1. Introduction
Monoclonal antibody (mAb)-based therapies have revolutionized the treatment paradigms in a multitude of disease areas [
1,
2]. With antibody gene transfer, the nucleotide sequence that encodes the therapeutic protein is administered to patients, instead of the therapeutic protein itself. The human body is turned into a mAb “factory”, resulting in the production and secretion of mAbs into the bloodstream for a prolonged period of time [
3]. This treatment strategy has multiple advantages over conventional antibody therapy. The less frequent administrations and gradual in vivo mAb production and buildup are anticipated to improve patient comfort and safety. Compared to conventional recombinant proteins, nucleic-acid based therapies can be rapidly designed and produced at large scale, saving both time and resources. The stability of pDNA at room temperature negates the current need for cold-chain storage and shipment, facilitating dissemination. Overall, these advantages can increase the accessibility and implementation of mAb therapy.
Our research group previously demonstrated proof of concept for DNA-based intramuscular gene transfer in mice, hamsters and sheep [
4,
5,
6,
7,
8]. pDNA is a favorable expression platform in terms of immunogenicity, biosafety, and payload capacity compared to viral vectors. However, it has a low transfection efficiency, which generally results in lower protein expression levels [
3,
9]. Physical methods, such as electroporation, are therefore required to overcome the low transfection efficiency [
3]. Hence, intramuscular pDNA injection is followed by the application of electrical pulses, leading to temporal cell membrane poration, allowing uptake of the pDNA in the muscle cells [
10]. Expression levels improve after this procedure, but are still lower compared to viral vector delivery. Despite its lower protein expression, DNA-based antibody gene transfer has shown preclinical efficacy and is currently under clinical evaluation (NCT01138410 and NCT03831503).
The current pharmacokinetic (PK) profile of mAbs expressed in vivo in mice after DNA-based delivery is typically characterized by a steady increase in plasma mAb concentration in the first two to three weeks. Peak levels reach single-digit to double-digit µg/mL in mice. However, thereafter a decline in mAb levels is typically observed, although mAb remains detectable for many months after the initial pDNA injection [
4]. This is comparable to the levels observed in sheep, where peak plasma levels reached 5 µg/mL after administration of 12 mg pOVAC in the absence of anti-drug antibodies. Sheep are more similar to humans in terms of body weight, musculature, and blood volume, and therefore these data provide valuable insights towards clinical translation [
7]. For a broad implementation of DNA-based mAb delivery, a more stable expression profile at high mAb concentrations would be beneficial. Ideally, these levels need to be maintained above or around 5–10 μg/mL for a longer time, in line with trough levels for most therapeutic mAbs in humans [
11].
The limited mAb expression that can be attained in vivo—only peak levels reaching mAbs trough levels—hampers the clinical development of DNA-based mAb therapeutics. Despite its significance for therapeutic applications, little is known about the factors that drive the in vivo expression of mAbs following intramuscular electroporation of mAb-encoding plasmids. Further improvement of in vivo mAb expression requires insight into the fate of the plasmids post-electroporation.
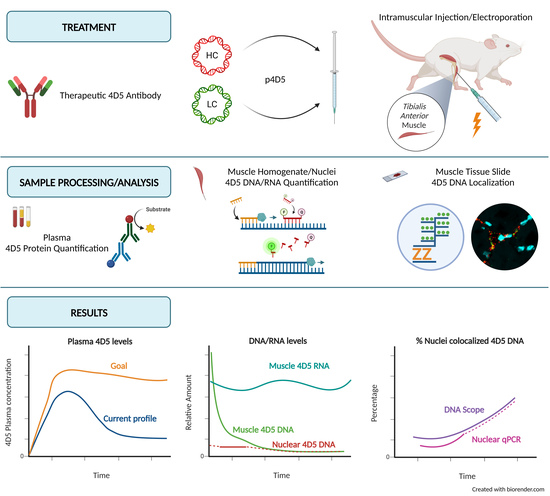
The current study aims to characterize the in vivo expression of antibodies after pDNA administration on a broader basis than only plasma protein expression. To the best of our knowledge, it is the first study to investigate pDNA levels, mAb mRNA levels, and pDNA localization over an extensive time period after mAb gene transfer. This will lead to a better understanding of the different factors driving protein expression. The findings of this study can contribute to the optimization of DNA-based mAb delivery. On top of that, the techniques described can also be used as a toolbox for evaluating future plasmid constructs or alternative DNA-based delivery methods.
