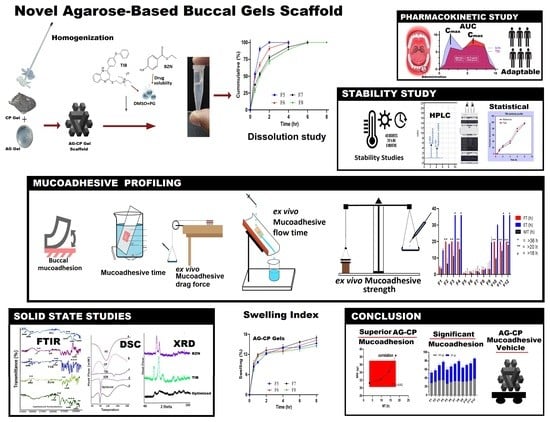
Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Design
2.3. Formulation Technique
2.4. Solid State Studies
2.4.1. Fourier Transform Infrared (FTIR)
2.4.2. Differential Scanning Calorimetry (DSC)
2.4.3. X-ray Powder Diffraction (PXRD)
2.5. Physicochemical Characterization
2.5.1. General Appearance
2.5.2. pH
2.5.3. Spreadibility (SP)
2.5.4. Contents Uniformity
2.5.5. Swelling Index (SI)
2.5.6. Matrix Erosion (ME)
2.5.7. Ex Vivo Mucoadhesive Strength (MS)
2.5.8. Ex Vivo Mucoadhesive Time (ET)
2.5.9. Ex Vivo Mucoadhesive Flow Time (FT)
2.5.10. Ex Vivo Mucoadhesive Drag Force (DF)
2.5.11. Mucoadhesive Time in Healthy Volunteers (MT)
2.5.12. In Vitro Drugs Release
2.5.13. HPLC Instrumental Settings
2.5.14. In Vitro Drugs Release Kinetics
2.5.15. In Vivo Volunteer Adaptability Response
2.5.16. In Vivo Salivary Drug Concentration
2.5.17. Stability Study
3. Results and Discussion
3.1. Solid State Characterization
3.1.1. FTIR Spectral Analysis
3.1.2. DSC Analysis
3.1.3. PXRD Analysis
3.2. Physicochemical Evaluation of Buccal Gels
3.2.1. General Appearance
3.2.2. pH
3.2.3. Spreadibility (SP)
3.2.4. Contents Uniformity
3.2.5. Swelling Index (SI)
3.2.6. Matrix Erosion (ME)
3.2.7. Ex Vivo Mucoadhesive Strength (MS)
3.2.8. Ex Vivo Mucoadhesive Drag Force (DF)
3.2.9. Ex Vivo Mucoadhesive Time (ET)
3.2.10. Ex Vivo Mucoadhesive Flow Time (FT)
3.2.11. Mucoadhesive Time in Healthy Volunteers (MT)
3.2.12. In Vitro Drugs Release
3.2.13. In Vitro Drugs Release Kinetics
3.2.14. In Vivo Volunteer Adaptability Response
3.2.15. In Vivo Salivary Drug Concentration
3.2.16. Stability Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hua, S. Advances in oral drug delivery for regional targeting in the gastrointestinal tract-influence of physiological, pathophysiological and pharmaceutical factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef] [PubMed]
- Macedo, A.S.; Castro, P.M.; Roque, L.; Thomé, N.G.; Reis, C.P.; Pintado, M.E.; Fonte, P. Novel and revisited approaches in nanoparticle systems for buccal drug delivery. J. Control. Release 2020, 320, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, J.; Landfester, K.; Mailänder, V. The challenges of oral drug delivery via nanocarriers. Drug Deliv. 2018, 25, 1694–1705. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Ng, K.W.; Lau, W.M.; Khutoryanskiy, V.V.J.P. Silica nanoparticles in transmucosal drug delivery. Pharmaceutics 2020, 12, 751. [Google Scholar] [CrossRef]
- Berillo, D.; Zharkinbekov, Z.; Kim, Y.; Raziyeva, K.; Temirkhanova, K.; Saparov, A. Stimuli-Responsive Polymers for Transdermal, Transmucosal and Ocular Drug Delivery. Pharmaceutics 2021, 13, 2050. [Google Scholar] [CrossRef]
- Deepak, A.; Goyal, A.K.; Rath, G. Nanofiber in transmucosal drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 379–387. [Google Scholar] [CrossRef]
- Syed, M.A.; Zahoor, A.F.; Iqbal, M.S.; Syed, H.K.; Khan, I.U.; Shah, M.A.; Hanif, S.; Mohsin, N.A.; Islam, N.; Ikram, M.; et al. In vitro-Ex vivo Characterization of Agarose–Carbopol 934® Based Buccal Mucoadhesive Tablets Containing Benzocaine and Tibezonium Iodide as Model Drugs. Lat. Am. J. Pharm. 2022, 41, 1–10. [Google Scholar]
- Alawdi, S.; Solanki, A.B. Mucoadhesive Drug Delivery Systems: A Review of Recent Developments. J. Sci. Res. Med. Biol. Sci. 2021, 2, 50–64. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, K.; Wang, J.; Zhao, R.; Zhang, Q.; Kong, X. Poly(amidoamine)-modified mesoporous silica nanoparticles as a mucoadhesive drug delivery system for potential bladder cancer therapy. Colloids Surf. B Biointerfaces 2020, 189, 110832. [Google Scholar] [CrossRef]
