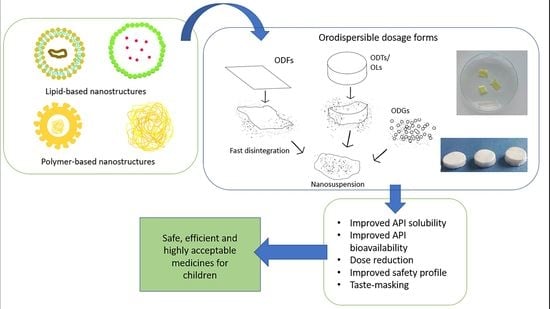
Orally Dispersible Dosage Forms for Paediatric Use: Current Knowledge and Development of Nanostructure-Based Formulations
Abstract
:1. Introduction
2. Orally Dispersible Dosage Forms (ODx)—General Aspects
2.1. Granules (ODGs)
2.2. Tablets (ODTs)
2.3. Minitablets (ODMTs)
2.4. Oral Lyophilisates (OLs)
2.5. Films (ODFs)
2.6. Shortcomings of ODx
2.7. Quality Profile of ODx for Paediatric Use
3. Nanotechnology-Based ODx
3.1. Nanostructures in Oral Drug Delivery
3.2. Benefits of the Use of Nanostructures in ODx
3.3. Preparation of ODx Formulations with Nanostructures
| ODx | ODx Formulation | API | Nanosystem/Preparation Method | Nanosystem Formulation | Benefits of the Inclusion of Nanosystems | Reference |
|---|---|---|---|---|---|---|
| ODTs | Filler: MCC, mannitol SD: croscarmellose sodium | Meloxicam | Nanofibres/electrospinning | Eudragit® E, PVP K30 | Improved API dissolution | [34] |
| Filler: MCC, lactose, mannitol SD: croscarmellose sodium Lubricant: magnesium stearate | Nitrendipine | Nanocrystals/antisolvent sonoprecipitation followed by freeze-drying | Stabiliser: HPMC E6 | Faster and complete release of API Improved oral bioavailability in rabbits | [142] | |
| Filler: dextrates, silicified MCC SD: sodium starch glycolate Sweetener: sodium saccharine Lubricant: sodium stearyl fumarate | Meclizine | Polymeric nanoparticles/antisolvent precipitation | Polymers: chitosan, shellac Stabiliser: Poloxamer 188 | Dual function compressed tablet whose core contained nanoparticles with prolonged release, while the outer layer contained the soluble free form for buccal absorption Higher bioavailability of API from novel formulation compared to commercial product | [139] | |
| Filler: lactose SD: L-HPC Lubricant: magnesium stearate | Promethazine | Polymer-coated nanoparticles/ionotropic gelation | Polymer: chitosan Coatings: PEG, PVP, polyethylene co-acrylic acid | Sustained release of the API; the API release decreased: ODTs free API > ODTs noncoated NP > ODTs coated NP | [119] | |
| Filler: MCC, mannitol, lactose SD: sodium starch glycolate Sweetener, diluent: xylitol Lubricant: magnesium stearate | Scopolamine hydrobromide | Polymeric nanoparticles/ionotropic gelation | Polymer: chitosan Cross-linker: tripolyphosphate | Improved sustained release profile when compared to formulations containing non-cross-linked polymer systems or free API | [151] | |
| ODMTs | - | Prednisone | Nanofibres/electrospinning | PVP | Increased solubility compared to the API powder | [138] |
| OLs | Filler: mannitol MFA: sodium alginate, sodium croscarmellose | Meloxicam | Nanocrystals/high pressure homogenisation | Stabiliser: Poloxamer 188 | Faster API dissolution | [55] |
| Filler: maltodextrin MFA: xanthan gum/croscarmellose/gelatin Co-binder: PEG 4000 | Piroxicam | Nanocrystals/high pressure homogenisation | Stabiliser: Poloxamer 188 | Improvement of API dissolution rate Improved dissolution profile compared to commercial product | [147,148] | |
| Filler: sucrose MFA, viscosity agent: PVA Co-binder: PEG 6000 | Silymarin | Mesoporous silica nanospheres/solvent evaporation method | Mesoporous material formers: Hexadecyltrimethyl ammonium chloride, tetraethyl orthosilicate | API fast dissolution rate, high saturation solubility | [149] | |
| MFA: Pullulan Binder: HPMC Sweetener: aspartame, xylitol SD: Plasdone XL | Rosuvastatin | Transfersomes/lipid film hydration | Phospholipid: soybean phosphatidylcholine Edge activator: Tween 80 Negative charge inducing agent: dicetyl phosphate | Superior pharmacokinetic and pharmacodynamic performance compared to commercial drug | [131] | |
| ODFs | FFA: HPMC Plasticizer: glycerol | Naproxen, anthraquinone | Polymer-coated nanoparticles/stirred media milling | Stabiliser: vinylpyrrolidone–vinyl acetate copolymer, sodium dodecyl sulphate | Improved API dissolution | [143,144] |
| FFA: HPMC E6, PVA SD: croscarmellose Plasticizer: PEG 400 | Nitrendipine | Nanocrystals/antisolvent sonoprecipitation | Stabiliser: HPMC E6 | Improved API solubility API in the amorphous state | [141] | |
| FFA: HPMC SD: L-HPC Filler: MCC Sweetener: mannitol Plasticizer: PEG–400 | Herpetrione | Polymer-coated nanoparticles/high pressure homogenisation | Stabiliser: povidone K30 and sodium dodecyl sulphate | Improved API dissolution API in the amorphous state | [145] | |
| FFA: Kollicoat IR/Kollicoat Pr/PVA Plasticizer: glycerol | Aripirazole | Nanoaggregates/high energy ball milling | Stabiliser: Poloxamer 407 | 100-fold improvement in API solubility when compared to raw API | [146] | |
| FFA: PVA Plasticizer: PEG 1500 | Vaccinium arctostaphyllos extract | Liposomes/lipid film hydration | Soybean phosphatidylcholine | Better active ingredient release from liposome formulation compared to the pure extract formulation | [140] | |
| FFA: guar gum Sweetener: sorbitol Penetration enhancer: citric acid | Alpha-casozepine | Polymeric nanoparticles/antisolvent sonoprecipitation | PLGA | Enhancement of buccal and intestinal permeation compared to the API dispersion Protection of the API along the GI tract | [156] | |
| - | Amlodipine | Nanofibres/electrospinning | Carboxymethylated curdlan, poly(ethylene oxide) | Faster onset of action, improved API absorption and bioavailability compared to the commercial product | [72] | |
| FFA: HPMC E15, PVA, maltodextrin Plasticizer: PEG 600, glycerol Sweetener: mannitol, aspartame | Dimethyl fumarate | Polymeric nanoparticles/ionotropic gelation | Core: sodium alginate Shell: chitosan | The formulation allowed dose reduction and enhanced bioavailability compared to pure drug | [134] | |
| - | Prednisolone | Nanofibres/electrospinning | PVA | Faster disintegration and drug release when incorporated into fibres compared to the solvent casted film | [136] | |
| FFA: HPMC E5/E15, PVA Plasticizer: PEG 400, TPGS 1000 | Lercanidipine | Nanosuspension/antisolvent precipitation | Stabiliser: PEG, Hypromellose, PVA, Alginate, TPGS, HPC, Methylcellulose | Increased API solubility when compared to the free drug | [137] | |
| - | Isoniazid | Nanofibres/electrospinning | Pullulan + Hypromellose/pectin/sodium caseinate | The pullulan/Hypromellose films disintegrated within 5 s and released the API content within 30 s | [125] | |
| FFA: HPMC E15, PVA Plasticizer: propylene glycol | Buspirone | Polymeric nanoparticles/antisolvent precipitation | Polymer: PLGA Stabiliser: Poloxamer 188 | Dual release: fast release of the free form and sustained release of API from nanoparticles | [73] | |
| - | Valsartan | Nanofibres/electrospinning | PVP K90 | Enhanced API release compared to the physical mixture or free drug | [157] | |
| - | Lopinavir and ritonavir | Polymer-coated nanoparticles/antisolvent precipitation | Coating: Eudragit® E PO | Taste-masking of the APIs has been proved with the aid of the electronic tongue | [74] |
4. Paediatric Orodispersible vs. Nanostructure-Containing Orodispersible Forms: Current Research Landscape and Commercially Available Products
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Regulation (EC) No. 1901/2006 of the European Parliament and of the Council of 12 December 2006 on Medicinal Products for Paediatric Use and Amending Regulation (EEC) No 1768/92, Directive 2001/20/EC, Directive 2001/83/EC and Regulation (EC) No. 726/2004. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32006R1901&qid=1621344437946 (accessed on 10 February 2022).
