Antiplasmodial Activity of Hydroalcoholic Extract from Jucá (Libidibia ferrea) Pods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Botanical Material
2.2. Chromatographic Analysis
2.3. Solubilization of the Compound for Tests of Biological Activity
2.4. Cultivation of Human Cell Lines
2.5. Cytotoxicity Assays
2.6. Assessment of Hemolytic Activity
2.7. In Vitro Culture of Plasmodium spp.
2.8. In Vitro Culture of Intraerythrocytic Stages of Plasmodium falciparum
2.9. Determination of Parasitemia
2.10. Synchronization of Plasmodium falciparum Cultivation
2.11. In Vitro Schizonticidal Testing with Plasmodium falciparum
2.12. Selectivity Index
2.13. Statistical Analysis
3. Results
3.1. Chromatographic Analysis
3.2. Antiplasmodial Activity and Cytotoxicity
3.3. Hemolytic Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reiners, A.A.O.; Azevedo, R.C.d.S.; Ricci, H.A.; de Souza, T.G. User Adherence and Reactions to Malaria Treatment: Implications for Health Education. Texto E Contexto Enferm. 2010, 19, 536–544. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Meneguetti, D.U.d.O.; Cunha, R.M.da; Lima, R.A.; Oliveira, F.A.d.S.; de Medeiros, D.S.S.; Passarini, G.M.; Medeiros, P.S.d.M.d.; Militão, J.S.L.T.; Facundo, V.A. Antimalarial Ethnopharmacology in the Brazilian Amazon. Rev. De Cienc. Farm. Basica E Apl. 2014, 35, 385–392. [Google Scholar]
- Gomes, A.P.; Vitorino, R.R.; Costa, A.D.P.; De Mendonça, E.G.; Oliveira, M.G.D.A.; Siqueira-Batista, R. Severe Plasmodium Falciparum Malaria. Rev. Bras. Ter. Intensiv. 2011, 23, 358–369. [Google Scholar] [CrossRef] [Green Version]
- França, T.C.C.; Dos Santos, M.G.; Figueroa-Villar, J.D. Malária: Aspectos Históricos e Quimioterapia. Quim Nova 2008, 31, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Ariey, F.; Witkowski, B.; Amaratunga, C.; Beghain, J.; Langlois, A.C.; Khim, N.; Kim, S.; Duru, V.; Bouchier, C.; Ma, L.; et al. A Molecular Marker of Artemisinin-Resistant Plasmodium Falciparum Malaria. Nature 2014, 505, 50–55. [Google Scholar] [CrossRef]
- Schulz, V.; Hänsel, R.; Tyler, V.E.; Schulz, V.; Hänsel, R.; Tyler, V.E. Medicinal Plants, Phytomedicines, and Phytotherapy. In Rational Phytotherapy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–42. [Google Scholar]
- Silva, É.B.S.; Barata, L.E.S.; Arevalo, M.R.; Vieira, L.Q.; Castro, W.; Ruiz, A.L.T.G.; Torre, A.della; Castro, K.C.F.; Sartoratto, A.; Baratto, L.C.; et al. Chemical Composition and Antiproliferative Activity of the Ethanolic Extract of Cyperus Articulatus L. (Cyperaceae). Plants 2021, 10, 2084. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, J.S., Jr.; da Silva, É.B.S.; Moraes, T.M.P.; Kasper, A.A.M.; Sartoratto, A.; Baratto, L.C.; de Oliveira, E.C.P.; Oliveira, E.; Barata, L.E.S.; Minervino, A.H.H.; et al. Anti-Inflammatory Potential of the Oleoresin from the Amazonian Tree Copaifera Reticulata with an Unusual Chemical Composition in Rats. Vet. Sci 2021, 8, 320. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural Products as Sources of New Drugs over the Period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Calixto, J.B.; Scheidt, C.; Otuki, M.; Santos, A.R.S. Biological Activity of Plant Extracts: Novel Analgesic Drugs. Expert Opin. Emerg. Drugs 2001, 6, 261–279. [Google Scholar] [CrossRef]