2. Materials and Methods
2.1. pDNA Constructs
Two previously designed pDNA constructs, pCAG-4D5-HC and pCAG-4D5-LC, encoding the heavy chain and light chain, respectively, of the murine anti-HER2 mAb, 4D5 were used [
12]. The plasmid backbone of both contained a CAG promoter, pUC origin of replication, ampicillin resistance gene, and TK poly(A) sequence [
4]. pDNA was produced in
E. coli (TOP10F’), purified using the NucleoBond Xtra Maxi EF kit (Machery—Nagel, Düren, Germany), eluted in sterile water, and stored at −20 °C until use. Plasmid purity and concentration were determined via UV spectrophotometry, and integrity was evaluated via agarose gel electrophoresis.
2.2. Mice
Female 7–8-week-old Balb/c mice (Balb/cAnNRj) with an approximate weight of 17–20 g were purchased at Janvier (Janvier, Le Genest-Saint-Isle, France). All experiments were approved by the KU Leuven Animal Ethics Committee (project P157/2017).
2.3. Intramuscular Electroporation
pDNA delivery to mice was performed as described previously [
5]. In short, mice were treated in both tibialis anterior muscles. The site of delivery was prepared using depilatory cream (Veet, Reckitt Benckiser, Slough, UK) approximately one week before treatment. On the day of treatment, mice received an intramuscular injection with 40 µL of 0.4 U/µL hyaluronidase from bovine testes (H4272, Sigma, St. Louis, MO, USA) (reconstituted in sterile saline), exactly one hour prior to pDNA electrotransfer. Intramuscular injection with an equimolar mixture of pCAG-4D5-HC and pCAG-4D5-LC pDNA, 30 µL of 2 µg/µL solution in sterile water, was immediately followed by in situ electroporation using the NEPA21 Electroporator (Sonidel, Dublin, Ireland) with CUY650P5 tweezer electrodes (Sonidel, Dublin, Ireland) at a fixed width of 5 mm. Signa Electrode Gel (Parker Laboratories, Fairfield, NJ) was applied to the muscle and electrodes to decrease tissue impedance below 0.3 kΩ. Three series of four 20-ms square-wave pulses of 120 V/cm with a 50-ms interval were applied with polarity switching after two of the four pulses. Pulse delivery was verified using the NEPA21 readout; if energy transfer was below 0.7 J, an extra pulse was administered.
2.4. Sample Collection
Sample collection was performed at multiple timepoints post treatment (1, 4, 7, 14, 21, 42, and 84 days). At each timepoint, muscle isolation was performed for 5 mice. Blood for mAb titer determination was collected via retro-orbital bleeding, with 0.2 mM sodium citrate buffer as anticoagulant (10% of blood volume), processed to plasma by centrifugation (10 min, 2000× g, 4 °C and 10 min, 16,000× g, 4 °C), and stored at −20 °C. Mice were euthanized with a Dolethal® (pentobarbital) (Vetoquinol, Niel, Belgium) overdose (150–200 mg/kg) injected peritoneally. Blood samples for anti-drug antibody determination were collected at the end of study follow-up, with a prefilled heparin (Leo Pharma, Ballerup, Denmark) syringe via terminal cardiac puncture, processed to plasma by centrifugation (10 min, 2000× g, 4 °C and 10 min, 16,000× g, 4 °C) and stored at −20 °C. Tibialis anterior muscles were collected after transcardial perfusion with heparinized PBS. Muscles were snap frozen in isopentane (Sigma, St. Louis, MO, USA), pre-chilled on a dry ice ethanol slurry, and stored at −80 °C until further processing.
