biodegradable block copolymers. J. Polym. Sci. 2020, 58, 2168–2180. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Polymeric micelle for tumor pH and folate-mediated targeting. J. Control. Release 2003, 91, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.G.; Lee, D.H.; Kim, D.I.; Bae, Y.H. Doxorubicin loaded pH-sensitive micelle targeting acidic extracellular pH of human ovarian A2780 tumor in mice. J. Drug Target. 2005, 13, 391–397. [Google Scholar] [CrossRef]
- Lee, E.S.; Na, K.; Bae, Y.H. Doxorubicin loaded pH-sensitive polymeric micelles for reversal of resistant MCF-7 tumor. J. Control. Release 2005, 103, 405–418. [Google Scholar] [CrossRef]
- Lee, E.S.; Oh, K.T.; Kim, D.; Youn, Y.S.; Bae, Y.H. Tumor pH-responsive flower-like micelles of poly(L-lactic acid)-b-poly(ethylene glycol)-b-poly(L-histidine). J. Control. Release 2007, 123, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Li, D.; He, B.; Xu, X.; Sheng, M.; Lai, Y.; Wang, G.; Gu, Z. Anti-tumor drug delivery of pH-sensitive poly(ethylene glycol)-poly(L-histidine-)-poly(L-lactide) nanoparticles. J. Control. Release 2011, 152, 49–56. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.; Chen, Q.; Hao, T.; Qiao, M.; Zhao, H.; Zhang, J.; Hu, H.; Zhao, X.; Chen, D.; et al. pH-sensitive nanoparticles of poly(L-histidine)-poly(lactide-co-glycolide)-tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater. 2015, 11, 137–150. [Google Scholar] [CrossRef]
- Jia, L.; Jia, N.; Gao, Y.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. Multi-Modulation of Doxorubicin Resistance in Breast Cancer Cells by Poly(l-histidine)-Based Multifunctional Micelles. Pharmaceutics 2019, 11, 385. [Google Scholar] [CrossRef]
- Johnson, R.P.; Jeong, Y.I.; Choi, E.; Chung, C.W.; Kang, D.H.; Oh, S.O.; Suh, H.; Kim, I. Biocompatible Poly (2-hydroxyethyl methacrylate)-b-poly(L-histidine) Hybrid Materials for pH-Sensitive Intracellular Anticancer Drug Delivery. Adv. Funct. Mater. 2012, 22, 1058–1068. [Google Scholar] [CrossRef]
- Johnson, R.P.; Uthaman, S.; John, J.V.; Lee, H.R.; Lee, S.J.; Park, H.; Park, I.-K.; Suh, H.; Kim, I. Poly(PEGA)-b-poly(l-lysine)-b-poly(l-histidine) Hybrid Vesicles for Tumoral pH-Triggered Intracellular Delivery of Doxorubicin Hydrochloride. ACS Appl. Mater. Interfaces 2015, 7, 21770–21779. [Google Scholar] [CrossRef]
- Hwang, J.H.; Choi, C.W.; Kim, H.W.; Kim, D.H.; Kwak, T.W.; Lee, H.M.; Kim, C.H.; Chung, C.W.; Jeong, Y.I.; Kang, D.H. Dextran-b-poly(L-histidine) copolymer nanoparticles for ph-responsive drug delivery to tumor cells. Int. J. Nanomed. 2013, 8, 3197–3207. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Chen, F.; Jia, L.; Xu, Q.; Gai, X.; Yu, Y.; Di, Y.; Zhu, Z.; Liang, Y.; et al. A novel pH-sensitive carrier for the delivery of antitumor drugs: Histidine-modified auricularia auricular polysaccharide nano-micelles. Sci. Rep. 2017, 7, 4751. [Google Scholar] [CrossRef]
- Kim, G.M.; Bae, Y.H.; Jo, W.H. pH-induced Micelle Formation of Poly(histidine-co-phenylalanine)-block-Poly(ethylene glycol) in Aqueous Media. Macromol. Biosci. 2005, 5, 1118–1124. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, L.; Torchilin, V.P. pH-sensitive poly(histidine)-PEG/DSPE-PEG co-polymer micelles for cytosolic drug delivery. Biomaterials 2013, 34, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.C.; Chang, W.H.; Lo, C.L.; Tsai, C.H.; Chang, C.H.; Ou, T.W.; Yen, T.C.; Hsiue, G.H. Graft and diblock copolymer multifunctional micelles for cancer chemotherapy and imaging. Biomaterials 2010, 31, 2293–2301. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.R.; Lee, H.J.; Kim, J.D. Histidine-conjugated poly(amino acid) derivatives for the novel endosomolytic delivery carrier of doxorubicin. J. Control. Release 2006, 114, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, Z.; Qiao, M.; Long, M.; Wang, M.; Zhang, X.; Tian, C.; Chen, D. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014, 10, 2024–2035. [Google Scholar] [CrossRef]