- van Riet-Nales, D.A.; Kozarewicz, P.; Aylward, B.; de Vries, R.; Egberts, T.C.G.; Rademaker, C.M.A.; Schobben, A.F.A.M. Paediatric drug development and formulation design—A european perspective. AAPS PharmSciTech 2017, 18, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Preis, M. Orally disintegrating films and mini-tablets—innovative dosage forms of choice for pediatric use. AAPS PharmSciTech 2015, 16, 234–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Riet-Nales, D.A.; Schobben, A.F.A.M.; Vromans, H.; Egberts, T.C.G.; Rademaker, C.M.A. Safe and effective pharmacotherapy in infants and preschool children: Importance of formulation aspects. Arch. Dis. Child. 2016, 101, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Slavkova, M.; Breitkreutz, J. Orodispersible drug formulations for children and elderly. Eur. J. Pharm. Sci. 2015, 75, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Cilurzo, F.; Musazzi, U.M.; Franzé, S.; Selmin, F.; Minghetti, P. Orodispersible dosage forms: Biopharmaceutical improvements and regulatory requirements. Drug Discov. Today 2018, 23, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granules-European Pharmacopoeia 10.8. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/0499E.htm?highlight=on&terms=granules (accessed on 11 February 2022).
- Tablets-European Pharmacopoeia 10.8. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/0478E.htm?highlight=off&terms=tablets&terms=tablets.%20tablets (accessed on 16 February 2022).
- Food and Drug Administration (FDA). Guidance for Industry Orally Disintegrating Tablets. Available online: http://www.fda.gov/cder/guidance/index.htm (accessed on 16 February 2022).
- Debruyne, F.M.J.; Gittelman, M.; Sperling, H.; Börner, M.; Beneke, M. Time to onset of action of vardenafil: A retrospective analysis of the pivotal trials for the orodispersible and film-coated tablet formulations. J. Sex. Med. 2011, 8, 2912–2923. [Google Scholar] [CrossRef]
- Sugár, D.; Francombe, D.; da Silva, T.; Hanid, S.; Hutchings, S. Comparative bioavailability study of a new orodispersible formulation of ibuprofen versus two existing oral tablet formulations in healthy male and female volunteers. Clin. Ther. 2019, 41, 1486–1498. [Google Scholar] [CrossRef]
- Almukainzi, M.; Araujo, G.L.B.; Löbenberg, R. Orally disintegrating dosage forms. J. Pharm. Investig. 2019, 49, 229–243. [Google Scholar] [CrossRef]
- Al-Khattawi, A.; Mohammed, A.R. Challenges and emerging solutions in the development of compressed orally disintegrating tablets. Expert Opin. Drug Discov. 2014, 9, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Advankar, A.; Maheshwari, R.; Tambe, V.; Todke, P.; Raval, N.; Kapoor, D.; Tekade, R.K. Specialized tablets: Ancient history to modern developments. In Drug Delivery Systems; Tekade, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 615–664. ISBN 9780128145081. [Google Scholar]
- Bowles, B.J.; Dziemidowicz, K.; Lopez, F.L.; Orlu, M.; Tuleu, C.; Edwards, A.J.; Ernest, T.B. Co-processed excipients for dispersible tablets–part 1: Manufacturability. AAPS PharmSciTech 2018, 19, 2598–2609. [Google Scholar] [CrossRef] [Green Version]
- Dziemidowicz, K.; Lopez, F.L.; Bowles, B.J.; Edwards, A.J.; Ernest, T.B.; Orlu, M.; Tuleu, C. Co-processed excipients for dispersible tablets—part 2: Patient acceptability. AAPS PharmSciTech 2018, 19, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Kokott, M.; Lura, A.; Breitkreutz, J.; Wiedey, R. Evaluation of two novel co-processed excipients for direct compression of orodispersible tablets and mini-tablets. Eur. J. Pharm. Biopharm. 2021, 168, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Petrovick, G.F.; Kleinebudde, P.; Breitkreutz, J. Orodispersible tablets containing taste-masked solid lipid pellets with metformin hydrochloride: Influence of process parameters on tablet properties. Eur. J. Pharm. Biopharm. 2018, 122, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.K.; Ahmad, S.A.; Beg, S.; Ara, T.J.; Hasnain, M.S. Drug delivery: Present, past, and future of medicine. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, A.M.A., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 255–282. [Google Scholar]
- Badgujar, B.P.; Mundada, A.S. The technologies used for developing orally disintegrating tablets: A review. Acta Pharm. 2011, 61, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Shan, X.; Du, Y.; Pang, S.; Hu, L. Development and evaluation of novel innovative multi-channel aripiprazole orally disintegrating tablets. J. Drug Deliv. Sci. Technol. 2020, 55, 101446. [Google Scholar] [CrossRef]
- Yu, J.; Shan, X.; Chen, S.; Sun, X.; Song, P.; Zhao, R.; Hu, L. Preparation and evaluation of novel multi-channel orally disintegrating tablets. Eur. J. Pharm. Sci. 2020, 142, 105108. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Tang, H.; Ren, W.; Gao, X.; Sun, Y.; Zhao, Q.X.; Wang, F.; Liu, J. Development and optimization of levodopa and benzylhydrazine orally disintegrating tablets by direct compression and response surface methodology. Drug Dev. Ind. Pharm. 2020, 46, 42–49. [Google Scholar] [CrossRef]
- Vlad, R.A.; Antonoaea, P.; Todoran, N.; Muntean, D.L.; Rédai, E.M.; Silași, O.A.; Tătaru, A.; Bîrsan, M.; Imre, S.; Ciurba, A. Pharmacotechnical and analytical preformulation studies for cannabidiol orodispersible tablets. Saudi Pharm. J. 2021, 29, 1029–1042. [Google Scholar] [CrossRef]