2.5. DNA/RNA Extraction from Muscle
DNA and RNA extraction were performed using the AllPrep DNA/RNA/miRNA Universal Kit (Qiagen, Hilden, Germany). Homogenization of muscle tissue was performed in Buffer RLT supplemented with 2-mercaptoethanol (Sigma, St. Louis, MO, USA), using metal bead lysing matrix S (MP Biomedicals, Irvine, CA, USA) and a FastPrep-24 classic homogenizer (6.5 m/s, 45 s) (MP Biomedicals, Irvine, CA, USA). Homogenate corresponding to maximum 30 mg of muscle tissue was used for further processing, according to the manufacturer’s instructions. DNA and RNA samples were stored at −20 °C and −80 °C, respectively, until analysis.
2.6. Nuclei Isolation from Muscle
The protocol for nuclei isolation from frozen muscle was based on a protocol by Santol et al. [
13]. Muscles were thawed on ice for 5 min before adding 0.3 mL of ice-cold lysis buffer (10 mM Tris-HCl (Sigma, St. Louis, MO, USA), 10 mM NaCl (Fisher Scientific, Waltham, MA, USA), 3 mM MgCl
2 (Sigma, St. Louis, MO, USA), and 0.1% NonidetTM P40 (Alfa Aesar, Haverhill, MA, USA) in nuclease-free H
2O (Invitrogen, Waltham, MA, USA)). Tissue was cut into small pieces (2–3 mm), 4 extra volumes of lysis buffer were added, and the mixture was incubated for 3 min on ice with gentle shaking. Wash buffer (2.7 mL, PBS + 2% bovine serum albumin (BSA) (Sigma, St. Louis, MO, USA)) was added, and the mixture was Dounce homogenized on ice. The cell suspension was filtered through a 70-µm and 40-µm cell strainer (VWR, Leuven, Belgium). Subsequently, the filtrate was centrifuged for 5 min, 500×
g at 4 °C. The nuclei pellet was washed three times with wash buffer, followed by centrifugation for 5 min, 500×
g at 4 °C.
2.7. DNA Extraction from Nuclei
Isolated nuclei were lysed by incubation in 0.6 mL nuclei lysis buffer (0.5% SDS (Sigma, St. Louis, MO, USA), 100 µg/mL proteinase K (Thermo Fisher, Waltham, MA, USA), and 20 µg/mL RNase (Qiagen, Hilden, Germany) in nuclease-free H2O) for 5 h at 50 °C. DNA was extracted from lysates by one extraction in one volume of TE-saturated phenol (Sigma, St. Louis, MO, USA), one extraction in one volume of mixture TE-saturated phenol:chloroform (Acros Organics, Waltham, MA, USA) 50:50, and one extraction in one volume of chloroform. Total DNA was precipitated with 3 volumes of 95% ethanol (Fisher Scientific, Waltham, MA, USA) containing 0.12 M sodium acetate (Sigma, St. Louis, MO, USA) overnight at −20 °C, washed with 80% ethanol, and stored in elution buffer (Qiagen, Hilden, Germany) at −20 °C until analysis.