- Wu, J.-L.; Liu, C.-G.; Wang, X.-L.; Huang, Z.-H. Preparation and characterization of nanoparticles based on histidine–hyaluronic acid conjugates as doxorubicin carriers. J. Mater. Sci. Mater. Med. 2012, 23, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.T.; Leng, Q.; Scaria, P.; Woodle, M.; Mixson, A.J. Selective modification of HK peptides enhances siRNA silencing of tumor targets in vivo. Cancer Gene Ther. 2011, 18, 707–716. [Google Scholar] [CrossRef]
- He, J.; Xu, S.; Mixson, A.J. The Multifaceted Histidine-Based Carriers for Nucleic Acid Delivery: Advances and Challenges. Pharmaceutics 2020, 12, 774. [Google Scholar] [CrossRef]
- Imtiyaz, Z.; He, J.; Leng, Q.; Agrawal, A.K.; Mixson, A.J. pH-Sensitive Targeting of Tumors with Chemotherapy-Laden Nanoparticles: Progress and Challenges. Pharmaceutics 2022, 14, 2427. [Google Scholar] [CrossRef] [PubMed]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Zhu, W.J.; Yang, S.D.; Qu, C.X.; Zhu, Q.L.; Chen, W.L.; Li, F.; Yuan, Z.Q.; Liu, Y.; You, B.G.; Zhang, X.N. Low-density lipoprotein-coupled micelles with reduction and pH dual sensitivity for intelligent co-delivery of paclitaxel and siRNA to breast tumor. Int. J. Nanomed. 2017, 12, 3375–3393. [Google Scholar] [CrossRef] [PubMed]
- Cummings, N.A.; Nordby, G.L. Measurement of synovial fluid pH in normal and arthritic knees. Arthritis Rheum 1966, 9, 47–56. [Google Scholar] [CrossRef]
- Rajamaki, K.; Nordstrom, T.; Nurmi, K.; Akerman, K.E.; Kovanen, P.T.; Oorni, K.; Eklund, K.K. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 2013, 288, 13410–13419. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Noda, R.; Sato, K.; Harasawa, H.; Kurosaki, T.; Nakagawa, H.; Nakamura, T.; Kitahara, T.; Muro, T.; Sasaki, H. Methotrexate-Coated Complexes of Plasmid DNA and Polyethylenimine for Gene Delivery. Biol. Pharm. Bull. 2018, 41, 1537–1542. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhuang, W.; Wang, Y.; Luo, R.; Wang, Y. pH-sensitive doxorubicin-conjugated prodrug micelles with charge-conversion for cancer therapy. Acta Biomater. 2018, 70, 186–196. [Google Scholar] [CrossRef]
- Qiu, L.; Hong, C.Y.; Pan, C.Y. Doxorubicin-loaded aromatic imine-contained amphiphilic branched star polymer micelles: Synthesis, self-assembly, and drug delivery. Int. J. Nanomed. 2015, 10, 3623–3640. [Google Scholar] [CrossRef]
- Bachelder, E.M.; Beaudette, T.T.; Broaders, K.E.; Dashe, J.; Frechet, J.M. Acetal-derivatized dextran: An acid-responsive biodegradable material for therapeutic applications. J. Am. Chem. Soc. 2008, 130, 10494–10495. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhao, M.; Wu, L.; Luo, K.; Pu, Y.; He, B. Tumor-pH-Sensitive PLLA-Based Microsphere with Acid Cleavable Acetal Bonds on the Backbone for Efficient Localized Chemotherapy. Biomacromolecules 2018, 19, 3140–3148. [Google Scholar] [CrossRef]