- Muntean, A.C.; Negoi, O.I.; Rus, L.L.; Vonica, A.L.; Tomuță, I. Formulation of orodispersible tablets containing paracetamol and their in vitro characterization-a QbD approach. Farmacia 2020, 68, 436–446. [Google Scholar] [CrossRef]
- Conceição, J.; Adeoye, O.; Cabral-Marques, H.; Concheiro, A.; Alvarez-Lorenzo, C.; Sousa Lobo, J.M. Orodispersible carbamazepine/hydroxypropyl-β-cyclodextrin tablets obtained by direct compression with five-in-one co-processed excipients. AAPS PharmSciTech 2020, 21, 39. [Google Scholar] [CrossRef]
- Brniak, W.; Maślak, E.; Jachowicz, R. Orodispersible films and tablets with prednisolone microparticles. Eur. J. Pharm. Sci. 2015, 75, 81–90. [Google Scholar] [CrossRef]
- Suñé-Negre, J.M.; Roig, M.; Fuster, R.; Hernández, C.; Ruhí, R.; García-Montoya, E.; Pérez-Lozano, P.; Miñarro, M.; Ticó, J.R. New classification of directly compressible (DC) excipients in function of the SeDeM diagarm expert system. Int. J. Pharm. 2014, 470, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, J.E.; Montoya, E.G.; Lozano, P.P.; Negre, J.M.S.; Carmona, M.M.; Grau, J.R.T. New SeDeM-ODT expert system: An expert system for formulation of orodispersible tablets obtained by direct compression. In Formulation Tools for Pharmaceutical Development; Woodhead Publishing: Sawston, UK, 2013; pp. 137–154. ISBN 9781907568992. [Google Scholar]
- Genina, N.; Boetker, J.P.; Rantanen, J. 3D printing in oral drug delivery. In Nanotechnology for Oral Drug Delivery; Martins, J.P., Santos, H.A., Eds.; Elsevier: London, UK, 2020; pp. 359–386. [Google Scholar]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- El Aita, I.; Rahman, J.; Breitkreutz, J.; Quodbach, J. 3D-printing with precise layer-wise dose adjustments for paediatric use via pressure-assisted microsyringe printing. Eur. J. Pharm. Biopharm. 2020, 157, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Szabó, P.; Kállai-Szabó, B.; Sebe, I.; Zelkó, R. Preformulation study of fiber formation and formulation of drug-loaded microfiber based orodispersible tablets for in vitro dissolution enhancement. Int. J. Pharm. 2014, 477, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Casian, T.; Borbás, E.; Ilyés, K.; Démuth, B.; Farkas, A.; Rapi, Z.; Bogdan, C.; Iurian, S.; Toma, V.; Știufiuc, R.; et al. Electrospun amorphous solid dispersions of meloxicam: Influence of polymer type and downstream processing to orodispersible dosage forms. Int. J. Pharm. 2019, 569, 118593. [Google Scholar] [CrossRef]
- Draksiene, G.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Psyllium (Plantago Ovata Forsk) husk powder as a natural superdisintegrant for orodispersible formulations: A study on meloxicam tablets. Molecules 2019, 24, 3255. [Google Scholar] [CrossRef] [Green Version]
- Thomson, S.A.; Tuleu, C.; Wong, I.C.K.; Keady, S.; Pitt, K.G.; Sutcliffe, A.G. Minitablets: New modality to deliver medicines to preschool-aged children. Pediatrics 2009, 123, e235–e238. [Google Scholar] [CrossRef]
- Ranmal, S.R.; Barker, S.A.; Tuleu, C. Paediatric solid formulations. In Pediatric Formulations: A Roadmap; Bar-Shalom, D., Rose, K., Eds.; Springer: New York, NY, USA, 2014; Volume 11, pp. 153–170. ISBN 978-1-4899-8010-6. [Google Scholar]
- Comoglu, T.; Dilek Ozyilmaz, E. Orally disintegrating tablets and orally disintegrating mini tablets–novel dosage forms for pediatric use. Pharm. Dev. Technol. 2019, 24, 902–914. [Google Scholar] [CrossRef]
- Lura, A.; Luhn, O.; Suarez Gonzales, J.; Breitkreutz, J. New orodispersible mini-tablets for paediatric use—A comparison of isomalt with a mannitol based co-processed excipient. Int. J. Pharm. 2019, 572, 118804. [Google Scholar] [CrossRef]
- Lura, A.; Elezaj, V.; Kokott, M.; Fischer, B.; Breitkreutz, J. Transfer and scale-up of the manufacturing of orodispersible mini-tablets from a compaction simulator to an industrial rotary tablet press. Int. J. Pharm. 2021, 602, 120636. [Google Scholar] [CrossRef]
- Wiedey, R.; Kokott, M.; Breitkreutz, J. Orodispersible tablets for pediatric drug delivery: Current challenges and recent advances. Expert Opin. Drug Deliv. 2021, 18, 1873–1890. [Google Scholar] [CrossRef]
- Soulairol, I.; Sanchez-Ballester, N.M.; Aubert, A.; Tarlier, N.; Bataille, B.; Quignard, F.; Sharkawi, T. Evaluation of the super disintegrant functionnalities of alginic acid and calcium alginate for the design of orodispersible mini tablets. Carbohydr. Polym. 2018, 197, 576–585. [Google Scholar] [CrossRef]
- Suárez-González, J.; Magariños-Triviño, M.; Díaz-Torres, E.; Cáceres-Pérez, A.R.; Santoveña-Estévez, A.; Fariña, J.B. Individualized orodispersible pediatric dosage forms obtained by molding and semi-solid extrusion by 3D printing: A comparative study for hydrochlorothiazide. J. Drug Deliv. Sci. Technol. 2021, 66, 102884. [Google Scholar] [CrossRef]
- Eduardo, D.T.; Ana, S.E.; José, B.F. A Micro-extrusion 3D printing platform for fabrication of orodispersible printlets for pediatric use. Int. J. Pharm. 2021, 605, 120854. [Google Scholar] [CrossRef]
- Hejduk, A.; Lulek, J. Dispensing of minitablets–has the problem been resolved? Int. J. Pharm. 2022, 619, 121666. [Google Scholar] [CrossRef]
- Sympfiny® Oral Delivery System for Multiparticulate Drug Formulations-Pharmaceutical Technology. Available online: https://www.pharmaceutical-technology.com/products/sympfiny-oral-delivery-system-for-multiparticulate-drug-formulations/ (accessed on 2 January 2021).