- Delmacia, G.M.; Daiany, A.R.; Henrique, D.M.C.; Irwin, R.A.M.; Marta, M.A.S. Práticas Terapêuticas Tradicionais: Uso e Conhecimento de Plantas Do Cerrado No Estado de Pernambuco (Nordeste Do Brasil). Bol. Latinoam. Y Del Caribe De Plantas Med. Y Aromat. 2015, 14, 491–508. [Google Scholar]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas Nativas Do Brasil; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2002; ISBN 8586714143. [Google Scholar]
- De Freitas, A.C.C. Atividades Biológicas de Preparações Obtidas de Libidibia (Caesalpinia) Ferrea Var. Parvifolia (Mart. Ex Tul.) LP Queiroz; Universidade Federal de Pernambuco: Recife, Brazil, 2012. [Google Scholar]
- Oliveira, A.F.; Batista, J.S.; Paiva, E.S.; Silva, A.E.; Farias, Y.J.M.D.; Damasceno, C.A.R.; Brito, P.D.; Queiroz, S.A.C.; Rodrigues, C.M.F.; Freitas, C.I.A. Avaliação Da Atividade Cicatrizante Do Jucá (Caesalpinia Ferrea Mart. Ex Tul. Var. Ferrea) Em Lesões Cutâneas de Caprinos. Rev. Bras. De Plantas Med. 2010, 12, 302–310. [Google Scholar] [CrossRef] [Green Version]
- Paiva, W.D.S.; Neto, F.E.D.S.; Bandeira, M.G.L.; Abrantes, M.R.; De Lima Batista, A.C.; Da Silva, J.B.A. Atividade Antibacteriana Da Casca Do Jucá (Libidibia Ferrea (Mart. Ex Tul.) l. p. Queiroz), Frente a Staphylococcus Spp. Isolados Do Leite de Cabras Com Mastite. Arch. Veter. Sci. 2015, 20, 141–146. [Google Scholar] [CrossRef]
- de Sousa, M.J.B. Evaluation of the Genotoxic and Mutagenic Potential of Standardized Extracts of Caesalpinia Ferrea (Jucá) and Brosimum Gaudi-chaudii (Inharé). Master’s Dissertation, Stricto Sensu Graduate Program in Genetics, Pontifícia Universidade Católica de Goiás, Goiania, Brazil, 2017. [Google Scholar]
- Gonzalez, F.G. Estudo Farmacognóstico e Farmacológico de Caesalpinia Ferrea Martius; Universidade de São Paulo: São Paulo, Brazil, 2005. [Google Scholar]
- Borges, C.d.S.; Cuchiara, C.C.; Maculan, K.; Sopezki, M.d.S.; Bobrowski, V.L. Descrição Morfológica Da Plântula e Diásporos de Caesalpinia Ferrea Mart. Rev. Bras. De Biociências 2007, 5, 747–749. [Google Scholar]
- Ferreira, M.R.A. Triagem Antifúngica de Extratos Obtidos de Espécies Vegetais do Nordeste Brasileiro; Universidade Federal do Rio Grande do Norte: Natal, Brazil, 2012. [Google Scholar]
- Oliveira, I.V.P.D.M.; Dias, R.V.D.C.; Calado, E.; Lucena, R.; Costa, A.L.; Sakamoto, S.M.; Pimentel, M.M.L. Evaluation Macroscopically Scar of the String Bean and the Hull of the Caesalpinia Ferrea (Tul.) Martius (“Jucá”) on the Cutaneous Wounds of the Asinines (Equus Asinus). Acta Veter. Bras. 2014, 8, 129–135. [Google Scholar] [CrossRef]
