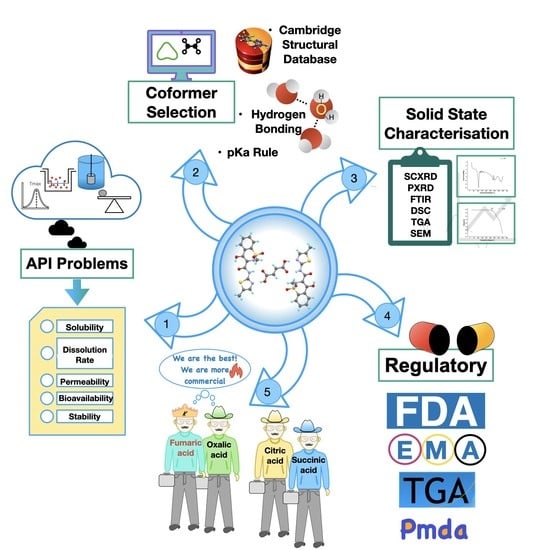
Cocrystals by Design: A Rational Coformer Selection Approach for Tackling the API Problems
Abstract
:1. Introduction
2. Selection of Coformer
2.1. Cambridge Structural Database (CSD)
2.2. Hydrogen-Bond Rules
2.3. pKa Rule
3. Coformer Impact on Pharmaceutical Attributes
3.1. Role of Coformers in Solubility, Dissolution, and Bioavailability
3.2. Role of Coformers in Improving the Mechanical Properties of Drug Molecule
3.3. Role of Coformers in Stabilizing the Drug Molecule
3.4. Role of Coformers in Enhancing the Permeability of Cocrystals
| Drug | Coformer(s) | Model | Effect on Permeability | Mechanism | Reference |
|---|---|---|---|---|---|
| Dapsone (DAP) | Caffeine | Calu-3 human bronchial epithelial cells | Greater DAP permeability from apical to basolateral direction compared to DAP alone Reduction in efflux rate, i.e., basolateral to apical direction of DAP compared to pure DAP | Possible effect of membrane transporters by caffeine and its metabolite theophylline | [79] |
| Ethenzamide | 2,4-dihydroxybenzoic acid | Diffusion apparatus with cellulose nitrate membrane | Improved permeability of cocrystals compared to pure ethenzamide | Higher solubility of cocrystal | [81] |
| Entacapone (ETP) |
| Diffusion apparatus with dialysis membrane-135 | Cocrystals of ETP with theophylline and pyrazinamide exhibited higher diffusion rate compared to pure drug ETP-THP cocrystal hydrate had better diffusion rate compared to ETP-PYZ cocrystal | Higher solubility and higher permeability of the coformer (Theophylline BCS Class I and pyrazinamide BCS Class III) | [82] |
| 1,2,4-Thiadiazole derivative | Vanillic acid | Franz-type diffusion apparatus with regenerated cellulose membrane | Increased flux with no change in apparent permeability coefficient values | Higher amount of dissolved drug leading to higher concentration gradient | [83] |
| Adefovir dipivoxil | Stearic acid | Caco-2 cells monolayers | Improved cell permeability | Stearic acid acts as P-gp inhibitor | [69] |
| Hydrochlorothiazide |
| Franz diffusion apparatus with cellulose nitrate membrane | Higher cumulative amount permeated with high diffusion rate | Higher solubility leading to increased concentration gradient | [84] |
| Lower cumulative amount permeated with marginal increase in initial flux | |||||
| Marginal increase in diffusion rate with marginal increase in flux | |||||
| Furosemide |
| Franz diffusion cell apparatus through a cellulose nitrate membrane | Higher cumulative amount permeated with high diffusion rate | Decrease in lattice energy with presence of weaker intermolecular interaction leading to increased solubility and ultimately higher concentration gradient | [44] |
| Lower cumulative amount permeated with lower flux | High lattice energy contributing to poor solubility and lesser concentration in donor compartment | ||||
| 5-Fluorouracil |
| Franz-type diffusion apparatus with a silicon membrane | Higher cumulative amount permeated with high diffusion rate | Presence of weaker intermolecular interaction between drug and coformer. Lipophilicity of coformer. | [78] |
4. Coformers Reported in the Literature
5. Coformers Used in High Demand
6. Commercially Available Drug Products Based on Cocrystals
6.1. Depakote®
6.2. Entresto®
6.3. Suglat®
6.4. Steglatro®
6.5. Lexapro® & ESIX-10®
6.6. Beta-Chlor®
6.7. Cafcit®
6.8. Zafatek®
6.9. Lamivudine/Zidovudine Teva®
6.10. Odomzo®
6.11. Mayzent®
6.12. Seglentis®
6.13. Dimenhydrinate
6.14. Ibrutinib
6.15. E-58425 (Clinical Trial Phase 3)
6.16. TAK-020 (Clinical Trial Phase 1)
7. Most Popular Coformers Utilized in Cocrystal-Based Marketed Formulations
7.1. Fumaric Acid (FA)
7.1.1. Pharmaceutical Properties
7.1.2. Fumaric Acid as a Coformer
7.2. Oxalic Acid (OA)
Oxalic Acid as a Coformer
7.3. Succinic Acid (SA)
7.3.1. Succinic Acid as a Coformer
7.3.2. Other Applications
7.4. Citric Acid
Citric Acid as a Coformer
8. Comparison of Coformers
9. Patentability Issue Criteria and Regulatory Guidelines of Pharmaceutical Cocrystals
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koranne, S.; Krzyzaniak, J.F.; Luthra, S.; Arora, K.K.; Suryanarayanan, R. Role of Coformer and Excipient Properties on the Solid-State Stability of Theophylline Cocrystals. Cryst. Growth Des. 2019, 19, 868–875. [Google Scholar] [CrossRef]
- Ullah, M.; Hussain, I.; Sun, C.C. The development of carbamazepine-succinic acid cocrystal tablet formulations with improved in vitro and in vivo performance. Drug Dev. Ind. Pharm. 2016, 42, 969–976. [Google Scholar] [CrossRef]
- Chattoraj, S.; Shi, L.; Chen, M.; Alhalaweh, A.; Velaga, S.; Sun, C.C. Origin of Deteriorated Crystal Plasticity and Compaction Properties of a 1:1 Cocrystal between Piroxicam and Saccharin. Cryst. Growth Des. 2014, 14, 3864–3874. [Google Scholar] [CrossRef]
- Rahman, Z.; Agarabi, C.; Zidan, A.S.; Khan, S.R.; Khan, M.A. Physico-mechanical and stability evaluation of carbamazepine cocrystal with nicotinamide. AAPS PharmSciTech 2011, 12, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Vandana, K.R.; Prasanna Raju, Y.; Harini Chowdary, V.; Sushma, M.; Vijay Kumar, N. An overview on in situ micronization technique—An emerging novel concept in advanced drug delivery. Saudi Pharm. J. 2014, 22, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Dun, J.; Chen, J.M.; Liu, S.; Sun, C.C. Improving solid-state properties of berberine chloride through forming a salt cocrystal with citric acid. Int. J. Pharm. 2019, 554, 14–20. [Google Scholar] [CrossRef]
- Yang, D.; Cao, J.; Jiao, L.; Yang, S.; Zhang, L.; Lu, Y.; Du, G. Solubility and Stability Advantages of a New Cocrystal of Berberine Chloride with Fumaric Acid. ACS Omega 2020, 5, 8283–8292. [Google Scholar] [CrossRef] [Green Version]
- Rasenack, N.; Müller, B.W. Micron-size drug particles: Common and novel micronization techniques. Pharm Dev Technol 2004, 9, 1–13. [Google Scholar] [CrossRef]
- Issa, N.; Barnett, S.A.; Mohamed, S.; Braun, D.E.; Copley, R.C.B.; Tocher, D.A.; Price, S.L. Screening for cocrystals of succinic acid and 4-aminobenzoic acid. CrystEngComm 2012, 14, 2454–2464. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, Q.; Li, X.; Ma, X. Preparation of 2:1 urea-succinic acid cocrystals by sublimation. J. Cryst. Growth 2017, 469, 114–118. [Google Scholar] [CrossRef]
- European Medicines Agency. Reflection Paper on the Use of Cocrystals of Active Substances in Medicinal Products; European Medicines Agency: Amsterdam, The Netherlands, 2015.
- USFDA. Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry. 7. Available online: https://www.fda.gov/files/drugs/published/Regulatory-Classification-of-Pharmaceutical-Co-Crystals.pdf. (accessed on 22 December 2022).
- Childs, S.L.; Stahly, G.P.; Park, A. The salt−cocrystal continuum: The influence of crystal structure on ionization state. Mol. Pharm. 2007, 4, 323–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Kumar Bandaru, R.; Rout, S.R.; Kenguva, G.; Gorain, B.; Alhakamy, N.A.; Kesharwani, P.; Dandela, R. Recent Advances in Pharmaceutical Cocrystals: From Bench to Market. Front Pharm. 2021, 12, 780582. [Google Scholar] [CrossRef] [PubMed]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal engineering and pharmaceutical crystallization. In Hot Topics in Crystal Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 157–229. [Google Scholar]
- Cheney, M.L.; Weyna, D.R.; Shan, N.; Hanna, M.; Wojtas, L.; Zaworotko, M.J. Coformer selection in pharmaceutical cocrystal development: A case study of a meloxicam aspirin cocrystal that exhibits enhanced solubility and pharmacokinetics. J. Pharm. Sci. 2011, 100, 2172–2181. [Google Scholar] [CrossRef] [PubMed]
- Mondal, P.K.; Mirmehrabi, M.; Rohani, S. Insight into the Formation of Heteromolecular Hydrogen Bonds between Dasatinib and GRAS Molecules. Org. Process Res. Dev. 2021, 25, 1579–1588. [Google Scholar] [CrossRef]
- Wathoni, N.; Sari, W.A.; Elamin, K.M.; Mohammed, A.F.A.; Suharyani, I. A Review of Coformer Utilization in Multicomponent Crystal Formation. Molecules 2022, 27, 8693. [Google Scholar] [CrossRef]
- Dhondale, M.R.; Thakor, P.; Nambiar, A.G.; Singh, M.; Agrawal, A.K.; Shastri, N.R.; Kumar, D. Co-Crystallization Approach to Enhance the Stability of Moisture-Sensitive Drugs. Pharmaceutics 2023, 15, 189. [Google Scholar] [CrossRef]
- Putra, O.D.; Umeda, D.; Nugraha, Y.P.; Nango, K.; Yonemochi, E.; Uekusa, H. Simultaneous Improvement of Epalrestat Photostability and Solubility via Cocrystallization: A Case Study. Cryst. Growth Des. 2017, 18, 373–379. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, J.; Liu, Z.; Kan, H.; Zu, H.; Sun, W.; Zhang, J.; Qian, S. Coformer selection based on degradation pathway of drugs: A case study of adefovir dipivoxil-saccharin and adefovir dipivoxil-nicotinamide cocrystals. Int. J. Pharm. 2012, 438, 327–335. [Google Scholar] [CrossRef]
- Fei, T.; Lv, P.; Liu, Y.; He, C.; Sun, C.; Pang, S. Design and Synthesis of a Series of CL-20 Cocrystals: Six-Membered Symmetrical N-Heterocyclic Compounds as Effective Coformers. Cryst. Growth Des. 2019, 19, 2779–2784. [Google Scholar] [CrossRef]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Supramolecular synthons in crystal engineering—A new organic synthesis. Angew. Chem. Int. Ed. Engl. 1995, 34, 2311–2327. [Google Scholar] [CrossRef]
- Fábián, L. Cambridge Structural Database Analysis of Molecular Complementarity in Cocrystals. Cryst. Growth Des. 2009, 9, 1436–1443. [Google Scholar] [CrossRef]
- Manin, A.N.; Drozd, K.V.; Churakov, A.V.; Perlovich, G.L. Hydrogen Bond Donor/Acceptor Ratios of the Coformers: Do They Really Matter for the Prediction of Molecular Packing in Cocrystals? The Case of Benzamide Derivatives with Dicarboxylic Acids. Cryst. Growth Des. 2018, 18, 5254–5269. [Google Scholar] [CrossRef]
- Kumar, S. Pharmaceutical cocrystals: An overview. Indian J. Pharm. Sci. 2018, 79, 858–871. [Google Scholar] [CrossRef]
- Surov, A.O.; Ramazanova, A.G.; Voronin, A.P.; Drozd, K.V.; Churakov, A.V.; Perlovich, G.L. Virtual Screening, Structural Analysis, and Formation Thermodynamics of Carbamazepine Cocrystals. Pharmaceutics 2023, 15, 836. [Google Scholar] [CrossRef]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient: Their Importance in Surface Coating Formulation. Ph.D. Dissertation, Danish Technical, Copenhagen, Denmark, 1967. [Google Scholar]
- Qiao, N.; Li, M.; Schlindwein, W.; Malek, N.; Davies, A.; Trappitt, G. Pharmaceutical cocrystals: An overview. Int. J. Pharm. 2011, 419, 1–11. [Google Scholar] [CrossRef]
- Vishweshwar, P.; McMahon, J.A.; Bis, J.A.; Zaworotko, M.J. Pharmaceutical co-crystals. J. Pharm. Sci. 2006, 95, 499–516. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; Bahia de Oliveira da Rocha, A.; Goncalves Cardoso, S.; Rodriguez-Hornedo, N. Cocrystals to facilitate delivery of poorly soluble compounds beyond-rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef] [Green Version]
- CCDC. Current Structures in the Cambridge Structural Database. Available online: https://www.ccdc.cam.ac.uk/ (accessed on 3 January 2023).
