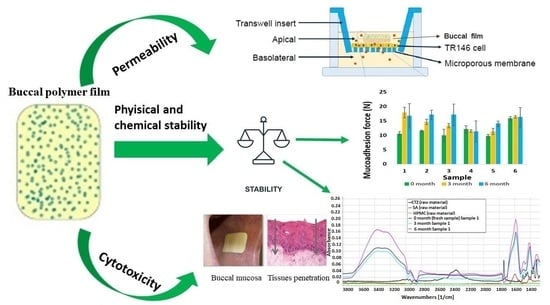
Stability, Permeability and Cytotoxicity of Buccal Films in Allergy Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Buccal Films
2.3. Stability Test
2.3.1. Breaking Hardness of Films
2.3.2. In Vitro Mucoadhesivity of Films
2.3.3. Active Agent Content
2.3.4. FT-IR Spectroscopy
2.4. Permeation Test
2.4.1. Permeation Test across Artificial Membrane
2.4.2. Cell Culturing
2.4.3. In Vitro Permeation Test
2.5. Cytotoxicity Test
2.6. Statistical Analyses
3. Results
3.1. Stability Test
3.1.1. Breaking Hardness of Films
3.1.2. In Vitro Mucoadhesivity of Films
3.1.3. Active Agent Content
3.1.4. FT-IR Spectroscopy
3.2. Cytotoxicity Test
3.3. Permeation Test
3.3.1. Permeation Test across Artificial Membrane
3.3.2. In Vitro Permeation Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McConnell, T.H. The Nature of Disease: Pathology for the Health Professions; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2007; ISBN 978-0-7817-5317-3. [Google Scholar]
- Dahl, R.; Stender, A.; Rak, S. Specific Immunotherapy with SQ Standardized Grass Allergen Tablets in Asthmatics with Rhinoconjunctivitis. Allergy 2006, 61, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, L.M.; Togias, A. Allergic Rhinitis. N. Engl. J. Med. 2015, 372, 456–463. [Google Scholar] [CrossRef] [Green Version]
- Larsen, J.N.; Broge, L.; Jacobi, H. Allergy Immunotherapy: The Future of Allergy Treatment. Drug Discov. Today 2016, 21, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Holgate, S.T.; Polosa, R. Treatment Strategies for Allergy and Asthma. Nat. Rev. Immunol. 2008, 8, 218–230. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, G.; Kumar, D.; Singh, M. Formulation Development and Evaluation of Fast Disintegrating Tablets of Salbutamol Sulphate, Cetirizine Hydrochloride in Combined Pharmaceutical Dosage Form: A New Era in Novel Drug Delivery for Pediatrics and Geriatrics. J. Drug Deliv. 2015, 2015, 640529. [Google Scholar] [CrossRef] [PubMed]
- Bizikova, P.; Papich, M.G.; Olivry, T. Hydroxyzine and Cetirizine Pharmacokinetics and Pharmacodynamics after Oral and Intravenous Administration of Hydroxyzine to Healthy Dogs. Vet. Dermatol. 2008, 19, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Durda, J.; Wedi, B.; Martin, V.; Breuer, K. Hyperresponsiveness to Antihistamines in Spontaneous Urticaria and Heat Urticaria. Allergol Sel. 2017, 1, 222–226. [Google Scholar] [CrossRef]
- May, J.R.; Dolen, W.K. Management of Allergic Rhinitis: A Review for the Community Pharmacist. Clin. Ther. 2017, 39, 2410–2419. [Google Scholar] [CrossRef] [Green Version]
- Avachat, A.M.; Gujar, K.N.; Wagh, K.V. Development and Evaluation of Tamarind Seed Xyloglucan-Based Mucoadhesive Buccal Films of Rizatriptan Benzoate. Carbohydr. Polym. 2013, 91, 537–542. [Google Scholar] [CrossRef]
- Orlu, M.; Ranmal, S.R.; Sheng, Y.; Tuleu, C.; Seddon, P. Acceptability of Orodispersible Films for Delivery of Medicines to Infants and Preschool Children. Drug Deliv. 2017, 24, 1243–1248. [Google Scholar] [CrossRef] [Green Version]