- Magalhães, L.S.; Pussente, G.; Rodrigues de Azevedo, L.; Maria S Crespo, J.R. Avaliação Da Atividade Antibacteriana Do Extrato de Caesalpinia Ferrea Martius e Desenvolvimento de Uma Formulação Fitocosmética. Rev. Científica Da Faminas 2015, 1, 11. [Google Scholar]
- Henrique, C.H. Avaliação Da Atividade Antimicrobiana e Moduladora Do Extrato Etanólico de Libidibia Ferrea (Mart. Ex Tul.) L.P. Queiroz. Rev. Cuba. Plantas Med. 2016, 21, 71–82. [Google Scholar]
- Kobayashi, Y.T.d.S.; de Almeida, V.T.; Bandeira, T.; de Alcántara, B.N.; da Silva, A.S.B.; Barbosa, W.L.R.; da Silva, P.B.; Monteiro, M.V.B.; de Almeida, M.B. Avaliação Fitoquímica e Potencial Cicatrizante Do Extrato Etanólico Dos Frutos de Jucá (Libidibia Ferrea) Em Ratos Wistar. Braz. J. Veter. Res. Anim. Sci. 2015, 52, 34–40. [Google Scholar] [CrossRef]
- Américo, Á.V.L.D.S.; Nunes, K.M.; Assis, F.F.V.; Dias, S.R.; Passos, C.T.S.; Morini, A.C.; Araújo, J.A.; Castro, K.C.F.; Escher, S.K.S.; Barata, L.E.S.; et al. Efficacy of Phytopharmaceuticals from the Amazonian Plant Libidibia Ferrea for Wound Healing in Dogs. Front. Veter. Sci. 2020, 7, 244. [Google Scholar] [CrossRef]
- Yarramraju, S.; Akurathi, V.; Wolfs, K.; Van Schepdael, A.; Hoogmartens, J.; Adams, E. Investigation of sorbic acid volatile degradation products in pharmaceutical formulations using static headspace gas chromatography. J. Pharm. Biomed. Anal. 2007, 44, 456–463. [Google Scholar] [CrossRef]
- Patra, N.; De, U.; Kang, J.A.; Kim, J.M.; Ahn, M.Y.; Lee, J.; Jung, J.H.; Chung, H.Y.; Moon, H.R.; Kim, H.S. A Novel Epoxypropoxy Flavonoid Derivative and Topoisomerase II Inhibitor, MHY336, Induces Apoptosis in Prostate Cancer Cells. Eur. J. Pharm. 2011, 658, 98–107. [Google Scholar] [CrossRef]
- Pereira, J.R.C.S.; Hilário, F.F.; Lima, A.B.; Silveira, M.L.T.; Silva, L.M.; Alves, R.B.; de Freitas, R.P.; Varotti, F.P.; Viana, G.H.R. Cytotoxicity Evaluation of Marine Alkaloid Analogues of Viscosaline and Theonelladin C. Biomed. Prev. Nutr. 2012, 2, 145–148. [Google Scholar] [CrossRef]
- Matsuzaki, W.S.; Rodrigues, F.C.M.; Malheiros, C.A.; Rahal, F. Uso de Teste de Químio-Sensibilidade Para Escolha Da Quimioterapia Adjuvante No Câncer Gástrico Avançado. Rev. Col. Bras. Cir. 2006, 33, 228–234. [Google Scholar] [CrossRef]
- Park, J.G.; Kramer, B.S.; Carmichael, J.; Minna, J.D.; Gazdar, A.F.; Steinberg, S.M.; Collins, J.M. Chemosensitivity Testing of Human Colorectal Carcinoma Cell Lines Using a Tetrazolium-Based Colorimetric Assay. Cancer Res. 1987, 47, 5875–5879. [Google Scholar]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a Tetrazolium-Based Semiautomated Colorimetric Assay: Assessment of Radiosensitivity. Cancer Res. 1987, 47, 943–946. [Google Scholar] [PubMed]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Modifications to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Assis, F.F.V.; Silva, N.C.; Moraes, W.P.; Barata, L.E.S.; Minervino, A.H.H. Chemical Composition and in Vitro Antiplasmodial Activity of the Ethanolic Extract of Cyperus Articulatus Var. Nodosus Residue. Pathogens 2020, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, A.; Manzoor, N.; Khan, L.A. Evolution of Ergosterol Biosynthesis Inhibitors as Fungicidal against Candida. Microb. Pathog. 