- Thakuria, R.; Sarma, B.; Nangia, A. 7.03—Hydrogen Bonding in Molecular Crystals. In Comprehensive Supramolecular Chemistry II; Atwood, J.L., Ed.; Elsevier: Oxford, UK, 2017; pp. 25–48. [Google Scholar]
- Kumar, A.; Nanda, A. In-silico methods of cocrystal screening: A review on tools for rational design of pharmaceutical cocrystals. J. Drug Deliv. Sci. Technol. 2021, 63, 102527. [Google Scholar] [CrossRef]
- Bennett, A.J.; Matzger, A.J. Progress in Predicting Ionic Cocrystal Formation: The Case of Ammonium Nitrate. Chem. Eur. J. 2023, e202300076. [Google Scholar] [CrossRef] [PubMed]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Etter, M.C. Hydrogen bonds as design elements in organic chemistry. J. Phys. Chem. 1991, 95, 4601–4610. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Goswami, P.K.; Thaimattam, R.; Ramanan, A. Crystal Engineering of Multicomponent Crystal Forms of p-Aminosalicylic Acid with Pyridine Based Coformers. Cryst. Growth Des. 2016, 16, 1268–1281. [Google Scholar] [CrossRef]
- Tsume, Y.; Mudie, D.M.; Langguth, P.; Amidon, G.E.; Amidon, G.L. The Biopharmaceutics Classification System: Subclasses for in vivo predictive dissolution (IPD) methodology and IVIVC. Eur. J. Pharm. Sci. 2014, 57, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Banik, M.; Gopi, S.P.; Ganguly, S.; Desiraju, G.R. Cocrystal and Salt Forms of Furosemide: Solubility and Diffusion Variations. Cryst. Growth Des. 2016, 16, 5418–5428. [Google Scholar] [CrossRef]
- Surov, A.O.; Voronin, A.P.; Manin, A.N.; Manin, N.G.; Kuzmina, L.G.; Churakov, A.V.; Perlovich, G.L. Pharmaceutical cocrystals of diflunisal and diclofenac with theophylline. Mol. Pharm. 2014, 11, 3707–3715. [Google Scholar] [CrossRef]
- Nugrahani, I.; Utami, D.; Ibrahim, S.; Nugraha, Y.P.; Uekusa, H. Zwitterionic cocrystal of diclofenac and l-proline: Structure determination, solubility, kinetics of cocrystallization, and stability study. Eur. J. Pharm. Sci. 2018, 117, 168–176. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Cocrystallization with flufenamic acid: Comparison of physicochemical properties of two pharmaceutical cocrystals. CrystEngComm 2014, 16, 5793–5801. [Google Scholar] [CrossRef]
- Goyal, P.; Rani, D.; Chadha, R. Crystal Engineering: A Remedy To Tailor the Biopharmaceutical Aspects of Glibenclamide. Cryst. Growth Des. 2017, 18, 105–118. [Google Scholar] [CrossRef]
- Mehta, B.K.; Singh, S.S.; Chaturvedi, S.; Wahajuddin, M.; Thakur, T.S. Rational Coformer Selection and the Development of New Crystalline Multicomponent Forms of Resveratrol with Enhanced Water Solubility. Cryst. Growth Des. 2018, 18, 1581–1592. [Google Scholar] [CrossRef]
- Yang, C.; Guo, W.; Lin, Y.; Lin, Q.; Wang, J.; Wang, J.; Zeng, Y. Experimental and DFT simulation study of a novel felodipine cocrystal: Characterization, dissolving properties and thermal decomposition kinetics. J. Pharm. Biomed. Anal. 2018, 154, 198–206. [Google Scholar] [CrossRef]
- Kerr, H.E.; Softley, L.K.; Suresh, K.; Nangia, A.; Hodgkinson, P.; Evans, I.R. A furosemide–isonicotinamide cocrystal: An investigation of properties and extensive structural disorder. CrystEngComm 2015, 17, 6707–6715. [Google Scholar] [CrossRef] [Green Version]
- Khare, S.G.; Jena, S.K.; Sangamwar, A.T.; Khullar, S.; Mandal, S.K. Multicomponent Pharmaceutical Adducts of α-Eprosartan: Physicochemical Properties and Pharmacokinetic Study. Cryst. Growth Des. 2017, 17, 1589–1599. [Google Scholar] [CrossRef]
- Sarkar, A.; Rohani, S. Cocrystals of acyclovir with promising physicochemical properties. J. Pharm. Sci. 2015, 104, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sanphui, P.; Devi, V.K.; Clara, D.; Malviya, N.; Ganguly, S.; Desiraju, G.R. Cocrystals of Hydrochlorothiazide: Solubility and Diffusion/Permeability Enhancements through Drug-Coformer Interactions. Mol. Pharm. 2015, 12, 1615–1622. [Google Scholar] [CrossRef]
- Shete, A.; Murthy, S.; Korpale, S.; Yadav, A.; Sajane, S.; Sakhare, S.; Doijad, R. Cocrystals of itraconazole with amino acids: Screening, synthesis, solid state characterization, in vitro drug release and antifungal activity. J. Drug Deliv. Sci. Technol. 2015, 28, 46–55. [Google Scholar] [CrossRef]
- Wang, J.-R.; Ye, C.; Zhu, B.; Zhou, C.; Mei, X. Pharmaceutical cocrystals of the anti-tuberculosis drug pyrazinamide with dicarboxylic and tricarboxylic acids. CrystEngComm 2015, 17, 747–752. [Google Scholar] [CrossRef]
- Chattoraj, S.; Shi, L.; Sun, C.C. Understanding the relationship between crystal structure, plasticity and compaction behaviour of theophylline, methyl gallate, and their 1: 1 co-crystal. CrystEngComm 2010, 12, 2466–2472. [Google Scholar] [CrossRef]
- Karki, S.; Friščić, T.; Fabian, L.; Laity, P.R.; Day, G.M.; Jones, W. Improving mechanical properties of crystalline solids by cocrystal formation: New compressible forms of paracetamol. Adv. Mater. 2009, 21, 3905–3909. [Google Scholar] [CrossRef]
- Sun, C.C.; Hou, H. Improving mechanical properties of caffeine and methyl gallate crystals by cocrystallization. Cryst. Growth Des. 2008, 8, 1575–1579. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Shimpi, M.R.; Velaga, S.P. Relationship between mechanical properties and crystal structure in cocrystals and salt of paracetamol. Drug. Dev. Ind. Pharm. 2017, 43, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Rao Khandavilli, U.B.; Bhogala, B.R.; Maguire, A.R.; Lawrence, S.E. Symmetry assisted tuning of bending and brittle multi-component forms of probenecid. Chem. Commun. 2017, 53, 3381–3384. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.K.; Hazarika, M.; Gupta, P.; Ray, N.R.; Paul, A.K.; Nauha, E. Plastically bendable crystals of probenecid and its cocrystal with 4,4′-Bipyridine. J. Mol. Struct. 2018, 1160, 20–25. [Google Scholar] [CrossRef]
- Chow, S.F.; Chen, M.; Shi, L.; Chow, A.H.; Sun, C.C. Simultaneously improving the mechanical properties, dissolution performance, and hygroscopicity of ibuprofen and flurbiprofen by cocrystallization with nicotinamide. Pharm. Res. 2012, 29, 1854–1865. [Google Scholar] [CrossRef]
- Maeno, Y.; Fukami, T.; Kawahata, M.; Yamaguchi, K.; Tagami, T.; Ozeki, T.; Suzuki, T.; Tomono, K. Novel pharmaceutical cocrystal consisting of paracetamol and trimethylglycine, a new promising cocrystal former. Int. J. Pharm. 2014, 473, 179–186. [Google Scholar] [CrossRef]
- Sanphui, P.; Mishra, M.K.; Ramamurty, U.; Desiraju, G.R. Tuning mechanical properties of pharmaceutical crystals with multicomponent crystals: Voriconazole as a case study. Mol. Pharm. 2015, 12, 889–897. [Google Scholar] [CrossRef]
- Thipparaboina, R.; Kumar, D.; Mittapalli, S.; Balasubramanian, S.; Nangia, A.; Shastri, N.R. Ionic, Neutral, and Hybrid Acid–Base Crystalline Adducts of Lamotrigine with Improved Pharmaceutical Performance. Cryst. Growth Des. 2015, 15, 5816–5826. [Google Scholar] [CrossRef]
- Hiendrawan, S.; Veriansyah, B.; Widjojokusumo, E.; Soewandhi, S.N.; Wikarsa, S.; Tjandrawinata, R.R. Physicochemical and mechanical properties of paracetamol cocrystal with 5-nitroisophthalic acid. Int. J. Pharm. 2016, 497, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.R.; O’Connell, P.; Paluch, K.J.; Walsh, D.; Healy, A.M. Cocrystal habit engineering to improve drug dissolution and alter derived powder properties. J. Pharm. Pharm. 2016, 68, 665–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, J.-W.; Hwang, K.-M.; Lee, S.-H.; Kim, D.-W.; Park, E.-S. Preparation and characterization of adefovir dipivoxil–stearic acid cocrystal with enhanced physicochemical properties. Pharm. Dev. Technol. 2017, 23, 890–899. [Google Scholar] [CrossRef]
- Haneef, J.; Amir, M.; Sheikh, N.A.; Chadha, R. Mitigating Drug Stability Challenges Through Cocrystallization. AAPS PharmSciTech 2023, 24, 62. [Google Scholar] [CrossRef] [PubMed]
- Trask, A.V.; Motherwell, W.S.; Jones, W. Pharmaceutical cocrystallization: Engineering a remedy for caffeine hydration. Cryst. Growth Des. 2005, 5, 1013–1021. [Google Scholar] [CrossRef]
- Mittapalli, S.; Bolla, G.; Perumalla, S.; Nangia, A. Can we exchange water in a hydrate structure: A case study of etoricoxib. CrystEngComm 2016, 18, 2825–2829. [Google Scholar] [CrossRef]