- Pikal, M.J. Freeze drying. In Encyclopedia of Pharmaceutical Technology; Swarbick, J., Ed.; Informa Healthcare: New York, NY, USA, 2007; Volume 3, pp. 1807–1833. [Google Scholar]
- Seager, H. Drug-delivery products and the zydis fast-dissolving dosage form. J. Pharm. Pharmacol. 2011, 50, 375–382. [Google Scholar] [CrossRef]
- Borges, P.F.; García-Montoya, E.; Pérez-Lozano, P.; Jo, E.; Miñarro, M.; Manich, A.; Suñé-Negre, J.M. The role of SeDeM for characterizing the active substance and polyvinyilpyrrolidone eliminating metastable forms in an oral lyophilizate—A preformulation study. PLoS ONE 2018, 13, e0196049. [Google Scholar] [CrossRef]
- Casian, T.; Iurian, S.; Bogdan, C.; Rus, L.; Moldovan, M.; Tomuta, I. QbD for pediatric oral lyophilisates development: Risk assessment followed by screening and optimization. Drug Dev. Ind. Pharm. 2017, 43, 1932–1944. [Google Scholar] [CrossRef]
- Vanbillemont, B.; de Beer, T. Application of polyvinyl acetate in an innovative formulation strategy for lyophilized orally disintegrating tablets. Int. J. Pharm. 2020, 588, 119717. [Google Scholar] [CrossRef]
- Iurian, S.; Dinte, E.; Iuga, C.; Bogdan, C.; Spiridon, I.; Barbu-Tudoran, L.; Bodoki, A.; Tomuţă, I.; Leucuţa, S.E. The pharmaceutical applications of a biopolymer isolated from trigonella foenum-graecum seeds: Focus on the freeze-dried matrix forming capacity. Saudi Pharm. J. 2017, 25, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.S.R.; de Oliveira Cruvinel, K.; Oliveira-Nascimento, L. A mini-review on drug delivery through wafer technology: Formulation and manufacturing of buccal and oral lyophilizates. J. Adv. Res. 2019, 20, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Suciu, Ș.; Iurian, S.; Bogdan, C.; Rus, L.; Sebastian Porav, A.; Borodi, G.; Tomuță, I. Design of experiments approach to assess the impact of API particle size on freeze-drying bulking agents. Farmacia 2021, 69, 279–289. [Google Scholar] [CrossRef]
- Iurian, S.; Tomuta, I.; Bogdan, C.; Rus, L.; Tokes, T.; Barbu-Tudoran, L.; Achim, M.; Moldovan, M.; Leucuta, S. Defining the design space for freeze-dried orodispersible tablets with meloxicam. Drug Dev. Ind. Pharm. 2016, 42, 1977–1989. [Google Scholar] [CrossRef]
- Sandri, G.; Bonferoni, M.C.; Ferrari, F.; Rossi, S.; Caramella, C. Differentiating factors between oral fast-dissolving technologies. Am. J. Drug Deliv. 2012, 4, 249–262. [Google Scholar] [CrossRef]
- Douroumis, D. Orally disintegrating dosage forms and taste-masking technologies. Expert Opin. Drug Deliv. 2011, 8, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Oromucosal Preparations-European Pharmacopoeia 10.8. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/1807E.htm?highlight=on&terms=orodispersible%20films&terms=orodispersible%20films&terms=orodispersible%20films&terms=films&terms=films&terms=films&terms=films&terms=orodispersible%20films (accessed on 1 March 2022).
- Gupta, M.S.; Kumar, T.P.; Gowda, D.V.; Rosenholm, J.M. Orodispersible films: Conception to quality by design. Adv. Drug Deliv. Rev. 2021, 178, 113983. [Google Scholar] [CrossRef]
- Scarpa, M.; Stegemann, S.; Hsiao, W.K.; Pichler, H.; Gaisford, S.; Bresciani, M.; Paudel, A.; Orlu, M. Orodispersible films: Towards drug delivery in special populations. Int. J. Pharm. 2017, 523, 327–335. [Google Scholar] [CrossRef]
- Visser, J.C.; Woerdenbag, H.J.; Crediet, S.; Gerrits, E.; Lesschen, M.A.; Hinrichs, W.L.J.; Breitkreutz, J.; Frijlink, H.W. Orodispersible films in individualized pharmacotherapy: The development of a formulation for pharmacy preparations. Int. J. Pharm. 2015, 478, 155–163. [Google Scholar] [CrossRef]
- BASF Pharma|Kollicoat®, IR. Available online: https://pharma.basf.com/products/kollicoat-ir (accessed on 1 March 2022).
- Sheikh, F.A.; Aamir, M.N.; Haseeb, M.T.; Abbas Bukhari, S.N.; Farid ul Haq, M.; Akhtar, N. Design, physico-chemical assessment and pharmacokinetics of a non-toxic orodispersible film for potential application in musculo-skeletal disorder. J. Drug Deliv. Sci. Technol. 2021, 65, 102726. [Google Scholar] [CrossRef]
- Olechno, K.; Maciejewski, B.; Głowacz, K.; Lenik, J.; Ciosek-Skibí Nska, P.; Basa, A.; Winnicka, K. Orodispersible films with rupatadine fumarate enclosed in ethylcellulose microparticles as drug delivery platform with taste-masking effect. Materials 2022, 15, 2126. [Google Scholar] [CrossRef]
- Borges, A.F.; Silva, C.; Coelho, J.F.J.; Simões, S. Oral films: Current status and future perspectives ii—intellectual property, technologies and market needs. J. Control. Release 2015, 206, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, E.M.; Breitenbach, A.; Breitkreutz, J. Advances in orodispersible films for drug delivery. Expert Opin. Drug Deliv. 2011, 8, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Rodd, C.; Jean-Philippe, S.; Vanstone, C.; Weiler, H. Comparison of 2 vitamin D supplementation modalities in newborns: Adherence and preference. Appl. Physiol. Nutr. Metab. 2011, 36, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Morath, B.; Sauer, S.; Zaradzki, M.; Wagner, A.H. Orodispersible films–recent developments and new applications in drug delivery and therapy. Biochem. Pharmacol. 2022, 200, 115036. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.L.; Ernest, T.B.; Tuleu, C.; Gul, M.O. Formulation approaches to pediatric oral drug delivery: Benefits and limitations of current platforms. Expert Opin. Drug Deliv. 2015, 12, 1727–1740. [Google Scholar] [CrossRef] [PubMed]
- Iurian, S.; Bogdan, C.; Tomuță, I.; Szabó-Révész, P.; Chvatal, A.; Leucuța, S.E.; Moldovan, M.; Ambrus, R. Development of oral lyophilisates containing meloxicam nanocrystals using QbD approach. Eur. J. Pharm. Sci. 2017, 104, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Li, Y.; Wu, J.; Shi, Z.; Zhao, P.; Su, H.; Wang, Q.; Jin, L. Nanofiber orodispersible films based on carboxymethyl curdlan and PEO: New delivery system for amlodipine besylate. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128096. [Google Scholar] [CrossRef]
- Bharti, K.; Mittal, P.; Mishra, B. Formulation and characterization of fast dissolving oral films containing buspirone hydrochloride nanoparticles using design of experiment. J. Drug Deliv. Sci. Technol. 2019, 49, 420–432. [Google Scholar] [CrossRef]
- Deng, Y.; Shen, L.; Yang, Y.; Shen, J. Development of nanoparticle-based orodispersible palatable pediatric formulations. Int. J. Pharm. 2021, 596, 120206. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.K.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771. [Google Scholar] [CrossRef] [Green Version]
- International Conference on Harmonisation of Requirements for Registration of Pharmaceuticals (ICH). ICH Harmonised Tripartite Guideline-Pharmaceutical Development Q8. Available online: https://database.ich.org/sites/default/files/Q8_R2_Guideline.pdf (accessed on 9 March 2022).