- Chachlioutaki, K.; Tzimtzimis, E.K.; Tzetzis, D.; Chang, M.-W.; Ahmad, Z.; Karavasili, C.; Fatouros, D.G. Electrospun Orodispersible Films of Isoniazid for Pediatric Tuberculosis Treatment. Pharmaceutics 2020, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and Revisited Approaches in Nanoparticle Systems for Buccal Drug Delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.F.; Liu, F.; Brown, M.B. Advances in Oral Transmucosal Drug Delivery. J. Control. Release 2011, 153, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In Vitro Techniques to Evaluate Buccal Films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Hearnden, V.; Sankar, V.; Hull, K.; Juras, D.V.; Greenberg, M.; Kerr, A.R.; Lockhart, P.B.; Patton, L.L.; Porter, S.; Thornhill, M.H. New Developments and Opportunities in Oral Mucosal Drug Delivery for Local and Systemic Disease. Adv. Drug Deliv. Rev. 2012, 64, 16–28. [Google Scholar] [CrossRef]
- Gibaja, V.; Javot, L.; Tournebize, J.; Gillet, P. Données récentes de pharmacosurveillance du fentanyl d’action rapide: Alerte sur le mésusage. Therapies 2021, 76, 165. [Google Scholar] [CrossRef]
- Khan, S.; Boateng, J.S.; Mitchell, J.; Trivedi, V. Formulation, Characterisation and Stabilisation of Buccal Films for Paediatric Drug Delivery of Omeprazole. AAPS PharmSciTech 2015, 16, 800–810. [Google Scholar] [CrossRef] [Green Version]
- Tejada, G.; Lamas, M.C.; Svetaz, L.; Salomón, C.J.; Alvarez, V.A.; Leonardi, D. Effect of Drug Incorporation Technique and Polymer Combination on the Performance of Biopolymeric Antifungal Buccal Films. Int. J. Pharm. 2018, 548, 431–442. [Google Scholar] [CrossRef] [Green Version]
- Trastullo, R.; Abruzzo, A.; Saladini, B.; Gallucci, M.C.; Cerchiara, T.; Luppi, B.; Bigucci, F. Design and Evaluation of Buccal Films as Paediatric Dosage Form for Transmucosal Delivery of Ondansetron. Eur. J. Pharm. Biopharm. 2016, 105, 115–121. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Goomber, G.; Gupta, S. Buccal Films of Prednisolone with Enhanced Bioavailability. Drug Deliv. 2016, 23, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, U.D.; Mahalingam, R.; Li, X.; Pather, I.; Jasti, B. Effect of Experimental Temperature on the Permeation of Model Diffusants Across Porcine Buccal Mucosa. AAPS PharmSciTech 2011, 12, 579–586. [Google Scholar] [CrossRef] [Green Version]
- Morales, J.O.; McConville, J.T. Manufacture and Characterization of Mucoadhesive Buccal Films. Eur. J. Pharm. Biopharm. 2011, 77, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Hanif, M.; Shaheryar, Z.A. Development of Tizanidine HCl-Meloxicam Loaded Mucoadhesive Buccal Films: In-Vitro and in-Vivo Evaluation. PLoS ONE 2018, 13, e0194410. [Google Scholar] [CrossRef] [PubMed]
- Nair, A.B.; Al-Dhubiab, B.E.; Shah, J.; Vimal, P.; Attimarad, M.; Harsha, S. Development and Evaluation of Palonosetron Loaded Mucoadhesive Buccal Films. J. Drug Deliv. Sci. Technol. 2018, 47, 351–358. [Google Scholar] [CrossRef]
- Okeke, O.C.; Boateng, J.S. Nicotine Stabilization in Composite Sodium Alginate Based Wafers and Films for Nicotine Replacement Therapy. Carbohydr. Polym. 2017, 155, 78–88. [Google Scholar] [CrossRef]
- Tagami, T.; Ando, M.; Nagata, N.; Goto, E.; Yoshimura, N.; Takeuchi, T.; Noda, T.; Ozeki, T. Fabrication of Naftopidil-Loaded Tablets Using a Semisolid Extrusion-Type 3D Printer and the Characteristics of the Printed Hydrogel and Resulting Tablets. J. Pharm. Sci. 2019, 108, 907–913. [Google Scholar] [CrossRef]