- Vangala, V.R.; Chow, P.S.; Tan, R.B.H. Characterization, physicochemical and photo-stability of a co-crystal involving an antibioticdrug, nitrofurantoin, and 4-hydroxybenzoic acid. CrystEngComm 2011, 13, 759–762. [Google Scholar] [CrossRef]
- Babu, N.J.; Sanphui, P.; Nangia, A. Crystal engineering of stable temozolomide cocrystals. Chem. Asian J. 2012, 7, 2274–2285. [Google Scholar] [CrossRef]
- Geng, N.; Chen, J.-M.; Li, Z.-J.; Jiang, L.; Lu, T.-B. Approach of Cocrystallization to Improve the Solubility and Photostability of Tranilast. Cryst. Growth Des. 2013, 13, 3546–3553. [Google Scholar] [CrossRef]
- Jung, S.; Choi, I.; Kim, I. Liquid-Assisted Grinding to Prepare a Cocrystal of Adefovir Dipivoxil Thermodynamically Less Stable than Its Neat Phase. Crystals 2015, 5, 583–591. [Google Scholar] [CrossRef] [Green Version]
- Nechipadappu, S.K.; Ramachandran, J.; Shivalingegowda, N.; Lokanath, N.K.; Trivedi, D.R. Synthesis of cocrystals/salts of flucytosine: Structure and stability. New J. Chem. 2018, 42, 5433–5446. [Google Scholar] [CrossRef]
- Dai, X.-L.; Li, S.; Chen, J.-M.; Lu, T.-B. Improving the Membrane Permeability of 5-Fluorouracil via Cocrystallization. Cryst. Growth Des. 2016, 16, 4430–4438. [Google Scholar] [CrossRef]
- Do Amaral, L.H.; do Carmo, F.A.; Amaro, M.I.; de Sousa, V.P.; da Silva, L.; de Almeida, G.S.; Rodrigues, C.R.; Healy, A.M.; Cabral, L.M. Development and Characterization of Dapsone Cocrystal Prepared by Scalable Production Methods. AAPS PharmSciTech 2018, 19, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Balimane, P.V.; Chong, S. Evaluation of Permeability and P-glycoprotein Interactions: Industry Outlook. In Biopharmaceutics Applications in Drug Development; Krishna, R., Yu, L., Eds.; Springer: Boston, MA, USA, 2008; pp. 101–138. [Google Scholar]
- Khatioda, R.; Bora, P.; Sarma, B. Trimorphic Ethenzamide Cocrystal: In Vitro Solubility and Membrane Efflux Studies. Cryst. Growth Des. 2018, 18, 4637–4645. [Google Scholar] [CrossRef]
- Bommaka, M.K.; Mannava, M.K.C.; Suresh, K.; Gunnam, A.; Nangia, A. Entacapone: Improving Aqueous Solubility, Diffusion Permeability, and Cocrystal Stability with Theophylline. Cryst. Growth Des. 2018, 18, 6061–6069. [Google Scholar] [CrossRef]
- Surov, A.O.; Volkova, T.V.; Churakov, A.V.; Proshin, A.N.; Terekhova, I.V.; Perlovich, G.L. Cocrystal formation, crystal structure, solubility and permeability studies for novel 1,2,4-thiadiazole derivative as a potent neuroprotector. Eur. J. Pharm. Sci. 2017, 109, 31–39. [Google Scholar] [CrossRef]
- Gopi, S.P.; Banik, M.; Desiraju, G.R. New Cocrystals of Hydrochlorothiazide: Optimizing Solubility and Membrane Diffusivity. Cryst. Growth Des. 2016, 17, 308–316. [Google Scholar] [CrossRef]
- Sanphui, P.; Babu, N.J.; Nangia, A. Temozolomide Cocrystals with Carboxamide Coformers. Cryst. Growth Des. 2013, 13, 2208–2219. [Google Scholar] [CrossRef]
- Cho, M.-Y.; Kim, P.; Kim, G.-Y.; Lee, J.-Y.; Song, K.-H.; Lee, M.-J.; Yoon, W.; Yun, H.; Choi, G.J. Preparation and Characterization of Aripiprazole Cocrystals with Coformers of Multihydroxybenzene Compounds. Cryst. Growth Des. 2017, 17, 6641–6652. [Google Scholar] [CrossRef]
- Deka, P.; Gogoi, D.; Althubeiti, K.; Rao, D.R.; Thakuria, R. Mechanosynthesis, Characterization, and Physicochemical Property Investigation of a Favipiravir Cocrystal with Theophylline and GRAS Coformers. Cryst. Growth Des. 2021, 21, 4417–4425. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Crystal Engineering of Curcumin with Salicylic Acid and Hydroxyquinol as Coformers. Cryst. Growth Des. 2017, 17, 3974–3988. [Google Scholar] [CrossRef]
- Gołdyn, M.R.; Larowska, D.; Bartoszak-Adamska, E. Novel Purine Alkaloid Cocrystals with Trimesic and Hemimellitic Acids as Coformers: Synthetic Approach and Supramolecular Analysis. Cryst. Growth Des. 2020, 21, 396–413. [Google Scholar] [CrossRef]
- Jubeen, F.; Liaqat, A.; Amjad, F.; Sultan, M.; Iqbal, S.Z.; Sajid, I.; Khan Niazi, M.B.; Sher, F. Synthesis of 5-Fluorouracil Cocrystals with Novel Organic Acids as Coformers and Anticancer Evaluation against HCT-116 Colorectal Cell Lines. Cryst. Growth Des. 2020, 20, 2406–2414. [Google Scholar] [CrossRef]
- Hrinova, E.; Skorepova, E.; Cerna, I.; Kralovicova, J.; Kozlik, P.; Krizek, T.; Rousarova, J.; Rysanek, P.; Sima, M.; Slanar, O.; et al. Explaining dissolution properties of rivaroxaban cocrystals. Int. J. Pharm. 2022, 622, 121854. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Tan, F.; Yao, J.; Cui, Y.; Feng, Y.; Li, Z.; Wang, Y.; Yang, Y.; Gong, W.; Yang, M.; et al. Preparation, characterization, and pharmacokinetics of rivaroxaban cocrystals with enhanced in vitro and in vivo properties in beagle dogs. Int. J. Pharm. X 2022, 4, 100119. [Google Scholar] [CrossRef]
- Kale, D.P.; Puri, V.; Kumar, A.; Kumar, N.; Bansal, A.K. The Role of Cocrystallization-Mediated Altered Crystallographic Properties on the Tabletability of Rivaroxaban and Malonic Acid. Pharmaceutics 2020, 12, 546. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.P.; Ugale, B.; Nagaraja, C.M.; Dubey, G.; Bharatam, P.V.; Bansal, A.K. Molecular Basis of Water Sorption Behavior of Rivaroxaban-Malonic Acid Cocrystal. Mol. Pharm. 2019, 16, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Kuang, W.j.; Ji, S.c.; Xu, S.m.; Lan, P.; Liao, A.p.; Zhou, J.y.; Zhang, J.y. Thermodynamic and Crystallization of Lamotrigine Cocrystal. Cryst. Growth Des. 2019, 19, 6603–6610. [Google Scholar] [CrossRef]
- Lekšić, E.; Pavlović, G.; Meštrović, E. Cocrystals of Lamotrigine Based on Coformers Involving Carbonyl Group Discovered by Hot-Stage Microscopy and DSC Screening. Cryst. Growth Des. 2012, 12, 1847–1858. [Google Scholar] [CrossRef]
- Kuang, W.; Ji, S.; Wang, X.; Zhang, J.; Lan, P. Relationship between crystal structures and physicochemical properties of lamotrigine cocrystal. Powder Technol. 2021, 380, 18–25. [Google Scholar] [CrossRef]
- Kaur, R.; Cavanagh, K.L.; Rodriguez-Hornedo, N.; Matzger, A.J. Multidrug Cocrystal of Anticonvulsants: Influence of Strong Intermolecular Interactions on Physiochemical Properties. Cryst. Growth Des. 2017, 17, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wang, Y.; Wu, S.; Yu, B.; Shi, P.; Bian, L.; Zhang, D.; Hou, J.; Wang, J.; Gong, J. Two novel cocrystals of lamotrigine with isomeric bipyridines and in situ monitoring of the cocrystallization. Eur. J. Pharm. Sci. 2017, 110, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R.; Saini, A.; Arora, P.; Jain, D.S.; Dasgupta, A.; Guru Row, T.N. Multicomponent solids of lamotrigine with some selected coformers and their characterization by thermoanalytical, spectroscopic and X-ray diffraction methods. CrystEngComm 2011, 13, 6271–6284. [Google Scholar] [CrossRef]
- Zalte, A.G.; Saudagar, R.B. Preparation and Characterization of Etodolac Co-Crystals Using 32 Full Factorial Design. Res. J. Pharm. Technol. 2018, 11, 3781–3786. [Google Scholar] [CrossRef]
- Ahirrao, S.P.; Sonawane, M.P.; Bhambere, D.S.; Udavant, P.B.; Ahire, E.D.; Kanade, R.; kuber, D. Cocrystal Formulation: A Novel Approach to Enhance Solubility and Dissolution of Etodolac. Biosci. Biotechnol. Res. Asia 2022, 19, 111–119. [Google Scholar] [CrossRef]
- Zachariah, S.B.; Borges, E.F.; Varghese, R.; Cruz, A.R.; Ross, G.S. Positive response to oral divalproex sodium (Depakote) in patients with spasticity and pain. Am. J. Med. Sci. 1994, 308, 38–40. [Google Scholar] [CrossRef]
- McGraw, D. Therapeutic drug monitoring with valproate–Why product selection is an important factor. Ment. Health Clin. 2014, 4, 31–34. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical cocrystals: From serendipity to design to application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Shaikh, R.; Singh, R.; Walker, G.M.; Croker, D.M. Pharmaceutical Cocrystal Drug Products: An Outlook on Product Development. Trends Pharm. Sci 2018, 39, 1033–1048. [Google Scholar] [CrossRef]
- USFDA. Highlights of Prescribing Information Entresto. Available online: https://www.novartis.com/us-en/sites/novartis_us/files/entresto.pdf (accessed on 23 November 2022).