2.8. (RT)-qPCR
A DNA probe-based multiplex real-time quantitative PCR was designed to simultaneously measure pCAG-4D5-HC and pCAG-4D5-LC in total DNA extracted from muscle samples. The method was validated for target specificity, sensitivity, PCR efficiency, and linearity. Quantitative PCR (qPCR) was performed using a Lightcycler 480 (Roche, Basel, Switzerland). The reaction mix included 2× qPCRBIO Probe Mix (PCR Biosystems, Wayne, PA, USA), primers (final concentration 0.4 µM), and probes (final concentration 0.2 µM) (
Table 1). qPCR conditions were as follows: activation at 95 °C for 3 min, denaturation/amplification (40 cycles of 95 °C for 5 s and 62 °C for 30 s). Before data analysis, color compensation was applied to correct for bleed-through of the fluorescence signal. For RNA quantification of 4D5-HC and 4D5-LC, mRNA was reverse-transcribed using 10-fold diluted RNA samples, anchored oligodT primers (Integrated DNA Technologies, Leuven, Belgium), and Superscript II reverse transcriptase (Invitrogen, Waltham, MA, USA), according to the manufacturer’s instructions. cDNA was stored at −20 °C and analyzed with the above-described qPCR method. The 4D5 DNA and mRNA levels are reported as relative values, with the whole muscle data of the first mouse in the 24 h group as the control subject (100%).
2.9. ELISA for mAb Quantification
The 4D5 levels in plasma samples were quantified using a previously described in-house developed ELISA [
4], with minor adjustments to the protocol. Briefly, plates were coated overnight at 4 °C with 500 ng/mL human HER2 (10004-H08H, Sino Biologicals, Eschborn, Germany) in PBS. Plates were blocked with 1% BSA in PBS for two hours at room temperature (RT). Samples were diluted in PTAE (PBS 0.1% BSA, 0.002% Tween 80 (Sigma, St. Louis, MO, USA), 5 mM EDTA (Chemlab, Zedelgem, Belgium)) and incubated on the blocked HER2-coated plates for one hour at RT. Serial two-fold dilutions of purified 4D5, with concentrations ranging between 0.125 and 8 ng/mL, were used as the calibration curve. Detection of the captured 4D5 was performed with goat anti-mouse IgG–HRP (GAM IgG (H+L)-HRP, 1:2000 dilution in PTA (PBS 0.1% BSA, 0.002% Tween 80)) (Bio-Rad Laboratories, Hercules, CA, USA). Plates were developed for 30 min using o-phenylenediamine (TCI, Zwijndrecht, Belgium) and H
2O
2 (Merck, Rahway, NJ, USA) in citrate (Sigma, St. Louis, MO, USA) buffer. The reaction was stopped with 4 M H
2SO
4 (Merck, Rahway, NJ, USA). Absorbance was measured at 490 nm using an ELx808 Absorbance Microplate Reader (BioTek Instruments, Bad Friedrichshall, Germany). Each incubation step was preceded by a washing step with PBS 0.05% Tween 20 (Sigma, St. Louis, MO, USA). Sample concentrations were calculated based on the 4D5 calibration curve using a simple linear regression fit (GraphPad Prism 9.3, GraphPad Software, San Diego, CA, USA).
2.10. ELISA for Detection of Anti-Drug Antibodies
The presence of anti-drug antibodies (ADA) against the in vivo expressed mAb was assessed via an affinity capture elution (ACE) ELISA. Ninety-six-well plates were coated overnight at 4 °C with 5 µg/mL purified 4D5 diluted in 1 M sodium bicarbonate (VWR, Leuven, Belgium) buffer, pH 9.6. Plates were blocked with 1% BSA in PBS for one hour at RT, washed, and 25 µL per well of 1 M tris (Sigma, St. Louis, MO, USA) buffer (pH 9.5) was added to the plates. Plasma samples were diluted in PTAE and acidified by 1:10 dilution with 300 mM acetic acid (Honeywell, Charlotte, NC, USA) followed by 15 min incubation at RT. Then, 75 µL of the acidified samples was added to the buffered 4D5-coated plates followed by incubation for one hour at RT to capture any anti-drug antibodies (ADA) present in the samples. Plates were washed, followed by addition of 100 µL 300 mM acetic acid per well and incubation for 15 min at RT to elute bound ADAs. Fresh 96-well plates were loaded with 25 µL 1 M tris buffer (pH 9.5). Then, 75 µL of acid-eluted sample was transferred to the new buffer-containing plate, followed by a one-hour incubation at RT to allow for adsorption of the ADAs to the plate. Plates were blocked with 1% BSA in PBS for one hour at RT. Subsequently, biotinylated 4D5 (diluted to 1 µg/mL in PTA) was added and incubated one hour at RT. Detection was performed with streptavidin poly-HRP (1:5000 dilution in PTA) (Sanquin, Amsterdam, The Netherlands) and incubation for 30 min at RT. Each incubation step, except the two blocking steps, was preceded by a washing step with PBS 0.002% Tween 80. Plates were developed for 60 min using o-phenylenediamine and H2O2 in citrate buffer. The reaction was stopped with 4 M H2SO4. Absorbance was measured at 490 nm using an ELx808 Absorbance Microplate Reader (BioTek Instruments, Bad Friedrichshall, Germany).