- Walsh, J.; Masini, T.; Huttner, B.D.; Moja, L.; Penazzato, M.; Cappello, B. Assessing the appropriateness of formulations on the WHO model list of essential medicines for children: Development of a paediatric quality target product profile tool. Pharmaceutics 2022, 14, 473. [Google Scholar] [CrossRef] [PubMed]
- Naman, S.; Madhavi, N.; Singh, B.; Madan, J.; Baldi, A. Implementing risk-based quality by design for development and optimization of flavored oral disintegrating mini tablets. J. Drug Deliv. Sci. Technol. 2021, 66, 102799. [Google Scholar] [CrossRef]
- Charoo, N.A.; Shamsher, A.A.A.; Zidan, A.S.; Rahman, Z. Quality by design approach for formulation development: A case study of dispersible tablets. Int. J. Pharm. 2012, 423, 167–178. [Google Scholar] [CrossRef]
- Walsh, J.; Schaufelberger, D.; Iurian, S.; Klein, S.; Batchelor, H.; Turner, R.; Gizurarson, S.; Boltri, L.; Alessandrini, E.; Tuleu, C. Path towards efficient paediatric formulation development based on partnering with clinical pharmacologists and clinicians, a C4c expert group white paper. Br. J. Clin. Pharmacol. 2021. early view. [Google Scholar] [CrossRef]
- Suciu, Ș.; Iurian, S.; Bogdan, C.; Iovanov, R.; Rus, L.; Moldovan, M.; Tomuță, I. QbD approach in the development of oral lyophilisates with ibuprofen for paediatric use. Farmacia 2018, 66, 514–523. [Google Scholar] [CrossRef]
- Hejduk, A.; Teżyk, M.; Jakubowska, E.; Krüger, K.; Lulek, J. Implementing the design of experiments (DoE) concept into the development phase of orodispersible minitablets (ODMTs) containing melatonin. AAPS PharmSciTech 2022, 23, 60. [Google Scholar] [CrossRef]
- Ranmal, S.R.; Cram, A.; Tuleu, C. Age-appropriate and acceptable paediatric dosage forms: Insights into end-user perceptions, preferences and practices from the children’s acceptability of oral formulations (CALF) study. Int. J. Pharm. 2016, 514, 296–307. [Google Scholar] [CrossRef]
- Alessandrini, E.; Brako, F.; Scarpa, M.; Lupo, M.; Bonifazi, D.; Pignataro, V.; Cavallo, M.; Cullufe, O.; Enache, C.; Nafria, B.; et al. Children’s preferences for oral dosage forms and their involvement in formulation research via EPTRI (European paediatric translational research infrastructure). Pharmaceutics 2021, 13, 730. [Google Scholar] [CrossRef]
- Cohen, I.T.; Joffe, D.; Hummer, K.; Soluri, A. Ondansetron oral disintegrating tablets: Acceptability and efficacy in children undergoing adenotonsillectomy. Anesth. Analg. 2005, 101, 59–63. [Google Scholar] [CrossRef]
- Valovirta, E.; Scadding, G. Parental attitudes toward new dosage forms of desloratadine in an online survey: Results from four european countries. Curr. Med. Res. Opin. 2009, 25, 2061–2067. [Google Scholar] [CrossRef]
- Ogutu, B.R.; Onyango, K.O.; Koskei, N.; Omondi, E.K.; Ongecha, J.M.; Otieno, G.A.; Obonyo, C.; Otieno, L.; Eyase, F.; Johnson, J.D.; et al. Efficacy and safety of artemether-lumefantrine and dihydroartemisinin-piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: Results of an open-label, randomized, single-centre study. Malar. J. 2014, 13, 33. [Google Scholar] [CrossRef] [Green Version]
- Angwa, L.M.; Ouma, C.; Okoth, P.; Nyamai, R.; Kamau, N.G.; Mutai, K.; Onono, M.A. Acceptability, adherence, and clinical outcomes, of amoxicillin dispersible tablets versus oral suspension in treatment of children aged 2–59 months with pneumonia, Kenya: A cluster randomized controlled trial. Heliyon 2020, 6, e03786. [Google Scholar] [CrossRef]
- Thabet, Y.; Walsh, J.; Breitkreutz, J. Flexible and precise dosing of enalapril maleate for all paediatric age groups utilizing orodispersible minitablets. Int. J. Pharm. 2018, 541, 136–142. [Google Scholar] [CrossRef]
- Lottmann, H.; Froeling, F.; Alloussi, S.; El-Radhi, A.S.; Rittig, S.; Riis, A.; Persson, B.E. A randomised comparison of oral desmopressin lyophilisate (MELT) and tablet formulations in children and adolescents with primary nocturnal enuresis. Int. J. Clin. Pract. 2007, 61, 1454–1460. [Google Scholar] [CrossRef]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of orodispersible films for delivery of medicines to infants and preschool children. Drug Deliv. 2017, 24, 1243. [Google Scholar] [CrossRef] [Green Version]
- Klingmann, V.; Pohly, C.E.; Meissner, T.; Mayatepek, E.; Möltner, A.; Flunkert, K.; Breitkreutz, J.; Bosse, H.M. Acceptability of an orodispersible film compared to syrup in neonates and infants: A randomized controlled trial. Eur. J. Pharm. Biopharm. 2020, 151, 239–245. [Google Scholar] [CrossRef]
- Mistry, P.; Batchelor, H. Evidence of acceptability of oral paediatric medicines: A review. J. Pharm. Pharmacol. 2017, 69, 361–376. [Google Scholar] [CrossRef] [Green Version]
- Casian, T.; Bogdan, C.; Tarta, D.; Moldovan, M.; Tomuta, I.; Iurian, S. Assessment of oral formulation-dependent characteristics of orodispersible tablets using texture profiles and multivariate data analysis. J. Pharm. Biomed. Anal. 2018, 152, 47–56. [Google Scholar] [CrossRef]
- Hofmanová, J.K.; Mason, J.; Batchelor, H.K. Tribology provides an in vitro tool that correlated to in vivo sensory data on the mouthfeel of coated tablets. Int. J. Pharm. 2021, 597, 120323. [Google Scholar] [CrossRef]
- Asiri, A.; Hofmanová, J.; Batchelor, H. A review of in vitro and in vivo methods and their correlations to assess mouthfeel of solid oral dosage forms. Drug Discov. Today 2021, 26, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Malallah, O.; Rashid, Z.; Li, C.L.; Alqurshi, A.; Alhanan, M.A.; Forbes, B.; Royall, P.G. Digital image disintegration analysis: A novel quality control method for fast disintegrating tablets. AAPS PharmSciTech 2021, 22, 219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.S.; Kumar, T.P. Characterization of orodispersible films: An overview of methods and introduction to a new disintegration test apparatus using LDR-LED sensors. J. Pharm. Sci. 2020, 109, 2925–2942. [Google Scholar] [CrossRef]
- Khan, D.; Kirby, D.; Bryson, S.; Shah, M.; Rahman Mohammed, A. Paediatric specific dosage forms: Patient and formulation considerations. Int. J. Pharm. 2022, 616, 121501. [Google Scholar] [CrossRef]
- Walsh, J.; Cram, A.; Woertz, K.; Breitkreutz, J.; Winzenburg, G.; Turner, R.; Tuleu, C. Playing hide and seek with poorly tasting paediatric medicines: Do not forget the excipients. Adv. Drug Deliv. Rev. 2014, 73, 14–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woertz, K.; Tissen, C.; Kleinebudde, P.; Breitkreutz, J. Taste sensing systems (electronic tongues) for pharmaceutical applications. Int. J. Pharm. 2011, 417, 256–271. [Google Scholar] [CrossRef]