- Astellas. Launch of Suglat. Available online: https://www.astellas.com/en/system/files/news/2018-06/140417_1_Eg.pdf (accessed on 20 October 2022).
- European Medicines Agency. ANNEX I, Summary of Product Characteristics Steglatro. Available online: https://www.ema.europa.eu/en/documents/product-information/steglatro-epar-product-information_en.pdf (accessed on 20 October 2022).
- USFDA. Highlights of Prescribing Information Steglatro. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209803s000lbl.pdf (accessed on 20 October 2022).
- Estevez, K.L.A.R.R. Escitalopram. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557734/ (accessed on 20 October 2022).
- USFDA. Highlights of Prescribing Information Lexapro. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021323s047lbl.pdf (accessed on 20 October 2022).
- USFDA. Drug Approval Package. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/020793_000_CafcitTOC.cfm (accessed on 20 October 2022).
- Evaluation and Licensing Division, Pharmaceutical and Food Safety Bureau; Ministry of Health, Labour and Welfare. Japanese Review Report. Available online: https://www.pmda.go.jp/files/000213963.pdf (accessed on 20 October 2022).
- Bhatt, P.M.; Azim, Y.; Thakur, T.S.; Desiraju, G.R. Co-crystals of the anti-HIV drugs lamivudine and zidovudine. Cryst. Growth Des. 2009, 9, 951–957. [Google Scholar] [CrossRef]
- European Medicines Agency. European Public Assessment Report (EPAR). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/lamivudine-zidovudine-teva#product-information-section (accessed on 20 October 2022).
- European Medicines Agency. ANNEX I, Summary of Product Characteristics Lamivudine/Zidovudine. Available online: https://www.ema.europa.eu/en/documents/product-information/lamivudine/zidovudine-teva-epar-product-information_en.pdf (accessed on 21 October 2022).
- Alvani, A.; Shayanfar, A. Solution Stability of Pharmaceutical Cocrystals. Cryst. Growth Des. 2022, 22, 6323–6337. [Google Scholar] [CrossRef]
- San Martín, O.; Llombart, B.; Carretero Hernandez, G.; Flórez Menéndez, Á.; Botella-Estrada, R.; Herrera Ceballos, E.; Puig, S. Sonidegib in the Treatment of Locally Advanced Basal Cell Carcinoma. Actas Dermo-Sifiliográficas 2021, 112, 295–301. [Google Scholar] [CrossRef]
- USFDA. Highlights of Prescribing Information of ODOMZO®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/205266s006lbl.pdf (accessed on 22 October 2022).
- Diaz-Villamarin, X.; Pinar-Morales, R.; Barrero-Hernandez, F.J.; Antunez-Rodriguez, A.; Cabeza-Barrera, J.; Moron-Romero, R. Pharmacogenetics of siponimod: A systematic review. Biomed. Pharm. 2022, 153, 113536. [Google Scholar] [CrossRef]
- O’Sullivan, A.; Long, B.; Verma, V.; Ryan, K.M.; Padrela, L. Solid-state and particle size control of pharmaceutical cocrystals using atomization-based techniques. Int. J. Pharm. 2022, 621, 121798. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Highlights of Prescribing Information of MAYZENT®. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/209884Orig1s011lbl.pdf (accessed on 22 October 2022).
- USFDA. Opioid Analgesic REMS Program. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213426s000lbl.pdf (accessed on 23 October 2022).
- Putra, O.D.; Yoshida, T.; Umeda, D.; Higashi, K.; Uekusa, H.; Yonemochi, E. Crystal Structure Determination of Dimenhydrinate after More than 60 Years: Solving Salt–Cocrystal Ambiguity via Solid-State Characterizations and Solubility Study. Cryst. Growth Des. 2016, 16, 5223–5229. [Google Scholar] [CrossRef]
- USFDA. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/results_product.cfm?Appl_Type=A&Appl_No=085166#13840 (accessed on 23 October 2022).
- Maytal Piran, Teva Pharmaceutical Industries Ltd. Creatively Using Co-Crystals to Produce Alternative Generic Products. Available online: https://www.teva-api.com/knowledge-center/using-co-crystals-to-produce-alternative-generic-products/ (accessed on 24 October 2022).
- Banerjee, M.; Nimkar, K.; Naik, S.; Patravale, V. Unlocking the potential of drug-drug cocrystals—A comprehensive review. J. Control Release 2022, 348, 456–469. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Nanda, A. A Review about Regulatory Status and Recent Patents of Pharmaceutical Co-Crystals. Adv. Pharm. Bull. 2018, 8, 355–363. [Google Scholar] [CrossRef]
- Videla, S.; Gascón, N.; Plata-Salamán, C. Commentary on the “Co-Crystal of Tramadol-Celecoxib in Patients with Moderate to Severe Acute Post-Surgical Oral Pain: A Dose-Finding, Randomised, Double-Blind, Placebo-and Active-Controlled, Multicentre Phase II Trial”. Implications for Cardiovascular Safety. J. Cardiol. Cardiovasc. Sci. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Merlos, M.; Portillo-Salido, E.; Brenchat, A.; Aubel, B.; Buxens, J.; Fisas, A.; Codony, X.; Romero, L.; Zamanillo, D.; Vela, J.M. Administration of a co-crystal of tramadol and celecoxib in a 1: 1 molecular ratio produces synergistic antinociceptive effects in a postoperative pain model in rats. Eur. J. Pharmacol. 2018, 833, 370–378. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Co-Crystal E-58425 vs. Tramadol and Celecoxib for Moderate to Severe Acute Pain after Bunionectomy. Phase III Clinical Trial. Available online: https://clinicaltrials.gov/ct2/show/NCT03108482 (accessed on 24 October 2022).
- Kimoto, K.; Yamamoto, M.; Karashima, M.; Hohokabe, M.; Takeda, J.; Yamamoto, K.; Ikeda, Y. Pharmaceutical Cocrystal Development of TAK-020 with Enhanced Oral Absorption. Crystals 2020, 10, 211. [Google Scholar] [CrossRef] [Green Version]
- U.S. National Library of Medicine. In TAK-020 Relative Bioavailability and Food Effect Study in Healthy Participants. Available online: https://clinicaltrials.gov/ct2/show/NCT02723201 (accessed on 24 October 2022).
- Devarakonda, S.N.; Vyas, K.; Bommareddy, S.R.; Padi, P.R.; Raghupathy, B. ARIPIPRAZOLE CO-CRYSTALS. US 2009/0054455, 2009. [Google Scholar]
- National Library of Medicine-National Center for Biotechnology Information. Compound Summary of Fumaric acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fumaric-acid (accessed on 27 October 2022).
- Drugbank. Fumaric Acid. Available online: https://go.drugbank.com/drugs/DB01677 (accessed on 26 October 2022).
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2010. [Google Scholar]
- Roa Engel, C.A.; Straathof, A.J.; Zijlmans, T.W.; van Gulik, W.M.; van der Wielen, L.A. Fumaric acid production by fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef] [Green Version]
- National Library of Medicine-National Center for Biotechnology Information. Compound Summary of Oxalic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Oxalic-acid#section=Related-Compounds (accessed on 27 October 2022).
- Palmieri, F.; Estoppey, A.; House, G.L.; Lohberger, A.; Bindschedler, S.; Chain, P.S.; Junier, P. Oxalic acid, a molecule at the crossroads of bacterial-fungal interactions. Adv. Appl. Microbiol. 2019, 106, 49–77. [Google Scholar] [CrossRef]
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–630. [Google Scholar]
- Yan, Y.; Wang, L.; Si, Z.; Zhang, X.; Yuan, W. A novel cocrystal of metformin hydrochloride with citric acid: Systematic synthesis and computational simulation. Eur. J. Pharm. Biopharm. 2022, 179, 37–46. [Google Scholar] [CrossRef] [PubMed]
- National Library of Medicine-National Center for Biotechnology Information. Compound Summary of Citric Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Citric-Acid (accessed on 27 October 2022).