2010, 48, 35–41. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human Malaria Parasites in Continuous Culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Moreno-Pérez, D.A.; Ruíz, J.A.; Patarroyo, M.A. Reticulocytes: Plasmodium Vivax Target Cells. Biol. Cell 2013, 105, 251–260. [Google Scholar] [CrossRef]
- Aguiar, A.C.C.; de Santos, R.M.; Figueiredo, F.J.B.; Cortopassi, W.A.; Pimentel, A.S.; França, T.C.C.; Meneghetti, M.R.; Krettli, A.U. Antimalarial Activity and Mechanisms of Action of Two Novel 4-Aminoquinolines against Chloroquine-Resistant Parasites. PLoS ONE 2012, 7, e37259. [Google Scholar] [CrossRef] [Green Version]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium Falciparum Erythrocytic Stages in Culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and Inexpensive Fluorescence-Based Technique for High-Throughput Antimalarial Drug Screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, K.S.; Sahasrabudhe, K.; Lawlor, M.D.; Wilson, S.L.; Lang, C.H.; Scheuchenzuber, W.J. In Vitro and In Vivo Inhibition of LPS-Stimulated Tumor Necrosis Factor-α Secretion by the Gallotannin β-d-Pentagalloylglucose. Bioorganic Med. Chem. Lett. 2001, 11, 1813–1815. [Google Scholar] [CrossRef] [PubMed]
- Al-Halabi, R.; Chedid, M.B.; Merhi, R.A.; El-Hajj, H.; Zahr, H.; Schneider-Stock, R.; Bazarbachi, A.; Gali-Muhtasib, H. Gallotannin Inhibits NFĸB Signaling and Growth of Human Colon Cancer Xenografts. Cancer Biol. Ther. 2011, 12, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salas, M.G.; Zugasti Cruz, A.; Yesenia, S.; Belmares, S.; Urdiales, B.V.; Herrera, R.R.; Noé, C.; González, A.; Chávez, J.M. Actividad Anticancerígena Del Ácido Gálico En Modelos Biológicos in Vitro. Acta Química Mex. 2013, 5, 5–11. [Google Scholar]
- GIll, R. Pharmacological studies on the gastrointestinal and behavioral effects of lupeol and valoneic acid dilactone, isolated from Cenostigma macrophyllum Tul., in rodents. Ph.D. Thesis, Faculty of Medicine, Federal University of Ceará, Fortaleza, Brazil, 2010. [Google Scholar]
- Amorim, C.M. Desenvolvimento de Sistemas de Liberação Nanoemulsionados Mucoadesivos Contendo Ácido Elágico Para Administração Nasal Visando o Tratamento Da Doença de Alzheimer. Ph.D. Thesis, Universidade Federal de Santa Catarina, Centro Centro de Ciências da Saúde, Programa Programa de Pós-Graduação em Nanotecnologia Farmacêutica, Florianópolis, Brazil, 2014. [Google Scholar]
- Lima, K.G. Avaliação Do Efeito Do Ácido Gálico No Tratamento De Células De Hepatocarcinoma Hepg2. Pontifícia Univ. Católica Do Rio Gd. Do Sul 2014, 1, 57. [Google Scholar]