- Pein, M.; Preis, M.; Eckert, C.; Kiene, F.E. Taste-masking assessment of solid oral dosage forms—A critical review. Int. J. Pharm. 2014, 465, 239–254. [Google Scholar] [CrossRef]
- Amelian, A.; Wasilewska, K.; Wesoły, M.; Ciosek-Skibińska, P.; Winnicka, K. Taste-masking assessment of orally disintegrating tablets and lyophilisates with cetirizine dihydrochloride microparticles. Saudi Pharm. J. 2017, 25, 1144–1150. [Google Scholar] [CrossRef]
- Abdelhakim, H.E.; Williams, G.R.; Craig, D.Q.M.; Orlu, M.; Tuleu, C. Human mouthfeel panel investigating the acceptability of electrospun and solvent cast orodispersible films. Int. J. Pharm. 2020, 585, 119532. [Google Scholar] [CrossRef]
- Desai, N.; Redfearn, A.; Macleod, G.; Tuleu, C.; Hanson, B.; Orlu, M. How do orodispersible tablets behave in an in vitro oral cavity model: A pilot study. Pharmaceutics 2020, 12, 651. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Masen, M.; Cann, P.; Hanson, B.; Tuleu, C.; Orlu, M. Modernising orodispersible film characterisation to improve palatability and acceptability using a toolbox of techniques. Pharmaceutics 2022, 14, 732. [Google Scholar] [CrossRef]
- Ruhil, S.; Dahiya, M.; Kaur, H.; Singh, J. New insights into the disintegration mechanism and disintegration profiling of rapidly disintegrating tablets (RDTs) by thermal imaging. Int. J. Pharm. 2022, 611, 121283. [Google Scholar] [CrossRef]
- Peltonen, L. Design space and QbD approach for production of drug nanocrystals by wet media milling techniques. Pharmaceutics 2018, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Rose, F.; Wern, J.E.; Ingvarsson, P.T.; van de Weert, M.; Andersen, P.; Follmann, F.; Foged, C. Engineering of a novel adjuvant based on lipid-polymer hybrid nanoparticles: A quality-by-design approach. J. Control. Release 2015, 210, 48–57. [Google Scholar] [CrossRef]
- Freitas, C.; Müller, R.H. Correlation between long-term stability of solid lipid nanoparticles (SLNTM) and crystallinity of the lipid phase. Eur. J. Pharm. Biopharm. 1999, 47, 125–132. [Google Scholar] [CrossRef]
- Diefenthaeler, H.S.; Bianchin, M.D.; Marques, M.S.; Nonnenmacher, J.L.; Bender, E.T.; Bender, J.G.; Nery, S.F.; Cichota, L.C.; Külkamp-Guerreiro, I.C. Omeprazole nanoparticles suspension: Development of a stable liquid formulation with a view to pediatric administration. Int. J. Pharm. 2020, 589, 119818. [Google Scholar] [CrossRef]
- Andretto, V.; Rosso, A.; Briançon, S.; Lollo, G. Nanocomposite systems for precise oral delivery of drugs and biologics. Drug Deliv. Transl. Res. 2021, 11, 445–470. [Google Scholar] [CrossRef]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Zahin, N.; Anwar, R.; Tewari, D.; Kabir, M.T.; Sajid, A.; Mathew, B.; Uddin, M.S.; Aleya, L.; Abdel-Daim, M.M. Nanoparticles and its biomedical applications in health and diseases: Special focus on drug delivery. Environ. Sci. Pollut. Res. 2019, 27, 19151–19168. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, H.; Liu, C.; Zhu, J.; Xu, Y.; Zhang, S.; Fan, M.; Zhang, D.; Zhang, Y.; Zhang, Z.; et al. Fabrication of stable zein nanoparticles by chondroitin sulfate deposition based on antisolvent precipitation method. Int. J. Biol. Macromol. 2019, 139, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, E.; Lulek, J. The application of freeze-drying as a production method of drug nanocrystals and solid dispersions–a review. J. Drug Deliv. Sci. Technol. 2021, 62, 102357. [Google Scholar] [CrossRef]
- Elwerfalli, A.M.; Al-Kinani, A.; Alany, R.G.; Elshaer, A. Nano-engineering chitosan particles to sustain the release of promethazine from orodispersables. Carbohydr. Polym. 2015, 131, 447–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabiano, V.; Mameli, C.; Zuccotti, G.V. Paediatric pharmacology: Remember the excipients. Pharmacol. Res. 2011, 63, 362–365. [Google Scholar] [CrossRef]
- Lim, K.; Hamid, Z.A.A. Polymer nanoparticle carriers in drug delivery systems: Research trend. In Applications of Nanocomposite Materials in Drug Delivery; Inamuddin, A.M.A., Ali, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 217–237. [Google Scholar]
- Turanlı, Y.; Acartürk, F. Preparation and characterization of colon-targeted PH/time-dependent nanoparticles using anionic and cationic polymethacrylate polymers. Eur. J. Pharm. Sci. 2022, 171, 106122. [Google Scholar] [CrossRef]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- Krieser, K.; Emanuelli, J.; Daudt, R.M.; Bilatto, S.; Willig, J.B.; Guterres, S.S.; Pohlmann, A.R.; Buffon, A.; Correa, D.S.; Külkamp-Guerreiro, I.C. Taste-masked nanoparticles containing saquinavir for pediatric oral administration. Mater. Sci. Eng. C 2020, 117, 111315. [Google Scholar] [CrossRef]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun orodispersible films of isoniazid for pediatric tuberculosis treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef]
- Nguyen, T.T.L.; Duong, V.A.; Maeng, H.J. Pharmaceutical formulations with p-glycoprotein inhibitory effect as promising approaches for enhancing oral drug absorption and bioavailability. Pharmaceutics 2021, 13, 1103. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, J.; Du, Y.Z.; Hu, F.Q.; Zeng, S.; Zhao, H.L. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf. B Biointerfaces 2007, 58, 157–164. [Google Scholar] [CrossRef]
- Hosny, K.M.; El-Say, K.M.; Alkhalidi, H.M. Quality by design approach to screen the formulation and process variables influencing the characteristics of carvedilol solid lipid nanoparticles. J. Drug Deliv. Sci. Technol. 2018, 45, 168–176. [Google Scholar] [CrossRef]
- Rajput, A.; Pingale, P.; Telange, D.; Chalikwar, S.; Borse, V. Lymphatic transport system to circumvent hepatic metabolism for oral delivery of lipid-based nanocarriers. J. Drug Deliv. Sci. Technol. 2021, 66, 102934. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A. Nanocarrier systems for oral drug delivery: Do we really need them? Eur. J. Pharm. Sci. 2013, 49, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.A. Study the pharmacokinetics, pharmacodynamics and hepatoprotective activity of rosuvastatin from drug loaded lyophilized orodispersible tablets containing transfersomes nanoparticles. J. Drug Deliv. Sci. Technol. 2021, 63, 102489. [Google Scholar] [CrossRef]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Castro, P.M.; Madureira, A.R.; Sarmento, B.; Pintado, M. Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides 2018, 101, 112–123. [Google Scholar] [CrossRef]