- Sebastian, J.; Osorio-Gonzalez, C.; Rouissi, T.; Hegde, K.; Brar, S.K. Bioderived fumaric acid for sustainable production of key active pharmaceutical ingredients: Dimethyl fumarate and Monomethyl fumarate. Process Biochem. 2022, 120, 35–40. [Google Scholar] [CrossRef]
- Borodi, G.; Turza, A.; Onija, O.; Bende, A. Succinic, fumaric, adipic and oxalic acid cocrystals of promethazine hydrochloride. Acta Cryst. C Struct. Chem. 2019, 75, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Sathisaran, I.; Dalvi, S.V. Engineering Cocrystals of PoorlyWater-Soluble Drugs to Enhance Dissolution in Aqueous Medium. Pharmaceutics 2018, 10, 108. [Google Scholar] [CrossRef] [Green Version]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical cocrystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Liu, L.; Zou, D.; Zhang, Y.; Zhang, Q.; Feng, Y.; Guo, Y.; Liu, Y.; Zhang, X.; Cheng, G.; Wang, C.; et al. Pharmaceutical salts/cocrystals of enoxacin with dicarboxylic acids: Enhancing in vitro antibacterial activity of enoxacin by improving the solubility and permeability. Eur. J. Pharm. Biopharm. 2020, 154, 62–73. [Google Scholar] [CrossRef]
- Childs, S.L.; Chyall, L.J.; Dunlap, J.T.; Smolenskaya, V.N.; Stahly, B.C.; Stahly, G.P. Crystal engineering approach to forming cocrystals of amine hydrochlorides with organic acids. Molecular complexes of fluoxetine hydrochloride with benzoic, succinic, and fumaric acids. J. Am. Chem. Soc. 2004, 126, 13335–13342. [Google Scholar] [CrossRef] [Green Version]
- Yogheshwari, P.; Sridhar, B.; Anitha, K. Experimental and theoretical studies on bis (6-nitroquinoline) fumaric acid co-crystal. J. Mol. Struct. 2022, 1249, 131561. [Google Scholar] [CrossRef]
- Pandey, N.; Ghosh, A. An outlook on permeability escalation through cocrystallization for developing pharmaceuticals with improved biopharmaceutical properties. J. Drug Deliv. Sci. Technol. 2022, 76, 103757. [Google Scholar] [CrossRef]
- Mohamed, M.P.; Sudha, S.; Jayaprakash, P.; Vinitha, G.; Nageshwari, M.; Sangeetha, P.; Kumari, C.R.T.; Caroline, M.L. Growth and characterization of L-histidinium fumarate fumaric acid monohydrate single crystal: A promising second and third order nonlinear optical material. Chin. J. Phys. 2019, 60, 581–597. [Google Scholar] [CrossRef]
- Prakash, M.; Geetha, D.; Caroline, M. Growth and Characterization of Nonlinear Optics (NLO) Active L-Phenylalanine Fumaric Acid (LPFA) Single Crystal. Mater. Manuf. Process. 2011, 27, 519–522. [Google Scholar] [CrossRef]
- Sawatdee, S.; Atipairin, A.; Rakkummerd, S.; Suriyaphol, O.; Harding, D.J.; Muenraya, P.; Harding, P. Preparation and physicochemical characterization of sildenafil cocrystals. J. Adv. Pharm. Technol. Res. 2021, 12, 408–419. [Google Scholar] [CrossRef]
- Kamble, R.N.; Bothiraja, C.; Mehta, P.P.; Varghese, V. Synthesis, solid state characterization and antifungal activity of ketoconazole cocrystals. J. Pharm. Investig. 2017, 48, 541–549. [Google Scholar] [CrossRef]
- Maheshwari, C.; André, V.; Reddy, S.; Roy, L.; Duarte, T.; Rodríguez-Hornedo, N. Tailoring aqueous solubility of a highly soluble compound via cocrystallization: Effect of coformer ionization, pH max and solute–solvent interactions. CrystEngComm 2012, 14, 4801–4811. [Google Scholar] [CrossRef]
- Fernandes, R.P.; do Nascimento, A.L.C.S.; Carvalho, A.C.S.; Teixeira, J.A.; Ionashiro, M.; Caires, F.J. Mechanochemical synthesis, characterization, and thermal behavior of meloxicam cocrystals with salicylic acid, fumaric acid, and malic acid. J. Therm. Anal. Calorim. 2019, 138, 765–777. [Google Scholar] [CrossRef]
- Li, L.; Yin, X.H.; Diao, K.S. Improving the solubility and bioavailability of anti-hepatitis B drug PEC via PEC-fumaric acid cocrystal. RSC Adv. 2020, 10, 36125–36134. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R. Novel Cocrystals of Glipizide: Green Supramolecular Mechanosynthesis. Arch. Pharm. Pharmacol. Res. 2018, 1, 1–13. [Google Scholar] [CrossRef]
- Bruni, G.; Maietta, M.; Maggi, L.; Mustarelli, P.; Ferrara, C.; Berbenni, V.; Milanese, C.; Girella, A.; Marini, A. Preparation and physicochemical characterization of acyclovir cocrystals with improved dissolution properties. J. Pharm. Sci. 2013, 102, 4079–4086. [Google Scholar] [CrossRef] [PubMed]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Pharmaceutical cocrystals of ethenzamide: Structural, solubility and dissolution studies. CrystEngComm 2012, 14, 8515–8524. [Google Scholar] [CrossRef]
- Dayo Owoyemi, B.C.; da Silva, C.C.P.; Souza, M.S.; Diniz, L.F.; Ellena, J.; Carneiro, R.L. Fluconazole: Synthesis and Structural Characterization of Four New Pharmaceutical Cocrystal Forms. Cryst. Growth Des. 2019, 19, 648–657. [Google Scholar] [CrossRef]
- Gadade, D.D.; Pekamwar, S.S.; Shirsat, M.D. Crystal Engineering of Antiviral Agent Efavirenz for Solubility Enhancement. J. Drug Deliv. Ther. 2018, 8, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, L.; Yao, J.; Ma, Y.-Y.; Chen, J.-M.; Lu, T.-B. Improving the Solubility and Bioavailability of Apixaban via Apixaban–Oxalic Acid Cocrystal. Cryst. Growth Des. 2016, 16, 2923–2930. [Google Scholar] [CrossRef]
- Kanakaraju, K.; Lavanya, V.; Nangia, A. Temozolomide Cocrystals Exhibit Drug Sensitivity in Glioblastoma Cells. Proc. Natl. Acad. Sci. India Sect. A Phys. Sci. 2014, 84, 321–330. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wu, H.; Guo, C.Y.; Zhu, B.; Ren, G.B. Enhancing the solubility of natural compound xanthotoxin by modulating stability via cocrystallization engineering. Int. J. Pharm. 2019, 572, 118776. [Google Scholar] [CrossRef]
- Jindal, A.; Singh, R.; Tomar, S.; Dureja, J.; Karan, M.; Chadha, R. Engineering a Remedy to Modulate and Optimize Biopharmaceutical Properties of Rebamipide by Synthesizing New Cocrystal: In Silico and Experimental Studies. Pharm. Res. 2021, 38, 2129–2145. [Google Scholar] [CrossRef]
- Alatas, F.; Ratih, H.; Soewandhi, S.N. Enhancement of solubility and dissolution rate of telmisartan by telmisartan-oxalic acid co-crystal formation. Int. J. Pharm. Pharm. Sci. 2015, 7, 423–426, ISSN- 0975-1491. [Google Scholar]
- Aher, S.; Dhumal, R.; Mahadik, K.; Ketolainen, J.; Paradkar, A. Effect of cocrystallization techniques on compressional properties of caffeine/oxalic acid 2:1 cocrystal. Pharm. Dev. Technol. 2011, 18, 55–60. [Google Scholar] [CrossRef]
- Amin, S.; Sholihah, R.t.; Megantara, S.; Budiman, A. Synthesis of Glibenclamide-Oxalic Acid Cocrystal using ThermalSolvent-Free Method. Int. J. Pharm. Qual. Assur. 2020, 11, 404–408. [Google Scholar] [CrossRef]
- Tretter, L.; Patocs, A.; Chinopoulos, C. Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochim. Biophys. Acta 2016, 1857, 1086–1101. [Google Scholar] [CrossRef] [PubMed]
- Ninawe, A.; Kumar, A.; Mohadikar, P.; Shende, D.; Wasewar, K. Separation of succinic acid from aqueous phase using nontoxic solvents. Chem. Data Collect. 2022, 39, 100866. [Google Scholar] [CrossRef]
- Fuessl, A.; Yamamoto, M.; Schneller, A. Opportunities in Bio-Based Building Blocks for Thermoplastic Polymers. Ref. Modul. Mater. Sci. Mater. Eng. 2016, 1–25. [Google Scholar] [CrossRef]
- Marketsandmarkets. Succinic Acid Market. Available online: https://www.marketsandmarkets.com/Market-Reports/succinic-acid-market-402.html (accessed on 24 October 2022).
- Alhalaweh, A.; George, S.; Boström, D.; Velaga, S.P. 1:1 and 2:1 Urea−Succinic Acid Cocrystals: Structural Diversity, Solution Chemistry, and Thermodynamic Stability. Cryst. Growth Des. 2010, 10, 4847–4855. [Google Scholar] [CrossRef]
- Ober, C.A.; Gupta, R.B. Formation of itraconazole–succinic acid cocrystals by gas antisolvent cocrystallization. Aaps Pharmscitech 2012, 13, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitriani, L.; Fitriandi, A.D.; Hasanah, U.; Zaini, E. Nano-Cocrystals of Piperine-Succinic Acid: Physicochemical Characterization and Dissolution Rate Studies. ChemistrySelect 2022, 7, e202104196. [Google Scholar] [CrossRef]
- Lemmerer, A.; Bernstein, J.; Kahlenberg, V. One-pot covalent and supramolecular synthesis of pharmaceutical co-crystals using the API isoniazid: A potential supramolecular reagent. CrystEngComm 2010, 12, 2856–2864. [Google Scholar] [CrossRef]
- Noonan, T.J.; Chibale, K.; Bourne, S.A.; Caira, M.R. A preformulation co-crystal screening case study: Polymorphic co-crystals of an imidazopyridazine antimalarial drug lead with the coformer succinic acid. J. Mol. Struct. 2020, 1204, 127561. [Google Scholar] [CrossRef]
- Rama, V.; Vidavulur, S.; Tadikonda, P.V.; Rajana, N.; Mittapalli, S. Novel cocrystals of brexpiprazole with improved solubility. J. Cryst. Growth 2020, 551, 125910. [Google Scholar] [CrossRef]
- Butreddy, A.; Almutairi, M.; Komanduri, N.; Bandari, S.; Zhang, F.; Repka, M.A. Multicomponent crystalline solid forms of aripiprazole produced via hot melt extrusion techniques: An exploratory study. J. Drug Deliv. Sci. Technol. 2021, 63, 102529. [Google Scholar] [CrossRef]
- Cao, F.; Rodriguez-Hornedo, N.; Amidon, G.E. Mechanistic Analysis of Cocrystal Dissolution, Surface pH, and Dissolution Advantage as a Guide for Rational Selection. J. Pharm. Sci. 2019, 108, 243–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chennuru, R.; Devarapalli, R.; Rengaraj, P.; Srinivas, P.L.; Dey, S.; Reddy, C.M. Improving Solubility of Poorly Soluble Abiraterone Acetate by Cocrystal Design Aided by In Silico Screening. Cryst. Growth Des. 2020, 20, 5018–5030. [Google Scholar] [CrossRef]
- Rahman, F.; Winantari, A.N.; Setyawan, D.; Siswandono, S. Comparison Study of Grinding and Slurry Method on Physicochemical Characteristic of Acyclovir—Succinic Acid Cocrystal. Asian J. Pharm. Clin. Res. 2017, 10, 153–158. [Google Scholar] [CrossRef]
- Poerwono, H.; Higashiyama, K.; Kubo, H.; Poernomo, A.T.; Suharjono; Sudiana, I.K.; Indrayanto, G.; Brittain, H.G. Citric Acid. In Analytical Profiles of Drug Substances and Excipients; Elsevier: Amsterdam, The Netherlands, 2001; Volume 45, pp. 1–76. [Google Scholar]
- Research and Markets-The World’s Largest Market Research Store. Citric Acid Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2022–2027. Available online: https://www.researchandmarkets.com/reports/5577854/citric-acid-market-global-industry-trends (accessed on 27 October 2022).