- Kumria, R.; Nair, A.B.; Al-Dhubiab, B.E. Loratidine Buccal Films for Allergic Rhinitis: Development and Evaluation. Drug Dev. Ind. Pharm. 2014, 40, 625–631. [Google Scholar] [CrossRef]
- Deshmukh, K.; Basheer Ahamed, M.; Deshmukh, R.R.; Khadheer Pasha, S.K.; Bhagat, P.R.; Chidambaram, K. Biopolymer Composites With High Dielectric Performance: Interface Engineering. In Biopolymer Composites in Electronics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–128. ISBN 978-0-12-809261-3. [Google Scholar]
- Paolicelli, P.; Petralito, S.; Varani, G.; Nardoni, M.; Pacelli, S.; Di Muzio, L.; Tirillò, J.; Bartuli, C.; Cesa, S.; Casadei, M.A.; et al. Effect of Glycerol on the Physical and Mechanical Properties of Thin Gellan Gum Films for Oral Drug Delivery. Int. J. Pharm. 2018, 547, 226–234. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Rossi, S.; Ferrari, F.; Gibin, S.; Caramella, C. Chitosan Citrate as Multifunctional Polymer for Vaginal Delivery. Eur. J. Pharm. Sci. 2008, 33, 166–176. [Google Scholar] [CrossRef]
- Ibrahim, Y.H.-E.Y.; Regdon, G.; Kristó, K.; Kelemen, A.; Adam, M.E.; Hamedelniel, E.I.; Sovány, T. Design and Characterization of Chitosan/Citrate Films as Carrier for Oral Macromolecule Delivery. Eur. J. Pharm. Sci. 2020, 146, 105270. [Google Scholar] [CrossRef] [PubMed]
- Kelemen, A.; Gottnek, M.; Regdon, G.; Pintye-Hódi, K. New Equipment for Measurement of the Force of Adhesion of Mucoadhesive Films. J. Adhes. Sci. Technol. 2015, 29, 1360–1367. [Google Scholar] [CrossRef] [Green Version]
- Gottnek, M.; Süvegh, K.; Pintye-Hódi, K.; Regdon, G. Effects of Excipients on the Tensile Strength, Surface Properties and Free Volume of Klucel® Free Films of Pharmaceutical Importance. Radiat. Phys. Chem. 2013, 89, 57–63. [Google Scholar] [CrossRef]
- Patel, V.F.; Liu, F.; Brown, M.B. Modeling the Oral Cavity: In Vitro and in Vivo Evaluations of Buccal Drug Delivery Systems. J. Control. Release 2012, 161, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Sander, C.; Nielsen, H.M.; Jacobsen, J. Buccal Delivery of Metformin: TR146 Cell Culture Model Evaluating the Use of Bioadhesive Chitosan Discs for Drug Permeability Enhancement. Int. J. Pharm. 2013, 458, 254–261. [Google Scholar] [CrossRef]
- Tetyczka, C.; Griesbacher, M.; Absenger-Novak, M.; Fröhlich, E.; Roblegg, E. Development of Nanostructured Lipid Carriers for Intraoral Delivery of Domperidone. Int. J. Pharm. 2017, 526, 188–198. [Google Scholar] [CrossRef]
- Nielsen, H.M.; Verhoef, J.C.; Ponec, M.; Rassing, M.R. TR146 Cells Grown on Filters as a Model of Human Buccal Epithelium: Permeability of Fluorescein Isothiocyanate-Labelled Dextrans in the Presence of Sodium Glycocholate. J. Control. Release 1999, 60, 223–233. [Google Scholar] [CrossRef]
- Azarmi, S.; Roa, W.; Löbenberg, R. Current Perspectives in Dissolution Testing of Conventional and Novel Dosage Forms. Int. J. Pharm. 2007, 328, 12–21. [Google Scholar] [CrossRef]
- Jacobsen, J.; van Deurs, B.; Pedersen, M.; Rassing, M.R. TR146 Cells Grown on Filters as a Model for Human Buccal Epithelium: I. Morphology, Growth, Barrier Properties, and Permeability. Int. J. Pharm. 1995, 125, 165–184. [Google Scholar] [CrossRef]