- Sinha, S.; Garg, V.; Singh, R.P.; Dutt, R. Chitosan-alginate core-shell-corona shaped nanoparticles of dimethyl fumarate in orodispersible film to improve bioavailability in treatment of multiple sclerosis: Preparation, characterization and biodistribution in rats. J. Drug Deliv. Sci. Technol. 2021, 64, 102645. [Google Scholar] [CrossRef]
- Castro, P.M.; Baptista, P.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Combination of PLGA nanoparticles with mucoadhesive guar-gum films for buccal delivery of antihypertensive peptide. Int. J. Pharm. 2018, 547, 593–601. [Google Scholar] [CrossRef]
- Tawfik, E.A.; Scarpa, M.; Abdelhakim, H.E.; Bukhary, H.A.; Craig, D.Q.M.; Barker, S.A.; Orlu, M. A potential alternative orodispersible formulation to prednisolone sodium phosphate orally disintegrating tablets. Pharmaceutics 2021, 13, 120. [Google Scholar] [CrossRef]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Strachan, C.; Broadbent, R.; Walker, G.F. A Minitablet formulation made from electrospun nanofibers. Eur. J. Pharm. Biopharm. 2017, 114, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Darwesh, A.Y.; El-Dahhan, M.S.; Meshali, M.M. A new dual function orodissolvable/dispersible meclizine HCL tablet to challenge patient inconvenience: In vitro evaluation and in vivo assessment in human volunteers. Drug Deliv. Transl. Res. 2021, 11, 2209–2223. [Google Scholar] [CrossRef]
- Ockun, M.A.; Baranauskaite, J.; Uner, B.; Kan, Y.; Kırmızıbekmez, H. Preparation, characterization and evaluation of liposomal-freeze dried anthocyanin-enriched Vaccinium arctostaphylos L. fruit extract incorporated into fast dissolving oral films. J. Drug Deliv. Sci. Technol. 2022, 72, 103428. [Google Scholar] [CrossRef]
- Gandhi, N.V.; Deokate, U.A.; Angadi, S.S. Formulation, optimization and evaluation of nanoparticulate oral fast dissolving film dosage form of nitrendipine. AAPS PharmSciTech 2021, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.V.; Deokate, U.A.; Angadi, S.S. Development of nanonized nitrendipine and its transformation into nanoparticulate oral fast dissolving drug delivery system. AAPS PharmSciTech 2021, 22, 113. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. Efficient production of nanoparticle-loaded orodispersible films by process integration in a stirred media mill. Int. J. Pharm. 2016, 511, 804–813. [Google Scholar] [CrossRef]
- Steiner, D.; Finke, J.H.; Kwade, A. Instant ODFs–development of an intermediate, nanoparticle-based product platform for individualized medication. Eur. J. Pharm. Biopharm. 2018, 126, 149–158. [Google Scholar] [CrossRef]
- Shen, B.D.; Shen, C.Y.; Yuan, X.D.; Bai, J.X.; Lv, Q.Y.; Xu, H.; Dai, L.; Yu, C.; Han, J.; Yuan, H.L. Development and characterization of an orodispersible film containing drug nanoparticles. Eur. J. Pharm. Biopharm. 2013, 85, 1348–1356. [Google Scholar] [CrossRef]
- Łyszczarz, E.; Hofmanová, J.; Szafraniec-Szczęsny, J.; Jachowicz, R. Orodispersible films containing ball milled aripiprazole-poloxamer®407 solid dispersions. Int. J. Pharm. 2020, 575, 118955. [Google Scholar] [CrossRef]
- Lai, F.; Pini, E.; Corrias, F.; Perricci, J.; Manconi, M.; Fadda, A.M.; Sinico, C. Formulation strategy and evaluation of nanocrystal piroxicam orally disintegrating tablets manufacturing by freeze-drying. Int. J. Pharm. 2014, 467, 27–33. [Google Scholar] [CrossRef]
- Lai, F.; Pini, E.; Angioni, G.; Manca, M.L.; Perricci, J.; Sinico, C.; Fadda, A.M. Nanocrystals as tool to improve piroxicam dissolution rate in novel orally disintegrating tablets. Eur. J. Pharm. Biopharm. 2011, 79, 552–558. [Google Scholar] [CrossRef]
- Ibrahim, A.H.; Smått, J.H.; Govardhanam, N.P.; Ibrahim, H.M.; Ismael, H.R.; Afouna, M.I.; Samy, A.M.; Rosenholm, J.M. Formulation and optimization of drug-loaded mesoporous silica nanoparticle-based tablets to improve the dissolution rate of the poorly water-soluble drug silymarin. Eur. J. Pharm. Sci. 2020, 142, 105103. [Google Scholar] [CrossRef] [PubMed]
- Khurana, R.K.; Gaspar, B.L.; Welsby, G.; Katare, O.P.; Singh, K.K.; Singh, B. Improving the biopharmaceutical attributes of mangiferin using vitami E-TPGS co-loaded self-assembled phosholipidic nano-mixed micellar systems. Drug Deliv. Transl. Res. 2018, 8, 617–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.Q.; Yan, C.; Bi, J.; Lv, W.L.; Ji, R.R.; Chen, X.; Su, J.C.; Hu, J.H. A novel spray-dried nanoparticles-in-microparticles system for formulating scopolamine hydrobromide into orally disintegrating tablets. Int. J. Nanomed. 2011, 6, 897–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanić, Ž.; Planinšek, O.; Škalko-Basnet, N.; Tho, I. Tablets of pre-liposomes govern in situ formation of liposomes: Concept and potential of the novel drug delivery system. Eur. J. Pharm. Biopharm. 2014, 88, 443–454. [Google Scholar] [CrossRef]
- Nakamura, S.; Fukai, T.; Sakamoto, T. Orally disintegrating tablet manufacture via direct powder compression using cellulose nanofiber as a functional additive. AAPS PharmSciTech 2022, 23, 37. [Google Scholar] [CrossRef]
- Friability of Uncoated…-European Pharmacopoeia 10.8. Available online: https://pheur.edqm.eu/app/10-8/content/10-8/20907E.htm (accessed on 18 April 2022).
- Gonçalves, L.M.D.; Maestrelli, F.; Mannelli, L.C.; Ghelardini, C.; Almeida, A.J.; Mura, P. Development of solid lipid nanoparticles as carriers for improving oral bioavailability of glibenclamide. Eur. J. Pharm. Biopharm. 2016, 102, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Baptista, P.; Zuccheri, G.; Madureira, A.R.; Sarmento, B.; Pintado, M.E. Film-nanoparticle composite for enhanced oral delivery of alpha-casozepine. Colloids Surf. B Biointerfaces 2019, 181, 149–157. [Google Scholar] [CrossRef]
- Abbaspour, M.; Iraji, P.; Mahmoudi, Z.; Rahiman, N.; Akhgari, A. Design and physico-mechanical evaluation of fast-dissolving valsartan polymeric drug delivery system by electrospinning method. Iran. J. Basic Med. Sci. 2021, 24, 1683. [Google Scholar] [CrossRef]
- Steiner, D.; Emmendörffer, J.F.; Bunjes, H. Orodispersible films: A delivery platform for solid lipid nanoparticles? Pharmaceutics 2021, 13, 2162. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Finke, J.H.; Kwade, A. Redispersion of nanoparticle-loaded orodispersible films: Preservation of particle fineness. Chem. Ing. Tech. 2017, 89, 1034–1040. [Google Scholar] [CrossRef]
- MRI-Home. Available online: https://mri.cts-mrp.eu/portal/home?domain=h (accessed on 31 March 2022).