- Hsu, P.-C.; Lin, H.-L.; Wang, S.-L.; Lin, S.-Y. Solid-state thermal behavior and stability studies of theophylline–citric acid cocrystals prepared by neat cogrinding or thermal treatment. J. Solid State Chem. 2012, 192, 238–245. [Google Scholar] [CrossRef]
- Deng, J.H.; Lu, T.B.; Sun, C.C.; Chen, J.M. Dapagliflozin-citric acid cocrystal showing better solid state properties than dapagliflozin. Eur. J. Pharm. Sci. 2017, 104, 255–261. [Google Scholar] [CrossRef]
- Salem, A.; Khanfar, E.; Nagy, S.; Szechenyi, A. Cocrystals of tuberculosis antibiotics: Challenges and missed opportunities. Int. J. Pharm. 2022, 623, 121924. [Google Scholar] [CrossRef] [PubMed]
- Rachmaniar, R.; Riasari, H.; Fauziah, L.; Kenti; Ferdiansyah, R. The effect of cocrystallization method and citric acid as coformer on water solubility of ethyl p-metoxycinnamate particle. In Proceedings of the 3rd International Conference on Condensed Matter and Applied Physics (Icc-2019), Solo, Indonesia, 5 May 2020. [Google Scholar]
- Khan, F.M.; Ahmad, M.; Batool, F. Enhancement of solubility and release profile of simvastatin by co-crystallization with citric acid. Trop. J. Pharm. Res. 2019, 18, 2465–2472. [Google Scholar] [CrossRef]
- Dhibar, M.; Chakraborty, S.; Basak, S.; Pattanayak, P.; Chatterjee, T.; Ghosh, B.; Raafat, M.; Abourehab, M.A.S. Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment. Pharmaceuticals 2023, 16, 284. [Google Scholar] [CrossRef]
- Buol, X.; Robeyns, K.; Caro Garrido, C.; Tumanov, N.; Collard, L.; Wouters, J.; Leyssens, T. Improving Nefiracetam Dissolution and Solubility Behavior Using a Cocrystallization Approach. Pharmaceutics 2020, 12, 653. [Google Scholar] [CrossRef]
- Narala, S.; Ampati, R.; Nanam, R. Solubility enhancement of ritonavir: Cocrystallization. J. Pharm. Res. 2019, 8, 630–637. [Google Scholar] [CrossRef]
- Alhalaweh, A.; George, S.; Basavoju, S.; Childs, S.L.; Rizvi, S.A.A.; Velaga, S.P. Pharmaceutical cocrystals of nitrofurantoin: Screening, characterization and crystal structure analysis. CrystEngComm 2012, 14, 5078–5088. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, D.; Chen, T.; Zhang, B.; Xing, C.; Zhang, L.; Lu, Y.; Du, G. Insights into the Solubility and Structural Features of Four Praziquantel Cocrystals. Cryst. Growth Des. 2021, 21, 6321–6331. [Google Scholar] [CrossRef]
- Intellectual Property India. Filing International Applications for Patent Under the Patent Cooperation Treaty. Available online: https://ipindia.gov.in/writereaddata/images/pdf/pct-filing.pdf, (accessed on 20 October 2022).





















| Drug | Coformer | Effect on Solubility (Comparison with Drug) | Effect on Dissolution (Comparison with Drug) | Mechanism | Reference |
|---|---|---|---|---|---|
| Epalrestat | Betaine | 2-fold increase | 3.5-fold increase | Presence of layered structure with alternate drug and coformer | [21] |
| Glibenclamide | Hippuric acid | 2.2-fold increase | 2.2-fold increase | Hydrogen-bonding interactions between drug and coformers Melting point of cocrystal Solubility of coformer | [48] |
| Nicotinic acid | 3-fold increase | 3-fold increase | |||
| Theophylline | 1.5-fold increase | 1.6-fold increase | |||
| Succinic acid | 3.5-fold increase | 3.7-fold increase | |||
| Diclofenac | l-proline | 7.69-fold increase | - | Formation of layered structure | [47] |
| Resveratrol | Piperazine | 2.48-fold increase | - | Hydrogen bond network | [49] |
| Methenamine | 1.46-fold increase | ||||
| Felodipine | Glutaric acid | - | 2.41-fold increase at pH 1.2 | Lowering of melting point | [50] |
| Furosemide | Isonicotinamide | 5.6-fold increase (Apparent Solubility-Phase transformation) | No change | Coformer solubility | [51] |
| Eprosartan mesylate | Salicylic acid | 6-fold increase | Increased IDR | Coformer solubility Hydrogen-bonding interaction | [52] |
| p-aminobenzoic acid | 32-fold increase | ||||
| Succinic acid | 61-fold increase | ||||
| Acyclovir | Fumaric acid | 5.5-fold increase | Increased dissolution rate | Coformer solubility Melting points of cocrystal | [53] |
| Malonic acid | 5.7-fold increase | Increased dissolution rate | |||
| DL-tartaric acid | 5.3-fold increase | Increased dissolution rate | |||
| Hydrochlorothiazide | Nicotinic acid | 0.72 decrease | - | Formation of higher polarity cocrystals | [54] |
| Nicotinamide | 1.27 increase | ||||
| 4-aminobenzoic acid | 0.21 decrease | ||||
| Succinamide | 2.4-fold increase | ||||
| Resorcinol | 1.3-fold increase | ||||
| Itraconazole | Aspartic acid | 3.2-fold increase | Improved dissolution rate | Hydrogen-bonding interaction | [55] |
| Proline | 2.2-fold increase | ||||
| Serine | 2.5-fold increase | ||||
| Glycine | 2.3-fold increase | ||||
| Succinic acid | 1.6-fold increase | ||||
| Pyrazinamide | Adipic acid | 0.7-fold decrease | 0.6-fold decrease | Hydrogen-bonding interaction | [56] |
| Sebacic acid | 0.7-fold decrease | 0.4-fold decrease | |||
| trans-Aconitic acid | 1.6-fold increase | 1.8-fold increase | |||
| Citric acid | 1.2-fold increase | 1.4-fold increase |
| Drug | Coformer | Issue of Drug/Objective of the Study | Parameter Improved | Inference | Reference |
|---|---|---|---|---|---|
| Caffeine | Methyl gallate | Severe lamination and over compaction | Powder compaction and tensile strength | Presence of slip planes resulted in improved compaction properties | [59] |
| Paracetamol form II |
| Poor tablet-forming ability | Tensile strength, elastic constant and lattice energies, elastic compliance tensor | Layered structure of cocrystals leads to superior tablet formation ability | [58] |
| Theophylline | Methyl gallate | To examine the effects of cocrystallization on crystal mechanical properties | Elastic modulus, indentation values, crystal slip planes and Burger’s vector | Tableting performance theophylline > co-crystal > methyl gallate | [57] |
| Ibuprofen and Flurbiprofen | Nicotinamide | To demonstrate improvement of pharmaceutical properties over pure drug crystal | Powder compaction analysis (tensile strength) | Tabletability of cocrystal is apparently higher due to its higher bonding strength. | [63] |
| Paracetamol | Trimethyl glycine | Poor tablet-forming ability | Hardness and particle-size distribution | Improved compression properties due to structural stability and changed crystal face | [64] |
| Voriconazole |
| Too soft for tableting and compacting | Nanoindentation, elastic modulus and hardness | Hardness improved in the order of oxalic acid salt > cocrystals > drug | [65] |
| Lamotrigine |
| Poor flow properties and capping | Flow (angle of repose) and compression properties | All cocrystals except with ferulic acid showed improved flow properties; capping exhibited by cocrystal with salicylic acid was weak than that of ferulic acid | [66] |
| Paracetamol | 5-nitroisophthalic acid | Poor mechanical properties | Tabletability – tensile strength | Superior tabletability of cocrystal than the drug | [67] |
| Sulfadimidine | 4-aminosalicylic acid | To investigate the cocrystal habit engineering effect on compaction properties | Density and Carr’s compressibility index | Crystal habit engineering of cocrystals leads to improved flow properties | [68] |
| Adefovir Dipivoxil | Stearic acid | To investigate the enhanced powder properties of cocrystal | Compressibility (powder rheology analysis), Heckel analysis | Tabletability enhanced due to altered crystal habit by coformer | [69] |
| Drug | Coformer(s) | Stability Issue of Drug | Parameter Assessed | Inference | Reference |
|---|---|---|---|---|---|
| Caffeine |
| Crystalline powder of anhydrous caffeine transforms to caffeine hydrate at high RH | Physical stability at storage conditions of 0, 43, 75, and 98% RH up to 7 weeks. | No cocrystal hydrates have been found. Only oxalic acid-cocrystal exhibited physical stability till 7 weeks. The rest of cocrystals dissociated during storage. | [71] |
| Nitrofurantoin | 4-hydroxybenzoic acid | Photosensitive and physicochemically unstable | Physical, chemical, and photostability at different conditions for 13 weeks | Improved physicochemical and photostability compared to pure drug | [73] |
| Adefovir dipivoxil |
| Degradation by hydrolysis and dimerization during storage | Chemical stability at 60 °C (60% RH) and 40 °C (75% RH) for a month | Saccharin cocrystal was stable for one month whereas nicotinamide was not stable | [22] |
| Temozolomide |
| Spontaneous degradation during storage under normal conditions and is stable at pH < 5 but labile at pH > 7 | Chemical stability at 40 °C and 75% RH for 28 weeks | Inhibited the hydrolytic degradation of the drug as cocrystal by providing the acidic environment with organic acid coformers | [74] |
| Tranilast |
| Photochemically unstable | Subjected to 25 °C and 60% for 96 h | Photostability improved after the formation of cocrystal due to the increase in the distance between the drug molecules in the cocrystal | [75] |
| Acyclovir |
| Hydration of drug during storage | Physical stability at storage conditions of 0%, 43%, 75%, and 98% RH for 3 weeks | Cocrystal showed improved stability except with tartaric acid | [53] |