- Pham Le Khanh, H.; Nemes, D.; Rusznyák, Á.; Ujhelyi, Z.; Fehér, P.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Bácskay, I. Comparative Investigation of Cellular Effects of Polyethylene Glycol (PEG) Derivatives. Polymers 2022, 14, 279. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric Acid Cross-Linking of Starch Films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Uranga, J.; Puertas, A.I.; Etxabide, A.; Dueñas, M.T.; Guerrero, P.; de la Caba, K. Citric Acid-Incorporated Fish Gelatin/Chitosan Composite Films. Food Hydrocoll. 2019, 86, 95–103. [Google Scholar] [CrossRef]
- Rangel-Marrón, M.; Mani-López, E.; Palou, E.; López-Malo, A. Effects of Alginate-Glycerol-Citric Acid Concentrations on Selected Physical, Mechanical, and Barrier Properties of Papaya Puree-Based Edible Films and Coatings, as Evaluated by Response Surface Methodology. LWT 2019, 101, 83–91. [Google Scholar] [CrossRef]
- Szekalska, M.; Wróblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole. Mar. Drugs 2019, 17, 692. [Google Scholar] [CrossRef] [Green Version]
- Juliano, C.; Gavini, E.; Cossu, M.; Bonferoni, M.C.; Giunchedi, P. Mucoadhesive Alginate Matrices Containing Sodium Carboxymethyl Starch for Buccal Delivery: In Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2004, 14, 159–163. [Google Scholar] [CrossRef]
- Majumder, J.; Deb, J.; Husain, A.; Jana, S.S.; Dastidar, P. Cetirizine Derived Supramolecular Topical Gel in Action: Rational Design, Characterization and in Vivo Self-Delivery Application in Treating Skin Allergy in Mice. J. Mater. Chem. B 2015, 3, 6634–6644. [Google Scholar] [CrossRef]
- Salimi, A.; Razian, M.; Pourahmad, J. Analysis of Toxicity Effects of Buspirone, Cetirizine and Olanzapine on Human Blood Lymphocytes: In Vitro Model. Curr. Clin. Pharmacol. 2018, 13, 120–127. [Google Scholar] [CrossRef]







| Samples | SA (w/w %) | HPMC (w/w %) | GLY (w/w %) | CA (w/w %) | CTZ (10 mg) |
|---|---|---|---|---|---|
| 1 | 3 | 0 | 1 | − | + |
| 2 | 3 | 0 | 1 | + | + |
| 3 | 3 | 0 | 3 | − | + |
| 4 | 3 | 0 | 3 | + | + |
| 5 | 1.5 | 1.5 | 1 | − | + |
| 6 | 1.5 | 1.5 | 1 | + | + |
| 7 | 1.5 | 1.5 | 3 | − | + |
| 8 | 1.5 | 1.5 | 3 | + | + |
| 9 | 2 | 1 | 1 | − | + |
| 10 | 2 | 1 | 1 | + | + |
| 11 | 2 | 1 | 3 | − | + |
| 12 | 2 | 1 | 3 | + | + |
| Samples | Cell Viability Compared to Control (% ±SD) |
|---|---|
| 1 | 67.1 ± 2.7 |
| 2 | 78.7 ± 0.6 |
| 3 | 92.8 ± 0.4 |
| 4 | 56.3 ± 1.0 |
| 5 | 99.3 ± 0.8 |
| 6 | 55.9 ± 0.2 |
| 7 | 33.6 ± 0.7 |
| 8 | 87.7 ± 0.1 |
| 9 | 87.2 ± 0.5 |
| 10 | 17.4 ± 0.3 |
| 11 | 56.4 ± 4.6 |
| 12 | 91.3 ± 1.5 |
| Triton X | 0.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pamlényi, K.; Regdon, G., Jr.; Nemes, D.; Fenyvesi, F.; Bácskay, I.; Kristó, K. Stability, Permeability and Cytotoxicity of Buccal Films in Allergy Treatment. Pharmaceutics 2022, 14, 1633. https://doi.org/10.3390/pharmaceutics14081633
Pamlényi K, Regdon G Jr., Nemes D, Fenyvesi F, Bácskay I, Kristó K. Stability, Permeability and Cytotoxicity of Buccal Films in Allergy Treatment. Pharmaceutics. 2022; 14(8):1633. https://doi.org/10.3390/pharmaceutics14081633
Chicago/Turabian StylePamlényi, Krisztián, Géza Regdon, Jr., Dániel Nemes, Ferenc Fenyvesi, Ildikó Bácskay, and Katalin Kristó. 2022. "Stability, Permeability and Cytotoxicity of Buccal Films in Allergy Treatment" Pharmaceutics 14, no. 8: 1633. https://doi.org/10.3390/pharmaceutics14081633