- Medicines|European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human (accessed on 31 March 2022).


| Quality Attribute | Target | Justification | ||
|---|---|---|---|---|
| Route of administration | Oral | Oral administration provides higher treatment compliance, especially in paediatric patients. | ||
| Patient age range | Pre-term neonates to 18 years; may be restricted to smaller groups depending on age or weight | Different dosage forms may be designed for different age groups and/or different dosage strengths. | ||
| Dosage form | ODT | ODTs are easily acceptable and can be safely administered to younger patients. | ||
| Dose | According to the API and target population | Dose increments/banding may be particularised according to the target age or weight range. | ||
| Pharmaceutical properties | Physical properties | Disintegration time | ≤3 min | Meeting the European Pharmacopoeia requirements on the disintegration times of ODTs; the USP requires that the disintegration time is limited to 30 s [9]. |
| Tensile strength | Sufficient to allow handling | There are no official values for the tensile strength of orodispersible dosage forms; the values mentioned previously have been used in practice. | ||
| Friability | ≤1% | Meeting the European Pharmacopoeia requirements as specified in monograph 2.9.7, Friability of uncoated tablets. | ||
| Uniformity of dosage units | Unless otherwise specified, meeting the European Pharmacopoeia requirements as specified in the monographs on Uniformity of dosage units (2.9.40), Uniformity of content (2.9.6), Uniformity of mass (2.9.5) and Dissolution test for solid dosage units (2.9.3). | |||
| Uniformity of content | ||||
| Uniformity of mass | ||||
| Dissolution | ||||
| Release profile | Immediate/prolonged release | According to the API and therapeutic indication of the drug product. | ||
| Acceptability | Size and appearance | Acceptable for the patient; size should not pose any risk to the patient | The acceptability of dosage forms is a subjective matter, influenced by the age, conditions and personal preferences of every patient. | |
| Taste and palatability | Acceptable for the patient | |||
| Ease of administration | Requiring minimal ex tempore preparation | The drug should be easy to handle and administer in accurate doses. | ||
| Safety of excipients | Safe for the target population and suitable for the dosage form | If details on excipient safety are unavailable, potential excipient-associated risks should be taken into consideration | ||
| Stability | 2 years minimum | Special storage conditions (e.g., fridge storage) are less preferred; the packaging should contribute to maintaining the structural integrity and stability of the dosage form. | ||
| Test Product | Age Range | API | Reference Product | Results | Reference |
|---|---|---|---|---|---|
| ODT | 5–11 years | Ondansetron | Placebo | The ondansetron ODTs were effective in reducing postoperative nausea and vomiting, but less palatable compared to the placebo ODTs. | [85] |
| ≤12 years | Desloratadine | Desloratadine syrup | Most of patients’ caregivers were open to trying the ODT formulation and preferred it to the syrup due to the convenience and lack of messiness of the administration. | [86] | |
| ≤5 years | Artemether-lumefantrine | Dihydroartemisinin-piperaquine tablets | Both formulations were similarly effective to antimalarial medication, but the artemether-lumefantrine ODTs showed an increased treatment adherence. | [87] | |
| 2–59 months | Amoxicillin | Amoxicillin oral suspension | The ODT formulation was equivalent to the suspension in terms of acceptability and clinical outcome, but it determined a better treatment adherence. | [88] | |
| ODMT | ≤12 years | Enalapril | Not applicable | The formulation is compatible with several vehicles commonly used to increase acceptability in younger children and is acceptable to older children. | [89] |
| OL | 5–15 years | Desmopressin | Desmopressin tablets | The OL was preferred over the tablet, significantly so in the patients aged 5 to 11 years; the efficacy and safety were similar to the tablet at lower doses. | [90] |
| ODF | newborns | Vitamin D | Vitamin D syrup | Even though it needed more frequent administration, the ODF was much more preferred by patients and parents alike. | [68] |
| 6 months–5 years | Placebo | Not applicable | The majority of children aged 3 and over, as well as the caregivers of the entire patient cohort, gave the ODF a positive rating in terms of acceptability. | [91] | |
| 2 days–12 months | Placebo | Placebo glucose syrup | The ODF was deemed as non-inferior to the syrup in terms of acceptability and superior in the palatability and swallowability assessments. | [92] |
| API | Drug Class | Dosage Form | Commercial Name(s) | Minimal Usage Conditions |
|---|---|---|---|---|
| Amoxicillin/clavulanic acid | Antibiotic | ODT | Amoksiklav Quicktab | ≥40 kg |
| Aripiprazole | Antipsychotic | ODT | Abilify, generics | ≥13 years |
| Bilastine | Antihistaminic | ODT | Borenar, generics | ≥6 years |
| Desloratadine | Antihistaminic | ODT OL | Aerius, Neoclarityn, generics | ≥6 years |
| Desmopressin | Hormone analogue | ODT OL | Minirin Melt Minurin Flas | ≥5 years |
| Dexamethasone | Steroidal anti-inflammatory | ODF | Isicort | ≥3 months and 7 kg |
| Domperidone | Prokinetic | ODT | Domotil | ≥12 years and 35 kg |
| Ebastine | Antihistaminic | OL ODT | Bactil Flas, Kestine, Kestinlyo generics | ≥12 years |
| Ibuprofen | Non-steroidal anti-inflammatory | ODT | Nurofen | ≥6 years |
| Lamotrigine | Antiepileptic | ODT | Lamictal | ≥2 years |
| Loperamide | Antidiarrheic | ODT OL | Imodium, generics | ≥2 years |
| Lorazepam | Anxiolytic | OL ODT | Tavor Expidet, Temesta Expidet, generics | ≥6 years |
| Montelukast | Leukotriene receptor antagonist | ODG | Singulair | ≥6 months |
| Morphine | Opioid analgesic | ODT | Carpos Akut | ≥6 months |
| Ondansetron | Antiemetic | ODF | Setofilm | ≥6 months |
| Oxycodone | Opioid analgesic | ODT | Oxygesic Dispersa | ≥12 years |
| Paracetamol | Analgesic/antipyretic | ODT | Pinex Smelt | ≥4 years |
| Prednisolone | Steroidal anti-inflammatory | ODT | Solupred | ≥10 kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornilă, A.; Iurian, S.; Tomuță, I.; Porfire, A. Orally Dispersible Dosage Forms for Paediatric Use: Current Knowledge and Development of Nanostructure-Based Formulations. Pharmaceutics 2022, 14, 1621. https://doi.org/10.3390/pharmaceutics14081621
Cornilă A, Iurian S, Tomuță I, Porfire A. Orally Dispersible Dosage Forms for Paediatric Use: Current Knowledge and Development of Nanostructure-Based Formulations. Pharmaceutics. 2022; 14(8):1621. https://doi.org/10.3390/pharmaceutics14081621
Chicago/Turabian StyleCornilă, Andreea, Sonia Iurian, Ioan Tomuță, and Alina Porfire. 2022. "Orally Dispersible Dosage Forms for Paediatric Use: Current Knowledge and Development of Nanostructure-Based Formulations" Pharmaceutics 14, no. 8: 1621. https://doi.org/10.3390/pharmaceutics14081621