2.11. DNA Scope
For the DNA scope assay, three animals were included from three time points post-pDNA electroporation (24 h, week 1 and week 6). Per animal, six longitudinal tissue slices, located in the middle of the tibialis anterior muscle tissue, were evaluated. Snap-frozen muscles were sectioned longitudinally at a thickness of 5 µm using a cryostat and mounted onto glass slides (Superfrost® plus, Fisher Scientific, Waltham, MA, USA). RNAScope Multiplex Fluorescent Reagent Kit v2 (ACD, Advanced Cell Diagnostics, Newark, CA, USA) was used following the manufacturer’s instructions for fresh frozen tissue, except for an extended fixation time (one hour) in 10% neutral buffered formalin (Sigma, St. Louis, MO, USA). Custom-designed probes targeting the variable domain of pCAG-4D5-HC and pCAG-4D5-LC were purchased from ACD (Advanced Cell Diagnostics, Newark, CA, USA). Opal dyes 570 (1:1500) and 690 (1:1000) (Akoya Biosciences, Marlborough, MA, USA) were used for developing the fluorescence signal originating from pCAG-4D5-HC and pCAG-4D5-LC, respectively. Images were collected by a Zeiss LSM 880 confocal microscope using a 40× oil-immersion objective (Zeiss, Oberkochen, Germany).
The amount of pDNA in the nuclei was quantified relative to the total amount of pDNA. Microscopy scanning was performed on the part of the muscle slices that contained the highest amount of pDNA, selected based on visual inspection. Z-stacks were analyzed in three dimensions using Imaris 9.6.13 software (Oxford Instruments, Abingdon, United Kingdom). Nuclei were detected as surface objects, DNA signal for pCAG-4D5-HC and pCAG-4D5-LC as spots. Colocalization was determined based on shortest distance calculations and expressed as percentage of nuclear spots for each channel separately.
2.12. Statistics
At the start of experiments, mice were randomized based on body weight. DNA/RNA quantification and DNA scope data are available from multiple timepoints. To compare these timepoints, data are analyzed using one-way ANOVA, when data is normally distributed (Shapiro–Wilk test), or Kruskal–Wallis test, when this is not the case, with Tukey’s/Dunn’s multiple comparisons test. The decrease of plasma 4D5 levels from week 3 onwards was analyzed using a linear mixed model in R. Nuclei pDNA quantification data is analyzed with unpaired t-tests, to investigate a possible difference between the two timepoints. Data with p-value below 0.05 are considered as statistically significantly different. Statistical analysis and figure drawing were completed using GraphPad Prism 9.3.1 (GraphPad Software, San Diego, CA, USA). All graphs display individual data points per animal or tissue slice and median values or average ± SD (specified in figure legends).