| Etoricoxib |
| Hemihydrate conversion of drug during manufacturing or upon exposure to moisture (30 min) | Exposed to water (slurry) conditions for hydration | Formed stable cocrystals by replacing the water molecule in the crystal lattice | [72] |
| Epalrestat | Betaine | Photo instability | Subjected to 25 °C for 24 h | Improved photostability of cocrystal due to decreased reaction cavity | [21] |
| Isoniazid |
| Reaction of isoniazid and rifampicin in fixed-dose combination | Physical stability of isoniazid in accelerated conditions | Stronger hydrogen bond interaction and cyclic O-H···O synthon in the crystal structure stabilized the cocrystal. | [76] |
| Flucytosine |
| Susceptible to hydration | Subjected to 70–75% RH and 90–95% RH at ambient temperature | Stable cocrystal may be due to strong acid–amide heterosynthon between drug and coformer | [77] |
| API Name | Coformer/API Name | Cocrystals | API:Coformer Ratio | Reference |
|---|---|---|---|---|
| Temozolomide (TMZ) | Nicotinamide (NCT) | TMZ-NCT Cocrystal | 2:1 | [85] |
| Isonicotinamide (INA) | TMZ-INA Cocrystal | 2:1 | ||
| Pyrazinamide (PYZ) | TMZ-PYZ Cocrystal | 1:1 | ||
| Saccharin (SAC) | TMZ-SAC Cocrystal | 2:1 | ||
| Caffeine (CAF) | TMZ-CAF Cocrystal | 1:1 | ||
| Aripiprazole (ARI) | Orcinol (ORC) | ARI-ORC Cocrystal | 1:1 | [86] |
| Catechol (CAT) | ARI-CAT Cocrystal | 1:1 | ||
| Resorcinol (RES) | ARI-RES Cocrystal | 1:1 | ||
| Phloroglucinol (PHL) | ARI-PHL Cocrystal | 1:1 | ||
| Favipiravir (FAV) | 4-hydroxybenzoic acid (4HBA) | FAV-4HBA Cocrystal | 1:1 | [87] |
| p-aminobenzoic acid (PABA) | FAV-PABA Cocrystal | 1:1 | ||
| Ferulic acid (FRA) | FAV-FRA Cocrystal | 1:1 | ||
| Gallic acid (GA) | FAV-GA Cocrystal | 1:1 | ||
| p-aminosalicylic acid (PAS) | Pyrazine (PYZ) | PAS-PYZ Cocrystal | 1:1 | [42] |
| Pyrimidine (PYM) | PAS-PYM Cocrystal | 1:1 | ||
| Pyridazine (PDZ) | PAS-PDZ Cocrystal | 2:1 | ||
| Phenazine (PHZ) | PAS-PHZ Cocrystal | 1:2 | ||
| 4,4′-dipyridyl disulfide (DPDS) | PAS-DPDS Cocrystal | 1:1 | ||
| 4-cyanopyridine (4-CYP) | PAS-4-CYP Cocrystal (9) | 1:1 | ||
| Curcumin (CUR) | Salicylic acid (SAA) | CUR-SAA Cocrystal | 1:2 | [88] |
| Hydroxyquinol (HXQ) | CUR-HXQ Cocrystal | 1:1, 1:2 | ||
| Resorcinol (RNL) | CUR-RNL Cocrystal | 1:1 | ||
| Pyrogallol (PYG) | CUR-PYG Cocrystal | 1:1 | ||
| 4,4′-bipyridine N, N′-dioxide (4,4 BPDO) | CUR-4,4 BPDO Cocrystal | |||
| Salicylic acid (SA) | Benzamide (BZ) | SA-BZ Cocrystal | 1:1, 1:2 | |
| Isonicotinamide (INA) | SA-INA Cocrystal | 1:1, 2:1 | ||
| Carbamazepine (CMP) | 4-aminobenzoic acid (4, ABA) | CMP-4 ABA Cocrystal | 1:1, 2:1, 4:1 | |
| Nicotinamide (NCT) | r-mandelic acid (r-MDLA) | NCT-r-MDLA Cocrystal | 1:2, 1:1, 4:1 | |
| Urea (UA) | Succinic acid (SA) | UA-SA Cocrystal | 1:1, 2:1 | |
| Indomethacin (IMC) | Saccharin (SAC) | IMC-SAC Cocrystal | ||
| CL-20 | Pyrazine (PYZ) | CL-20-PYZ Cocrystal | [23] | |
| Theobromine (TBR) | Trimesic acid (TMSA) | TBR-TMSA Cocrystal | 1:1 | [89] |
| Theophylline (TPH) | Trimesic acid (TMSA) | TPH-TMSA Cocrystal | 1:1 | |
| Caffeine (CAF) | Trimesic acid (TMSA) | CAF-TMSA Cocrystal | 1:2 | |
| Theobromine (TBR) | Hemimellitic acid (HMLA) | TBR-HMLA Cocrystal | 1:1 | |
| Theophylline (TPH) | Hemimellitic acid (HMLA) | TPH-HMLA Cocrystal | 1:1 | |
| Caffeine (CAF) | Hemimellitic acid (HMLA) | CAF-HMLA Cocrystal | 10:1 | |
| 5-Fluorouracil (5-FU) | Succinic acid (SA) | 5-FU-SA Cocrystal | 1:1 | [90] |
| Phenazine (PHZ) | 5-FU-PHZ Cocrystal | 2:1 | ||
| Acridine (ACD) | 5-FU-ACD Cocrystal | 2:1 | ||
| Benzoic acid (BA) | 5-FU-BA Cocrystal | 1:1 | ||
| Malic acid (MA) | 5-FU-MA Cocrystal | 1:1 | ||
| Cinnamic acid (CA) | 5-FU-CA Cocrystal | 1:1 | ||
| 4,4-bispyridylethene (4,4 BPYE) | 5-FU-4,4 BPYE Cocrystal | 4:1 | ||
| p-aminopyridine (p-APY) | 5-FU-p-APY Cocrystal | |||
| Rivaroxaban (RVB) | Malonic Acid (MA) & Oxalic Acid (OA) | RVB-MA Cocrystals and RVB-OA Cocrystals | 1:1, 2:1 | [91] |
| p-hydroxybenzoic acid (pHBA) | RVB-pHBA Cocrystals | 1:1 | [92] | |
| Isonicotinamide (INTA) | RVB-INTA Cocrystal | 1:1 | ||
| Nicotinamide (NTA) | RVB-NTA Cocrystal | 1:1 | ||
| 2,4 dihydroxybenzoic acid (2,4 DHBA) | RVB-2,4 DHBA Cocrystals | 1:1 | ||
| Succinic acid (SA) | RVB-SA Cocrystals | 1:1 | ||
| Malonic Acid (MA) | RVB-MA Cocrystals | 2:1 | [93] | |
| Malonic Acid (MA) | RVB-MA Cocrystals | 2:1 | [94] | |
| Lamotrigine (LTG) | Phthalimide (PTA) | LTG-PTA Cocrystals | 1:1 | [95] |
| Succinic acid (SA) | LTG-SA Cocrystals | |||
| Pyromellitic diimide (PDA) | LTG-PDA Cocrystals | 1:1 | [96] | |
| Caffeine (CAF) | LTG-CAF Cocrystals | 2:1 | ||
| Isophthaldehyde (IPA) | LTG-IPA Cocrystals | 1:1 | ||
| Glutarimide (GTA) | LTG-GTA Cocrystals | 1:1 | [97] | |
| Phenobarbital (PBT) (Multi drug cocrystals) | LTG-PBT Cocrystals | 1:3, 3:1 | [98] | |
| 2,2′-bipyridine (2,2 BPYD) | LTG-2,2 BPYD Cocrystals | 1:1.5 | [99] | |
| 4,4′-bipyridine (4,4 BPYD) | LTG-4,4 BPYD Cocrystals | 2:1 | ||
| Nicotinamide monohydrate (NTAM) | LTG-NTAM Cocrystal | 1:1:1 | [100] | |
| Acetamide (ACT) | LTG-ACT Cocrystal | 1:1 | ||
| Acetic acid (ATA) | LTG-ATA Cocrystal | 1:3 | ||
| 4-hydroxy-benzoic acid (4 HBA) | LTG-4 HBA Cocrystal | 1:1 | ||
| Saccharin (SAC) | LTG-SAC Cocrystal | 1:1 | ||
| Etodolac (ETD) | 4-amino benzoic acid (4 ABA) | ETD-4 ABA Cocrystal | 1:1 | [101] |
| Glutaric acid (CA) | ETD-GA Cocrystal | 1:2 | [102] |
| Drug Name | Approval | Components | Dosage Form | Indication | Manufacturer | Ref. No. |
|---|---|---|---|---|---|---|
| Depakote® | U.S. FDA 1983 | Valproic acid + Valproate sodium | Tablet, Capsule | Epilepsy | Abbott Laboratories, Illinois, United States | [15,16,103,104,105,106] |
| Entresto® | U.S. FDA 2015 | Sacubitril sodium + Valsartan sodium | Tablet | Heart failure | Novartis, Basel, Switzerland | [15,16,107] |
| Suglat® | Japan 2014 | Ipragliflozin + L-proline | Tablet | Diabetes | Kotobuki Pharmaceuticals, Nishina, Shizuoka, Japan and Astellas Pharma, Tokyo, Japan | [15,16,105,108] |
| Steglatro® | U.S. FDA 2017 | Ertugliflozin + L-pyroglutamic acid | Tablet | Diabetes | Pfizer, New York, United States | [15,105,109,110] |
| Lexapro® | U.S. FDA 2002 | Escitalopram oxalate + Oxalic acid | Tablet | Anxiety and depression | Allergan Inc., Dublin, Ireland | [15,16,111,112] |
| ESIX-10® | U.S. FDA 2009 | Escitalopram oxalate + Oxalic acid | Tablet | Anxiety and depression | Sag Health Science Pvt Ltd., New Delhi, India | |
| Beta-chlor® | U.S. FDA 1963 | Chloral hydrate + Betaine | Tablet | Sedation | Mead Johnson, Illinois, United States | [15,105] |
| Cafcit® | U.S. FDA 1999 | Caffeine + Citric acid | Injection | Infantile apnoea | Hikma Pharmaceuticals Plc, London, United Kingdom | [16,105,113] |
| Zafatek® | Japan 2015 | Trelagliptin + Succinic acid | Tablet | Diabetes | Takeda Pharmaceutical Company Limited, Tokyo, Japan | [16,114] |
| Lamivudine/zidovudine Teva ® | EMA 2011 | Lamivudine + Zidovudine | Tablet | HIV infection | Teva Pharma B.V., Tel Aviv-Yafo, Israel | [16,115,116,117] |
| Abilify ® | U.S. FDA 2002 | Aripiprazole + Fumaric acid | Tablet | Schizophrenia | Otsuka Pharmaceuticals, Tokyo, Japan | [128,129,135] |
| Odomzo® | U.S. FDA 2015 | Sonidegib + Phosphoric acid | Capsule | Basal Cell Carcinoma | Sun Pharma Global, Mumbai, India. | [118,119,120] |
| Mayzent® | U.S. FDA 2019 | Siponimod + Fumaric acid | Tablet | Multiple Sclerosis | Novartis, Basel, Switzerland | [121,122,123] |
| Seglentis® | U.S. FDA 2021 | Celecoxib + Tramadol | Tablet | Acute Pain | Kowa Pharmaceuticals, Alabama, United States | [122,124] |
| Dimenhydrinate | U.S. FDA 1982 (ANDA) | Diphenhydramine and 8-chlorotheophylline | Tablet | Motion sickness | Watson Laboratories Inc., New Jersey, United States | [17,125,126] |
| Ibrutinib fumaric acid cocrystals | Tentative approval | Ibrutinib + Fumaric acid | NA | Cancer | Teva Pharmaceutical Industries Ltd., Tel Aviv-Yafo, Israel | [15,127] |
| E-58425 (Clinical Trial Phase 3) | Approval Pending | Celecoxib and racemic tramadol hydrochloride | NA | Management of acute pain | Patented by Laboratorios Del., La Paz, Bolivia Development done by Enantia and Esteve, R&D, Spain | [128,129,130,131,132] |
| TAK-020 (Clinical Trial Phase 1) | Approval Pending | TAK-020 and Gentisic acid | NA | Rheumatoid arthritis | Takeda Pharmaceuticals, Tokyo, Japan | [128,129,133,134] |
| Coformer | Fumaric Acid [136,137,138,139] | Oxalic Acid [91,140,141] | Succinic acid [142] | Citric Acid [143,144] |
|---|---|---|---|---|
| Physical state | Colorless crystalline solid | Colorless crystalline solid | Colorless, odorless white crystals | Colorless crystalline solid |
| Melting point | 287 °C | 189.5 °C | 185–187 °C | 153 °C |
| Solubility in solvents | Soluble in ethanol, concentrated sulfuric acid. Slightly soluble in ethyl ether, acetone. Insoluble in chloroform and benzene. |