- Zhao, N.; Woodle, M.C.; Mixson, A.J. Advances in delivery systems for doxorubicin. J. Nanomed. Nanotechnol. 2018, 9, 519. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, J.; Zhao, X.; Zhang, Y.; Liu, J.; Xu, S.; Deng, L.; Dong, A.; Zhang, J. PEG-b-PCL Copolymer Micelles with the Ability of pH-Controlled Negative-to-Positive Charge Reversal for Intracellular Delivery of Doxorubicin. Biomacromolecules 2014, 15, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhong, Y.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Acetal-linked paclitaxel prodrug micellar nanoparticles as a versatile and potent platform for cancer therapy. Biomacromolecules 2013, 14, 2772–2780. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, Q.; Li, Y.; Li, P.; Yuan, J.; Meng, X.; Xiao, Y. DOX-Conjugated keratin nanoparticles for pH-Sensitive drug delivery. Colloids Surf B Biointerfaces 2019, 181, 1012–1018. [Google Scholar] [CrossRef]
- Feng, T.; Ai, X.; An, G.; Yang, P.; Zhao, Y. Charge-Convertible Carbon Dots for Imaging-Guided Drug Delivery with Enhanced in Vivo Cancer Therapeutic Efficiency. ACS Nano 2016, 10, 4410–4420. [Google Scholar] [CrossRef]
- Lee, Y.; Ishii, T.; Cabral, H.; Kim, H.J.; Seo, J.H.; Nishiyama, N.; Oshima, H.; Osada, K.; Kataoka, K. Charge-conversional polyionic complex micelles-efficient nanocarriers for protein delivery into cytoplasm. Angew. Chem. Int. Ed. 2009, 48, 5309–5312. [Google Scholar] [CrossRef]
- Jin, M.; Jin, G.; Kang, L.; Chen, L.; Gao, Z.; Huang, W. Smart polymeric nanoparticles with pH-responsive and PEG-detachable properties for co-delivering paclitaxel and survivin siRNA to enhance antitumor outcomes. Int. J. Nanomed. 2018, 13, 2405–2426. [Google Scholar] [CrossRef]
- Miyazaki, M.; Yuba, E.; Hayashi, H.; Harada, A.; Kono, K. Hyaluronic Acid-Based pH-Sensitive Polymer-Modified Liposomes for Cell-Specific Intracellular Drug Delivery Systems. Bioconjug. Chem. 2018, 29, 44–55. [Google Scholar] [CrossRef]
- Alswieleh, A.M.; Beagan, A.M.; Alsheheri, B.M.; Alotaibi, K.M.; Alharthi, M.D.; Almeataq, M.S. Hybrid Mesoporous Silica Nanoparticles Grafted with 2-(tert-butylamino)ethyl Methacrylate-b-poly(ethylene Glycol) Methyl Ether Methacrylate Diblock Brushes as Drug Nanocarrier. Molecules 2020, 25, 195. [Google Scholar] [CrossRef]
- Alotaibi, K.M.; Almethen, A.A.; Beagan, A.M.; Alfhaid, L.H.; Ahamed, M.; El-Toni, A.M.; Alswieleh, A.M. Poly(oligo(ethylene glycol) methyl ether methacrylate) Capped pH-Responsive Poly(2-(diethylamino)ethyl methacrylate) Brushes Grafted on Mesoporous Silica Nanoparticles as Nanocarrier. Polymers 2021, 13, 823. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Sun, Y.; Hu, Y.; Peng, Y.; Li, Y.; Yin, G.; Liu, H.; Xu, J.; Zhong, S. Synthesis of Size-Tunable Hollow Polypyrrole Nanostructures and Their Assembly into Folate-Targeting and pH-Responsive Anticancer Drug-Delivery Agents. Chem. A Eur. J. 2017, 23, 17279–17289. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Zintchenko, A.; Ogris, M.; Wagner, E. A dimethylmaleic acid-melittin-polylysine conjugate with reduced toxicity, pH-triggered endosomolytic activity and enhanced gene transfer potential. J. Gene Med. 2007, 9, 797–805. [Google Scholar] [CrossRef]
- Shen, C.L.; Liu, H.R.; Lou, Q.; Wang, F.; Liu, K.K.; Dong, L.; Shan, C.X. Recent progress of carbon dots in targeted bioimaging and cancer therapy. Theranostics 2022, 12, 2860–2893. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E.; Tajima, N.; Yoshizaki, Y.; Harada, A.; Hayashi, H.; Kono, K. Dextran derivative-based pH-sensitive liposomes for cancer immunotherapy. Biomaterials 2014, 35, 3091–3101. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.P.; Alves, D.S.; Scott, H.L.; Davis, F.L.; Barrera, F.N. A Novel Soluble Peptide with pH-Responsive Membrane Insertion. Biochemistry 2015, 54, 6567–6575. [Google Scholar] [CrossRef]