4. Discussion
Antibody gene transfer is a promising alternative for conventional antibody treatment. Despite being under clinical evaluation, little is currently known about the fate of the injected pDNA after intramuscular electroporation. In the context of pDNA vaccination, pDNA biodistribution following intramuscular electroporation is well-characterized both pre-clinically and clinically [
14,
15,
16]. Following intramuscular injection and electroporation, pDNA is transiently distributed through the body, but only persists at the site of injection. Moreover, no obvious risk of integration into the host genome has been reported in vaccination studies. pDNA is typically lost within a few months, likely due to plasmid loss consequent to cell turnover, plasmid silencing, and/or immune responses, including cellular immunity [
14,
15,
16]. It is currently unclear how these factors play a role in the context of antibody gene transfer, where, in contrast to vaccination, the absence of an immune response is pursued, both against the plasmid and the expressed transgene. Despite the large number of pre-clinical studies for DNA-based mAbs, there is little to no data available on the factors that impact transgene expression.
This study characterizes antibody gene transfer from a fundamental point of view. DNA, the starting point of the central dogma of molecular biology, is injected as part of the treatment. In muscle, pDNA levels decreased 90% between 24 h and one week post treatment. In contrast, mRNA levels remained stable over time. 4D5 antibody plasma concentrations reached peak levels at week two followed by a slow decrease (5% after 12 weeks). Evaluation of pDNA localization revealed that extranuclear pDNA was cleared fast, whereas the nuclear fraction remained relatively stable. This is in line with the observed mRNA and protein levels over time and indicates that only a minor fraction of the administered pDNA is ultimately responsible for the observed systemic mAb levels.
A previous study by Cappelletti et al. [
17] showed that within 48 h post injection, the pDNA levels decreased 10,000-fold compared to the injected dose. No pDNA quantification was performed at later time points. In the current study, the initial pDNA loss (within 24 h) appears to be similar to the pDNA loss within 48 h in the study by Cappelletti et al. [
17]. However, more interesting is the amount of pDNA that remains over a longer time period. We therefore evaluated the relative pDNA quantity starting from 24 h post treatment up to 12 weeks. This pDNA time profile shows a substantial decrease in pDNA not correlating with evolution of the transgene expression, implying that even a small amount of remaining pDNA is enough to result in substantial protein expression. A similar observation was reported by Molnar et al. [
18]. The main difference in study design was the use of SCID mice and a plasmid coding for the reporter gene β-galactosidase instead of a therapeutic antibody [
18]. pDNA levels decreased 50% between 10 and 90 days, a smaller decrease than observed in the current study, 90% pDNA decrease between week 2 and week 12. Possible explanations are the differences in study design and different sample processing, whole muscle pDNA extraction in this study versus pDNA extraction of 10 µM thick muscle cryosections in the study from Molnar et al. [
18].
Muscle tissue is terminally differentiated, and normal rodent adult muscle has a slow turnover rate, at most 1–2% of myonuclei are replaced per week [
19]. Therefore, this cannot explain the substantial loss of pDNA. Other possible reasons for the pDNA decline could be apoptosis of transfected cells or the lack of stability of extrachromosomal pDNA [
18]. A possible disadvantage of using qPCR for pDNA quantification is that not only intact but also degraded pDNA can be detected because only part of the pDNA is amplified during qPCR, resulting in a positive signal. However, data from the literature show that pDNA degradation is mainly a concern in the first hours post treatment and should not have a major influence on pDNA quantification at later time points [
17].
To elucidate the nature of the apparent contradiction (strong decrease of pDNA whereas mRNA and protein levels remain relatively stable over the time course studied), pDNA in slices of treated mouse muscle (24 h, 1 week, and 6 weeks) was visualized and quantified using DNA scope. An important consideration is that these data are based on the analysis of only a fraction of the treated muscle (region with highest pDNA content in six muscle sections (5 µm) per muscle). The DNA scope results confirm a pDNA decrease as observed with qPCR. On the other hand, the results show a significant increase in the percentage nuclear pDNA over time. This most probably indicates that the pDNA that disappears is mainly extranuclear pDNA, either extracellular or cytoplasmic. This finding was confirmed by quantifying nuclear pDNA 4 days and 2 weeks post treatment, indicating a stable pDNA level in nuclei, and as a consequence, an increasing percentage of nuclear pDNA over time. Both extracellular and cytoplasmic pDNA can be removed by nucleases. It is known that pDNA in cytoplasm has a limited stability, as shown by in vitro studies in which pDNA half-life in cytoplasm was determined to be only 90 min [
20,
21].