Very soluble in ethanol. Slightly soluble in ether. Insoluble in benzene, chloroform, and petroleum ether. | Slightly soluble in ethanol, ether, acetone, glycerin. Not soluble in benzene, carbon sulfide, carbon tetrachloride. | Freely soluble in ethanol. Insoluble in benzene, chloroform, carbon tetrachloride, toluene, and carbon disulfide. |
| Solubility in water | 7 g/L (25 °C) | 220 mg/mL (25 °C) | Soluble (71 mg/mL) | 592 mg/mL (20 °C) |
| Molar mass | 116.07 | 90.03 | 118.09 | 192.1 |
| Density | 1.64 g/cm3 | 1.9 g/cm3 | 1.56 g/cm3 | 1.66 g/cm3 |
| pKa | 3.03 | 1.2 | 4.24 | 2.79 |
| No. of hydrogen bond donors | 2 | 2 | 2 | 4 |
| No. of hydrogen bond acceptors | 4 | 4 | 4 | 7 |
| Stability | Stable under ambient conditions | Stable under ambient conditions | Stable under ambient conditions | Moisture-sensitive |
| Cocrystal | Method of Preparation | Impact on Solubility | Impact on Dissolution Rate | Impact on Bioavailability | Impact on Stability | References |
|---|---|---|---|---|---|---|
| Berberine–Fumaric acid (2:1) | Slurry method | ~9.5-fold at 15 min # | ~3.75-fold | ──── | Reduced hygroscopicity | [7] |
| Promethazine hydrochloride–Fumaric acid (2:1) | Mechanochemistry | Improved | ──── | ──── | Improved | [146] |
| Slow solvent evaporation | ||||||
| Gabapentin-lactam–Fumaric acid (1:1) | Reaction crystallization method | Improved | ──── | ──── | ──── | [147,158] |
| Fluoxetine HCl–Fumaric acid (2:1) | Cooling crystallization | Increased ~2-fold | No improvement | ──── | ──── | [151] |
| Enoxacin–Fumaric acid (1:2) @ | Slow solvent evaporation | 9.8-fold | 8.9-fold | ──── | ──── | [150] |
| Meloxicam–Fumaric acid (1:1) | Liquid-assisted grinding | 33–84% improvement | ──── | ──── | ──── | [159] |
| Sildenafil–Fumaric acid (1:2 and 1:3) | Slow solvent evaporation | Increased 5-fold | ──── | ──── | ──── | [156] |
| PEC–Fumaric acid (1:1) | Anti-solvent addition | Increased 4-fold | Improved | Improved | ──── | [160] |
| Glipizide–Fumaric acid (1:1) | Liquid-assisted grinding | Increased ~2.3-fold | Increased ~2-fold | Improved | ──── | [161] |
| Acyclovir–Fumaric acid (1:1) | Slow solvent evaporation & liquid-assisted grinding | Increased ~5.5-fold | Increased ~2-fold | ──── | Improved | [53] |
| Ketoconazole–Fumaric acid (1:1, 1:2, and 1:3) | Slow solvent evaporation | Increased ~1.6-fold | Improved ~1.65-fold | ──── | Improved | [157] |
| Acyclovir–Fumaric acid (1:1) | Dry grinding or co-grinding | Less effect | Increased ~2.2-fold | ──── | ──── | [162] |
| Ethenzamide–Fumaric acid (2:1) | Slow solvent evaporation | Increased 3.84-fold | Increased 1.71-fold | ──── | ──── | [163] |
| Fluconazole–Fumaric acid (1:1) | Slow solvent evaporation | Increased ~2.5-fold # | Improved | ──── | Improved | [164] |
| Efavirenz–Fumaric acid (1:1) | Neat grinding | Increased ~26-fold | Increased ~2-fold | ──── | ──── | [165] |
| Cocrystal | Method of Preparation | Impact on Solubility | Impact on Dissolution rate | Impact on Bioavailability | Impact on Stability | References |
|---|---|---|---|---|---|---|
| Promethazine HCl–Oxalic acid (2:1) | Slow solvent evaporation | Improved | ──── | ──── | Improved | [146] |
| Telmisartan–Oxalic acid | ──── | Increased 7-fold | Increased ~2.4-fold | ──── | ──── | [122,169] |
| Rebamipide–Oxalic acid (1:1) | Liquid-assisted grinding | Increased 7.29-fold | Increased 7.19-fold | Increased 1.6-fold | ──── | |
| Rivaroxaban–Oxalic acid (1:1) | Anti-solvent addition | Improved | Increased ~1.6-fold | Increased ~2.12-fold | ──── | [91] |
| Apixaban–Oxalic acid (4:3) | ──── | Increased approx. 2-fold | ──── | Enhanced 2.7-fold | ──── | [166] |
| Temozolomide–Oxalic acid (2:1) | ──── | ──── | ──── | ──── | Improved | [167] |
| Xanthotoxin–Oxalic acid (2:1) | Liquid assisted grinding & slow solvent evaporation | Increased 1.6-fold | Increased ~1.1-fold | ──── | Improved | [168] |
| Telmisartan–Oxalic acid (1:1) | Solvent-drop grinding & solvent evaporation method | Increased 11.7-fold | Increased ~7.2-fold | ──── | ──── | [170] |
| Caffeine–Oxalic acid (2:1) | Solvent precipitation and ultrasound-assisted solution cocrystallization | ──── | ──── | ──── | Improved | [171] |
| Glibenclamide–Oxalic acid (1:2) | Thermal method | Increased ~2.7-fold | Increased ~1.7-fold | ──── | ──── | [172] |
| API-Coformer | Method of Preparation | Impact on Solubility | Impact on Dissolution Rate | Impact on Bioavailability | Impact on Stability | References |
|---|---|---|---|---|---|---|
| Itraconazole–Succinic acid | Liquid anti-solvent | ──── | F1—Achieved 50% release in 2 h | ──── | Improved | [178] |
| Gas anti-solvent | F2—Achieved 92% release in 2 h | |||||
| Piperine–Succinic acid | Wet-milling | Increased 12.70-fold | Achieved 53.281% release in 1 h | ──── | Improved | [179] |
| Carbamazepine–Succinic acid (2:1) | Slurry crystallization | Improved | F1—Achieved 82% release in 1 h F2—Achieved 95% release in 1 h F3—Achieved 95% release in 1 h | Improved | Improved | [2] |
| Isoniazid–Succinic acid (2:1) | Slow solvent evaporation | ──── | Improved | ──── | ──── | [180] |
| Imidazopyridazine- Succinic acid (1:1) | Neat grinding method | Improved | Improved | ──── | ──── | [181] |
| Brexpiprazole–Succinic acid (1:1) | Solvent-drop grinding method | Increased 1.59-fold | ──── | ──── | ──── | [182] |
| Aripiprazole–Succinic acid | Hot melt extrusion (HME) | ──── | Improved | ──── | ──── | [183] |
| Ketoconazole–Succinic acid (1:1) | Reaction crystallization method | ──── | Decreased with the low pH of coformer | ──── | ──── | [184] |
| Abiraterone acetate–Succinic acid (2:1) | Solvent evaporation | ──── | Increased 4.7-fold | ──── | Improved | [185] |
| Acyclovir–Succinic acid (1:1) | Grinding method | Dissolution efficiency– 54.23% (grinding time 15 min) | ──── | ──── | [186] | |
| Slurry crystallization | Dissolution efficiency – 74.36% (solvent concentration 12 mL/g) | ──── | ──── | |||
| Fluoxetine HCl–Succinic acid (2:1) | Slow solvent evaporation | Increased ~1.5-fold | Increased 3-fold | ──── | ──── | [151] |
| Cocrystals | Method of Preparation | Impact on Solubility | Impact on Dissolution rate | Impact on Bioavailability | Impact on Stability | References |
|---|---|---|---|---|---|---|
| Rebamipide–Citric acid (1:1) | Liquid-assisted grinding | Increased 12.58-fold | Increased ~13.2-fold | Increased 2.5-fold | ──── | [169] |
| Metformin hydrochloride–Citric acid (1:1) | Solution crystallization, neat grinding, and liquid-assisted grinding | Increased 1–4-fold | ──── | Improved | ──── | [143] |
| Berberine chloride- Citric acid (1:1) | Liquid-assisted grinding | Improved | ──── | ──── | Improved | [6] |
| Theophylline–Citric acid (1:1) | Neat co-grinding | ──── | ──── | ──── | Improved | [189] |
| Dapagliflozin propanediol monohydrate–Citric acid (1:1) | Solution crystallization method | Improved | Increased ~1-fold | ──── | Improved | [190] |
| Pyrazinamide–Citric acid (1:1) | Slow solvent evaporation | Increased ~1.1-fold | Increased ~1.4-fold | ──── | ──── | [56] |
| Ethyl p-methoxycinnamate–Citric acid (1:1, 1:2, 1:3) | Liquid-assisted grinding | Increased 1.4-fold | ──── | ──── | ──── | [192] |
| Simvastatin–Citric acid (1:1) | Liquid-assisted grinding, slow solvent evaporation | Increased 1.4–3-fold | ──── | Improved | Improved | [193] |
| Nefiracetam–Citric acid (2:1) | Slow solvent evaporation | Increased ~1.4-fold | Improved | ──── | ──── | [195] |
| Ritonavir–Citric acid (1:2) | Dry grinding method | Improved | Improved | ──── | ──── | [196] |
| Nitrofurantoin–Citric acid (1:1) | Liquid-assisted grinding | Improved | ──── | ──── | Improved | [197] |
| Praziquantel–Citric acid (1:1) | Liquid-assisted grinding | Increased ~2- 4-fold | Improved | ──── | ──── | [198] |
| S. No. | Parameter | Fumaric Acid | Oxalic Acid | Succinic Acid | Citric Acid |
|---|---|---|---|---|---|
| 1. | Solubility | Improved | Improved | Improved | Improved |
| 2. | Dissolution rate | Improved | Improved | Improved | Improved |
| 3. | Permeability | Improved | No Impact | No Impact | No Impact |
| 4. | Bioavailability | Improved | Improved | Improved | Improved |
| 5. | Stability | Improved | Improved | Improved | Improved |
| 6. | No. of commercialized cocrystals | Two | Two | One | One |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, M.; Barua, H.; Jyothi, V.G.S.S.; Dhondale, M.R.; Nambiar, A.G.; Agrawal, A.K.; Kumar, P.; Shastri, N.R.; Kumar, D. Cocrystals by Design: A Rational Coformer Selection Approach for Tackling the API Problems. Pharmaceutics 2023, 15, 1161. https://doi.org/10.3390/pharmaceutics15041161
Singh M, Barua H, Jyothi VGSS, Dhondale MR, Nambiar AG, Agrawal AK, Kumar P, Shastri NR, Kumar D. Cocrystals by Design: A Rational Coformer Selection Approach for Tackling the API Problems. Pharmaceutics. 2023; 15(4):1161. https://doi.org/10.3390/pharmaceutics15041161
Chicago/Turabian StyleSingh, Maan, Harsh Barua, Vaskuri G. S. Sainaga Jyothi, Madhukiran R. Dhondale, Amritha G. Nambiar, Ashish K. Agrawal, Pradeep Kumar, Nalini R. Shastri, and Dinesh Kumar. 2023. "Cocrystals by Design: A Rational Coformer Selection Approach for Tackling the API Problems" Pharmaceutics 15, no. 4: 1161. https://doi.org/10.3390/pharmaceutics15041161