- Nguyen, V.P.; Palanikumar, L.; Kennel, S.J.; Alves, D.S.; Ye, Y.; Wall, J.S.; Magzoub, M.; Barrera, F.N. Mechanistic insights into the pH-dependent membrane peptide ATRAM. J. Control. Release 2019, 298, 142–153. [Google Scholar] [CrossRef]
- Chang, L.; Bao, H.; Yao, J.; Liu, H.; Gou, S.; Zhong, C.; Zhang, Y.; Ni, J. New designed pH-responsive histidine-rich peptides with antitumor activity. J. Drug Target. 2021, 29, 651–659. [Google Scholar] [CrossRef]
- Zhang, C.; Kermaniyan, S.; Smith, S.A.; Gillies, E.R.; Such, G.K. Acid-Responsive Poly(glyoxylate) Self-Immolative Star Polymers. Biomacromolecules 2021, 22, 3892–3900. [Google Scholar] [CrossRef]
- Yardley, R.E.; Kenaree, A.R.; Gillies, E.R. Triggering Depolymerization: Progress and Opportunities for Self-Immolative Polymers. Macromolecules 2019, 52, 6342–6360. [Google Scholar] [CrossRef]
- Chu, S.; Shi, X.; Tian, Y.; Gao, F. pH-Responsive Polymer Nanomaterials for Tumor Therapy. Front. Oncol. 2022, 12, 855019. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: Background and future prospects. Bioconjug. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR effect and beyond: Strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, J.; Meng, H. Transcytosis—An effective targeting strategy that is complementary to “EPR effect” for pancreatic cancer nano drug delivery. Theranostics 2019, 9, 8018–8025. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Chen, S.-H.; Chiang, W.-H.; Huang, C.-W.; Lo, C.-L.; Chern, C.-S.; Chiu, H.-C. Tumor Microenvironment-Responsive Nanoparticle Delivery of Chemotherapy for Enhanced Selective Cellular Uptake and Transportation within Tumor. Biomacromolecules 2016, 17, 3883–3892. [Google Scholar] [CrossRef]
- Kinoshita, R.; Ishima, Y.; Chuang, V.T.G.; Nakamura, H.; Fang, J.; Watanabe, H.; Shimizu, T.; Okuhira, K.; Ishida, T.; Maeda, H.; et al. Improved anticancer effects of albumin-bound paclitaxel nanoparticle via augmentation of EPR effect and albumin-protein interactions using S-nitrosated human serum albumin dimer. Biomaterials 2017, 140, 162–169. [Google Scholar] [CrossRef]
- Zhu, M.; Zhuang, J.; Li, Z.; Liu, Q.; Zhao, R.; Gao, Z.; Midgley, A.C.; Qi, T.; Tian, J.; Zhang, Z.; et al. Machine-learning-assisted single-vessel analysis of nanoparticle permeability in tumour vasculatures. Nat. Nanotechnol. 2023, 1–10. [Google Scholar] [CrossRef]








| Polymer | Drug | Cell Lines (In Vitro, In Vivo) | Comments | Reference |
|---|---|---|---|---|
| Release of Chemotherapeutic agents | ||||
| PBAE 1/Pluronic F-108 | PTX | NIH3T3 (+, −) 2 | PBAE polymers stabilized by Pluronic F-108. NPs of about 110 nm completely dissolved at pH 6.5. The pH-sensitive particles loaded with PTX inhibited cells more than pH-insensitive particles. | Lynn et al., 2000 [114] |
| D-α-tocopheryl-PEG-PBAE | DTX | A2780/A2780-T (+, −) | D-α-tocopheryl inhibits MDR transporter. Diblock polymer formed a NP (size, ~260 nm; ZP, −26 mV). Particle solution became translucent at pH 6.4. Nearly 50% of the drug was released at pH 7.4 in 48 h. Drug-loaded particles inhibited sensitive (IC50-0.27 vs. 3.95 μg/mL) and insensitive cells (IC50-0.82 vs. 17.7) than the free drug. | Zhao et al., 2013 [116] |