Overall, this study provides evidence that the efficiency of pDNA reaching the nucleus is rather low. Previous research was especially focused on facilitating DNA entry in the cell [
22]. However, to result in protein expression, pDNA needs to overcome three main obstacles, (1) the plasma membrane, (2) cytoplasm, and (3) the nuclear membrane [
21]. Crossing the first barrier is mediated by electroporation, which results in a temporal poration of the cell membrane, allowing the pDNA to enter the cell. The pDNA that reaches the cytoplasm ends up in a dense network of organelles and cytoskeletal elements, preventing the diffusion of large molecules. pDNA is assisted by the microtubule network and associated motor proteins to traffic towards the nucleus [
23]. Nevertheless, it is assumed that a lot of naked pDNA will be degraded in the cytoplasm by cytosolic nuclease(s) [
20]. The final goal is pDNA reaching the nucleus, where it can be transcribed into mRNA, which can then be translated into protein. Research to overcome this final hurdle is gaining attention as this seems to be the rate-limiting step for gene transfer in non-dividing cells [
22].
pDNA can enter the nucleus through the nuclear pore complex (NPC). As a consequence, enhanced transfection ability is observed in mitotic cells displaying a disassembled nuclear envelope [
20]. However, antibody gene transfer often targets tissues consisting of non-dividing cells, which further adds to the difficulty of pDNA entering the nucleus. pDNA molecules associate with polypeptides such as transcription factors that contain nuclear localization sequences (NLS), which consequently interact with nuclear import receptors [
21]. Strategies are being explored to facilitate nuclear translocation of pDNA [
24]. As NLS play a critical role in nuclear uptake, modification of plasmids with NLS peptides or covalent linking of the nuclear targeting sequences to the pDNA constructs could improve nuclear uptake [
23]. Other possibilities that have been explored are (1) coupling of the pDNA to recombinant importin β, which can bind to NPC [
25]; (2) attaching dexamethasone to plasmids, which could result in glucocorticoid receptor binding and could consequently lead to conformational changes of the receptor exposing an NLS [
26]; or (3) chemical modification of pDNA vectors with oligosaccharides, which can bind to the lectins in the NPC [
27]. All these strategies have a major impact on the complexity of the production process of these constructs and are therefore not viable options as they overturn one of the main advantages of antibody gene transfer, i.e., rapid design and production at large scale, saving both time and resources.
On the other hand, nuclear import of pDNA is a sequence-specific process. Even pDNA without NLS or other conjugates as described above is able to enter the nucleus to a certain extent. Sequence optimization can lead to increased pDNA uptake in the nucleus [
23]. Previous research has shown that when the SV40 promoter/enhancer region is inserted in a plasmid that cannot enter the nucleus, nuclear import was observed. A total of 72 bp of the SV40 enhancer, referred to as DNA nuclear targeting sequence (DTS), have shown to be sufficient to drive plasmid nuclear import in different cell lines [
23]. A limited number of in vivo studies has evaluated this improved nuclear uptake by adding this DTS to the pDNA, resulting in a 40–200-fold increase in gene expression for vascular gene transfer in rats [
28], a 16-fold higher luciferase reporter expression after intramuscular electroporation in mice [
29], and a 20-fold increase in the level of exogenous gene expression in muscle [
30]. However, the studies evaluating intramuscular gene transfer only evaluated the levels of the expressed transgene and did not report data at the level of the plasmid. The DNA scope data from this study endorse the need for improving nuclear pDNA uptake. In addition to the low percentage of nuclear pDNA, data show that only few nuclei contain pDNA, often multiple copies, and that the majority of the nuclei do not contain any pDNA. Possibly, these few well-transfected nuclei had a damaged nuclear envelope due to electroporation or mitosis.