- Santana, L.S. Ellagic Acid and Its Role in Cancer Prevention and Treatment: Integrative Review [Ácido Elágico e Seu Papel na Prevenção e No Tratamento do Câncer: Revisão Integrativa; Monography Faculdade Maria Milza, Bacharelado em Nutrição: Governador Mangabeira, Brazil, 2020. [Google Scholar]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, Antimalarial and Antimicrobial Activities of Tannin-Rich Fractions, Ellagitannins and Phenolic Acids from Punica granatum L. Planta Medica 2007, 73, 461–467. [Google Scholar] [CrossRef]
- Venancio, V.P.; Abrão, L.C.; Kim, H.; Talcott, S.T.; Mertens-Talcott, S.U. In Vitro Antimalarial Activity of Microbial Metabolites from Mango Tannins (Mangifera Indica L.). FASEB J. 2016, 30, 916-6. [Google Scholar] [CrossRef]
- Bankole, A.E.; Adekunle, A.A.; Sowemimo, A.A.; Umebese, C.E.; Abiodun, O.; Gbotosho, G.O. Phytochemical Screening and in Vivo Antimalarial Activity of Extracts from Three Medicinal Plants Used in Malaria Treatment in Nigeria. Parasitol. Res. 2016, 115, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Lutgen, P. Tannins in Artemisia: The Hidden Treasure of Prophylaxis. Pharm. Pharmacol. Int. J. 2018, 6, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.P.M.; Lautu, D.; Tavul, L.; Hackett, S.L.; Siba, P.; Karunajeewa, H.A.; Ilett, K.F.; Mueller, I.; Davis, T.M.E. In Vitro Sensitivity of Plasmodium Falciparum to Conventional and Novel Antimalarial Drugs in Papua New Guinea. Trop. Med. Int. Health 2010, 15, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.C.; Gonçalves, S.F.; de Araújo, L.S.; Kasper, A.A.M.; da Fonseca, A.L.; Sartoratto, A.; Castro, K.C.F.; Moraes, T.M.P.; Baratto, L.C.; Varotti, F.d.P.; et al. In Vitro and in Vivo Antimalarial Activity of the Volatile Oil of Cyperus Articulatus (Cyperaceae). Acta Amaz. 2019, 49, 334–342. [Google Scholar] [CrossRef] [Green Version]
- Alkadi, H.O. Antimalarial Drug Toxicity: A Review. Chemotherapy 2007, 53, 385–391. [Google Scholar] [CrossRef]
- Moreno, A.D.H.; Possebon, L.; Sant’ana, M.; Ribeiro Souza, H.; Pilon, M.M.I.; Girol, A.P. Avaliação Da Atividade Antimicrobiana e Citotoxicidade Hemolítica Em Diferentes Extratos Vegetais. Arq. De Ciências Da Saúde 2018, 25, 11. [Google Scholar] [CrossRef] [Green Version]
- Pasquini-Netto, H.; Manente, F.A.; Moura, E.L.; Regasini, L.O.; Pinto, M.E.F.; Bolzani, V.S.; Oliveira, O.M.M.F.; Vellosa, J.C.R. Avaliação Das Atividades Antioxidante, Anti e Pró-Hemolítica Do Extrato Etanólico Das Folhas de Pterogyne Nitens Tul. (Fabaceae-Caesalpinioideae). Rev. Bras. De Plantas Med. 2012, 14, 666–672. [Google Scholar] [CrossRef] [Green Version]