| mPEG-PBAE-PLA | Dox | −, − | Micelles were formed by triblock polymer (size, 150 nm, ZP, 9 mV). About 20% and 96% of the drug were released from particles at pH 5 after 48 h. Translucency of solution not observed at lower pHs. | Yang et al., 2017 [104] |
| mPEG—b-PDEAEMA-PMMA/PDEAEMA-b-PMMA | Dox | HepG2 (+, −) | Mixed micelles (~86 nm in size, ZP, ~9.6) formed by diblock and triblock polymers. pH-dependent release of Dox with 20% and 80% released at pH 7.4 and 5.0, respectively, in 60 h. Micelles had modestly less cytotoxicity toward cells than free Dox except at high concentrations. Dox loading content was 24%. | Chen et al., 2017 [118] |
| PCL-b-PDEAEMA-b-PPEGMA | PTX | NIH3T3 (+, −) | Modest pH-dependent increase in size and release of PTX at pH 5.0. pH-dependent micelles showed modestly greater cytotoxicity toward cells compared to pH-independent micelles. | Feng et al., 2019 [119] |
| PEG-Dox, PDPAEMA, H4R4 | Dox | HeLa (+, −) | Low release of Dox at pH 7.4, yet significant release (90%) of the drug at pH of 5.5 (24 h). H4R4 had no role in drug release but likely enhanced endosomal lysis. NP had markedly improved efficacy toward HeLa cells vs. free drug. | Liang et al., 2015 [103] |
| P(DEAEMA-r-DPAEMA) | Calcein | NIH/3T3 (+, −) | Mixed micelles between 130 to 160 nm had varied pKa based on the ratios of DEAEMA and DPAEMA incorporated into polymer. Calcein release assay determined endosomal leakage. | Kongkatigumjorn et al., 2018 [120] |
| PDMAEMA-PDMS (AB5) | Dox | HeLa (+, −) | Empty micelles of diblock polymer in which PDMAEMA had 5 monomeric units were less toxic than those with 13 units. Marked release of Dox at pH 5.5 compared to pH 7.4. At low concentrations, free Dox-inhibited cells more than Dox-loaded micelles. | Car et al., 2014 [121] |
| Polyiatronic acid-g-FA-PEG-g-PLH | Dox | HeLa (+, −) | Stable micelle at pH 7.4 that showed graded pH release of Dox at pH 7 and less. Greater than 90% of Dox is released at pH 5.0 (24 h). pH-dependent charge surface reversal. Folate-targeted micelle had greater cytotoxicity for HeLa cells compared to free Dox. | Sun et al., 2015 [122] |
| Star-shaped 5-armed PLGA-His | DTX/ Disulfiram | MCF-7 (+, −) | Marked size increase in micelles at pH 6.8 vs. pH 7.4. Consistent with size increase, micelles released most of the two drugs at pH 6.8. Additionally, the pH-dependent micelles showed increased penetration into MCF-7 spheroid. | Swetha et al., 2021 [123] |
| Release of Chemotherapy and Nucleic Acids | ||||
| PEI-ss-PCL-ss- PEI | Dox/P53-plasmid | HepG2 (+, −) | Dual pH- and redox-responsive NP. Triblock polymers formed a NP with plasmid and Dox of about 168 nm. Modest increase in Dox release in presence of DTT, but pH-responsiveness not done. Significant increase in apoptosis with the combination of Dox and p53 plasmid than either agent alone | Davoodi et al., 2016 [124] |
| Succinyl chitosan-g-polylysine-palmitic acid | Dox/siPGP | HepG2 (+, +) | pH-responsive micelles (size, ZP) made of graft copolymers. No Dox release studies done. In resistant cells, Dox/siPGP micelles were more cytotoxic (about 3 to 4-fold) than Dox-loaded micelles or free Dox. In vivo, Dox/siPGP micelles reduced tumor size by about 50% more than Dox-alone micelles. Biodistribution study showed tumor specificity of micelle. siPGP reduced resistant PGP levels in tumors in treated mice. | Zhang et al., 2016 [125] |