In addition to an elaborate investigation of the fate of pDNA after antibody gene transfer, this study measured the mRNA and protein levels in the same subjects. mRNA levels show huge variability, but no significant difference is observed between different time points. This contributes to the hypothesis that the amount of pDNA in the nuclei stays quite stable over time. The pharmacokinetic profile of the protein expression shows an initial increase in antibody titers up to week 3, followed by a steady decline in plasma protein levels.
Unfortunately, the data of this study do not allow to directly correlate the protein expression levels to pDNA and mRNA levels because of several factors, (1) mice are treated in both tibialis anterior muscles: only one is used for pDNA and mRNA quantification, while both contribute to protein expression; (2) isolating muscles for pDNA and mRNA extraction is a terminal procedure, thus no further plasma protein level follow-up in the same animals is possible. Nevertheless, it is likely that pDNA/mRNA levels correlate with plasma protein levels at a later timepoint given the plasma half-life of antibodies. The percentage of nuclear pDNA and the number of pDNA dots of the DNA scope assay do not show a correlation with protein expression either. However, as pointed out before, this data was extracted from one small part of one of two treated muscles, so this experiment cannot exclude the possible correlation either.
In conclusion, this study demonstrates that durable expression is dependent on the nuclear uptake of the pDNA and that efforts to increase the protein levels upon pDNA-based gene therapy should also focus on strategies to increase both cellular entry and migration of the pDNA into the nucleus. The generated toolbox can be used to guide the design and evaluation of future pDNA constructs or delivery methods, to achieve a robust and prolonged protein expression.





 : intranuclear pCAG-4D5-HC,
: intranuclear pCAG-4D5-HC,  : intranuclear pCAG-4D5-LC,
: intranuclear pCAG-4D5-LC,  : pCAG-4D5-HC whole muscle,
: pCAG-4D5-HC whole muscle,  : pCAG-4D5-LC whole muscle) was performed relative to the amount of the control subject at 24 h. Data are represented as individual data points per animal (average of triplicate). Curves represent the median per time point (line: pCAG-4D5-HC, dashed line: pCAG-4D5-LC) (n = 5, five mice per time point) for whole-muscle pDNA quantification, horizontal lines represent median intranuclear pDNA levels (day 4: n = 4; week 2: n = 5).
: pCAG-4D5-LC whole muscle) was performed relative to the amount of the control subject at 24 h. Data are represented as individual data points per animal (average of triplicate). Curves represent the median per time point (line: pCAG-4D5-HC, dashed line: pCAG-4D5-LC) (n = 5, five mice per time point) for whole-muscle pDNA quantification, horizontal lines represent median intranuclear pDNA levels (day 4: n = 4; week 2: n = 5).
 : intranuclear pCAG-4D5-HC,
: intranuclear pCAG-4D5-HC,  : intranuclear pCAG-4D5-LC,
: intranuclear pCAG-4D5-LC,  : pCAG-4D5-HC whole muscle,
: pCAG-4D5-HC whole muscle,  : pCAG-4D5-LC whole muscle) was performed relative to the amount of the control subject at 24 h. Data are represented as individual data points per animal (average of triplicate). Curves represent the median per time point (line: pCAG-4D5-HC, dashed line: pCAG-4D5-LC) (n = 5, five mice per time point) for whole-muscle pDNA quantification, horizontal lines represent median intranuclear pDNA levels (day 4: n = 4; week 2: n = 5).
: pCAG-4D5-LC whole muscle) was performed relative to the amount of the control subject at 24 h. Data are represented as individual data points per animal (average of triplicate). Curves represent the median per time point (line: pCAG-4D5-HC, dashed line: pCAG-4D5-LC) (n = 5, five mice per time point) for whole-muscle pDNA quantification, horizontal lines represent median intranuclear pDNA levels (day 4: n = 4; week 2: n = 5).