- Da Paz, I.P.; Coelho, A.A.M.; Do Nascimento, S.P.; De Sá Oliveira, S.A.; Rosa, D.S.; Da Costa, M.M.; Do Nascimento, J.M.L.; De Sá, M.d.C.A. Toxicidade Do Extrato Vegetal, Óleo Essencial e Hidrolato Das Plantas Zingiber Officinale Roscoe e Allium Sativum L./Toxicity of Plant Extract, Essential Oil and Hydrolate of Zingiber Officinale Roscoe and Allium Sativum L. Plants. Braz. J. Dev. 2022, 8, 14318–14329. [Google Scholar] [CrossRef]
- Imwong, M.; Dhorda, M.; Tun, K.M.; Thu, A.M.; Phyo, A.P.; Proux, S.; Suwannasin, K.; Kunasol, C.; Srisutham, S.; Duanguppama, J.; et al. Molecular epidemiology of resistance to antimalarial drugs in the Greater Mekong subregion: An observational study. Lancet Infect. Dis. 2020, 20, 1470. [Google Scholar] [CrossRef]
- Nsanzabana, C. Resistance to Artemisinin Combination Therapies (ACTs): Do Not Forget the Partner Drug! Trop. Med. Infect. Dis. 2019, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Bhagavathula, A.S.; Elnour, A.A.; Shehab, A. Alternatives to currently used antimalarial drugs: In search of a magic bullet. Infect. Dis. Poverty 2016, 5, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, T.; Borrmann, S.; Ramharter, M. Drug-induced hypersensitivity to artemisinin-based therapies for malaria. Trends Parasitol. 2022, 38, 136–146. [Google Scholar] [CrossRef]
- Ceravolo, I.P.; Aguiar, A.C.; Adebayo, J.O.; Krettli, A.U. Studies on Activities and Chemical Characterization of Medicinal Plants in Search for New Antimalarials: A Ten Year Review on Ethnopharmacology. Front. Pharmacol. 2021, 12, 2478. [Google Scholar] [CrossRef] [PubMed]


| rt (min) | Compound | Rel.% |
|---|---|---|
| 4.28 | Gallic acid | 14.75 |
| 6.05 | Gallotannin | 15.19 |
| 6.8 | Valoneic acid dilactone | 13.89 |
| 7.68 | Ellagic acid | 34.27 |
| TOTAL: | 78.1 | |
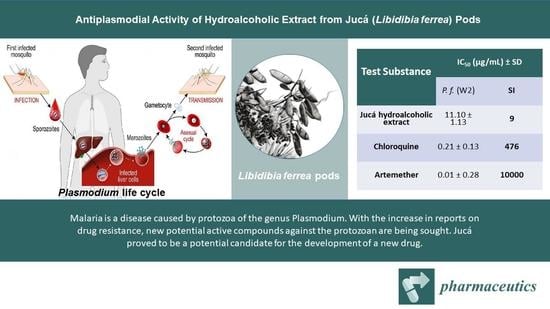
| Test Substance | IC50 (µg/mL) ± SD | ||
|---|---|---|---|
| P. f. (W2) | WI-26-VA4 | SI | |
| Jucá hydroalcoholic extract | 11.10 ± 1.13 | >100 | 9 |
| Chloroquine | 0.21 ± 0.13 | >100 | 476 |
| Artemether | 0.01 ± 0.28 | >100 | 10000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assis, F.F.V.d.; Almeida Junior, J.S.d.; Moraes, T.M.P.; Varotti, F.d.P.; Moraes, C.C.; Sartoratto, A.; Moraes, W.P.; Minervino, A.H.H. Antiplasmodial Activity of Hydroalcoholic Extract from Jucá (Libidibia ferrea) Pods. Pharmaceutics 2023, 15, 1162. https://doi.org/10.3390/pharmaceutics15041162
Assis FFVd, Almeida Junior JSd, Moraes TMP, Varotti FdP, Moraes CC, Sartoratto A, Moraes WP, Minervino AHH. Antiplasmodial Activity of Hydroalcoholic Extract from Jucá (Libidibia ferrea) Pods. Pharmaceutics. 2023; 15(4):1162. https://doi.org/10.3390/pharmaceutics15041162
Chicago/Turabian StyleAssis, Francisco Flávio Vieira de, José Sousa de Almeida Junior, Tânia Mara Pires Moraes, Fernando de Pilla Varotti, Camila Castilho Moraes, Adilson Sartoratto, Waldiney Pires Moraes, and Antonio Humberto Hamad Minervino. 2023. "Antiplasmodial Activity of Hydroalcoholic Extract from Jucá (Libidibia ferrea) Pods" Pharmaceutics 15, no. 4: 1162. https://doi.org/10.3390/pharmaceutics15041162