| TPGS/poloxamer-PEI conjugate | PTX/ shTw plasmid | 4T1 (+, +) | pH-responsive NP in charge, size, and release of Dox and shTw. Charge reversal of NP as pH was lowered. TPGS was necessary for stabilization. PTX-loaded and shTw-loaded particles reduced tumor size and lung metastasis significantly more than PTX-loaded particles in vivo. A biodistribution study showed tumor specificity of NP. | Shen et al., 2012, 2013 [126,127] |
| PEG-b-PAsp(AED)-b-PDPAEMA | Dox/siBCL-2 | SKOV-3 (+, +) | Dual pH- and redox-dependent NP. While 80% of Dox was released at pH 5, 90% was released at pH 5 and with DTT. Synergistic antitumor efficacy in vivo and prolonged survival observed with Dox- and siBCL-2-loaded NP. Biodistribution study showed tumor specificity of NP | Chen et al., 2014 [128] |
| PEG-b-PLA-PLH-ss-OEI | Dox/siPGP | MCF7/MDR-ADR (+, +) | Dual redox- and pH-dependent polyplex. Polyplex (size, ~120 nm, ZP, +25 mV at pH 7.4) also showed significant levels of cytotoxicity in MCF-7/ADR cells and marked synergistic tumor size suppression in mice. Additionally, biodistribution studies demonstrated tumor specificity of polyplex. | Gao et al., 2019 [129] |
| PEG-PEI/PEI-PCL | Methotrexate/ siNotch1 | Raw264.7 (+, − 3) | Non-tumor model in which polymeric NP (~160 nm in size) with a prolonged half-life in blood (~6 h). No pH-dependent studies done. Drug- and siRNA-loaded nanoparticles showed marked reduction in inflammation compared to methotrexate in an in vivo arthritic model. | Zhao and Zhang, 2018 [130] |
| Polymer | Drug/Payload | pH-Sensitive Bond | Cell Lines 1 (In Vitro, In Vivo) | Comments | Reference |
|---|---|---|---|---|---|
| Dextran | Fluorescein-labeled Dextran (FITC) 2 | Acetal | RAW macrophages (+, −) | Acetal groups conjugated to hydroxyl groups increased hydrophobicity. The average size of the microsphere was about 240 nm. The release half-life for FITC-dextran at pH 7.4 and 5.0 was about 15 days and 10 h, respectively. Also incorporated ovalbumin stimulated immune response. | Bachelder et al., 2008 [159] |
| PLLA backbone with interspersed acetal groups | Dox | Acetal | 4T1 (+,+) | Acetalized-PLLA microspheres with sizes ranging from 2 to 15 μm. About 30 and 70% of Dox release at 7.4 and 5.0, respectively. 12 days after the 4th intratumoral injection, subcutaneous tumors were inhibited by about 80%. | Li et al., 2018 [160] |
| Star polymer comprising DMAEMA co-MAEBA-co-DTDMA | Dox | Imine | HeLa, HepG2 (+, −) | Complex polymer synthesis with optimal micelle size of 170 nm. Two different imine interactions with Dox. Dox release at pH 7.4 less than 5% in 48 h, whereas 60% release at pH 5.0 and DTT 10 mM. Targeted Dox-loaded micelles less effective than free Dox except at high Dox concentrations. | Qiu et al., 2015 [158] |
| PMBC-Polylysine | Dox | Imine | 4T1 (+,+) | ε-amino group of lysine formed imine bonds with 4-CB or Dox. Micelles demonstrated pH-dependent charge reversal, size increase, and Dox release. In vivo Dox-loaded micelle inhibited tumors in mice more than free Dox. | Ma et al., 2018 [157] |
| PEG-b-(PCL-co-DCL) | Dox | β-carboxylic amide | HepG2 (+, −) | Release of pH-sensitive β-carboxylic acid resulted in negative to positive charge reversal micelle. Very pH-responsive micelle. About 10% and 90% of Dox were released at pH 7.4 and 5.3, respectively. Notably, micelles inhibited cells more effectively than pH non-responsive particles. | Deng et al., 2014 [162] |
| PEG-PAA | PTX, Dox | Acetal | A549 (+, −) | With pH release of PTX from PAA polymer, the negatively charged PAA micelle was disassembled. High loading capacity of 43% with PTX. In contrast to sensitive cells, micelles inhibited PTX-resistant cells significantly more than free PTX. Micelles were stable for months at 4 °C. Additionally, pH-dependent release of Dox was shown. | Gu et al., 2014 [163] |
| Iodoacetate-modified Keratin | Dox | Hydrazone | A549 (+ in vitro; H22, + in vivo) | Keratin-Dox NP formed by desolvation with size of 250 nm. About 60% of Dox released in 48 h at pH 5, while less than 5% released from particles in 11 days at pH 7.4. Negative to positive charge reversal as pH decreased. Dox-loaded NP inhibited H22 tumors in vivo more than free Dox. | Liu et al., 2019 [164] |
| Coating Polymer | Nanoparticle | Drug | Cell Lines (In Vitro, In Vivo) 1 | Comments | Reference |
|---|---|---|---|---|---|
| PAA | Zeolith imidazole NP | Dox | ND | Rhombic dodecahedron ZIF was formed by zinc and 2-methylhistidine. PAA-coated positively charged ZIF with size of about 170 nm. 2 Several components of NP (coating, zinc-imidazole, Dox) were pH-dependent. 25% and 85% of Dox released from NP at pH 7.4 and 5.0, respectively, in 100 h. | Tran and Lee, 2021 [96] |
| PAsp-PEG | PEI-PLA NP | PTX, siSurvivin | 4T1 (+, −); A549 (+,+) | Coated NPs of about 82 nm enhanced in vivo tumor efficacy with large tumors regressing and prolonged animal survival compared to non-coated NPs. Improved tumor accumulation of NP with coating polymer (biodistribution) | Jin et al., 2018 [167] |
| PEG-(PAH/DMMA) | CD | Cis-platinum | A2780 (+, −); HeLa (+, −); U14 (+,+) | Release of pH-sensitive dimethyl maleic acid results in the coating polymer release from CD. After decloaking, the cis-platinum conjugated to CD is sensitive to reducing conditions. A2780 and HeLa cells were more sensitive to cloaked NP at pH 6.8 than 7.4. Treated U14-bearing mice regressed with coated Dox-NPs, and pH-polymer was more effective than pH-independent polymer. | Feng et al., 2016 [165] |
| Chex50-HA | Liposomes | Dox | HeLa, MCF7, Colon 26, NIH3T3 (+, −) | 80% release of Chex50-HA in 10 min from NP at pH 4.5. Uptake of coated NP (size, 141 nm) was significantly greater in cells with high expression of the CD44 receptor (HeLa, Colon 26). Coated Dox-loaded NP showed significantly greater cytotoxicity toward HeLa cells than non-coated NP. | Miyazaki et al., 2018 [168] |
| ATRAM | Cross-linked BSA-PLGA NP | Dox-TPP | Neuro 2A (+, −); HeLa (+, −); MCF-7 (+, −); 4T1, (+,+) | Dual pH and redox-dependent NP about 100 nm in size. Less than 5% and about 80% of Dox-TPP released from NP at pH 7.4 and 5.0 in 24 h. The half-life of 7 h in blood in vivo with enhanced tumor accumulation of NP. Treatment with ATRAM-coated NPs regressed tumors and was more effective than uncoated NPs. | Palanikumar et al., 2020 [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, Q.; Imtiyaz, Z.; Woodle, M.C.; Mixson, A.J. Delivery of Chemotherapy Agents and Nucleic Acids with pH-Dependent Nanoparticles. Pharmaceutics 2023, 15, 1482. https://doi.org/10.3390/pharmaceutics15051482
Leng Q, Imtiyaz Z, Woodle MC, Mixson AJ. Delivery of Chemotherapy Agents and Nucleic Acids with pH-Dependent Nanoparticles. Pharmaceutics. 2023; 15(5):1482. https://doi.org/10.3390/pharmaceutics15051482
Chicago/Turabian StyleLeng, Qixin, Zuha Imtiyaz, Martin C. Woodle, and A. James Mixson. 2023. "Delivery of Chemotherapy Agents and Nucleic Acids with pH-Dependent Nanoparticles" Pharmaceutics 15, no. 5: 1482. https://doi.org/10.3390/pharmaceutics15051482