- Asati, S.; Jain, S.; Choubey, A. Bioadhesive or mucoadhesive drug delivery system: A potential alternative to conventional therapy. J. Drug Deliv. Ther. 2019, 9, 858–867. [Google Scholar]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Corral, J.R.; Mitrani, H.; Dade-Robertson, M.; Zhang, M.; Maiello, P. Agarose gel as a soil analogue for development of advanced bio-mediated soil improvement methods. Can. Geotech. J. 2020, 57, 2010–2019. [Google Scholar] [CrossRef]
- Hamzavi, N.; Dewavrin, J.Y.; Drozdov, A.D.; Birgersson, E. Nonmonotonic swelling of agarose-carbopol hybrid hydrogel: Experimental and theoretical analysis. J. Polym. Sci. B Polym. Phys. 2017, 55, 444–454. [Google Scholar] [CrossRef]
- Oyen, M. Mechanical characterisation of hydrogel materials. Int. Mater. Rev. 2014, 59, 44–59. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Hussain, Z. Smart mucoadhesive buccal chitosan/HPMC scaffold for sore throat: In vitro, ex vivo and pharmacokinetic profiling in humans. J. Drug Deliv. Sci. Technol. 2022, 71, 103271. [Google Scholar] [CrossRef]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Ali, S.; Iqbal, Z.; Shakir, R.; Iqbal, J. Formulation and Evaluation of Chitosan-Based Polymeric Biodegradable Mucoadhesive Buccal Delivery for Locally Acting Drugs: In Vitro, Ex Vivo and In Vivo Volunteers Characterization. Lat. Am. J. Pharm. 2021, 40, 670–681. [Google Scholar]
- Hanif, S.; Sarfraz, R.M.; Syed, M.A.; Mahmood, A.; Minhas, M.U.; Irfan, M. Development and optimization of tibezonium iodide and lignocaine hydrochloride containing novel mucoadhesive buccal tablets: A pharmacokinetic investigation among healthy humans. Drug Dev. Ind. Pharm. 2021, 47, 1209–1222. [Google Scholar] [CrossRef]
- Rathore, K.; Tyagi, C.; Pandey, H. Prepare and evaluate mucoadhesive formulations of lamivudine with better controlled/sustained drug release profile. J. Drug Deliv. Ther. 2019, 9, 694–700. [Google Scholar]
- Alves, M.C.; Chaves, D.S.; Benevenuto, B.R.; Farias, B.O.; Coelho, S.M.; Ferreira, T.P.; Pereira, G.A.; Santos, G.; Moreira, L.O.; Freitas, J.P. Chitosan gels for buccal delivery of Schinus molle L. essential oil in dogs: Characterization and antimicrobial activity in vitro. An. Acad. Bras. Ciências 2020, 92, e20200562. [Google Scholar] [CrossRef] [PubMed]
- Kenechukwu, F.C.; Attama, A.A.; Ibezim, E.C.; Nnamani, P.O.; Umeyor, C.E.; Uronnachi, E.M.; Gugu, T.H.; Momoh, M.A.; Ofokansi, K.C.; Akpa, P.A. Surface-modified mucoadhesive microgels as a controlled release system for miconazole nitrate to improve localized treatment of vulvovaginal candidiasis. Eur. J. Pharm. Sci. 2018, 111, 358–375. [Google Scholar] [CrossRef]
- Ashri, L.Y.; El Sayeh, F.A.; Ibrahim, M.A.; Alshora, D.H. Optimization and evaluation of chitosan buccal films containing tenoxicam for treating chronic periodontitis: In vitro and in vivo studies. J. Drug Deliv. Sci. Technol. 2020, 57, 101720. [Google Scholar] [CrossRef]
- Javed, Q.U.A.; Syed, M.A.; Arshad, R.; Rahdar, A.; Irfan, M.; Raza, S.A.; Shahnaz, G.; Hanif, S.; Díez-Pascual, A.M. Evaluation and Optimization of Prolonged Release Mucoadhesive Tablets of Dexamethasone for Wound Healing: In Vitro–In Vivo Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 807. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, S.; Hanif, S.; Syed, M.A.; Iqbal, J.; Raza, S.A.; Riaz, H.; Abid, F. Development and evaluation of mucoadhesive buccal tablet containing metronidazole for the treatment of periodontitis and gingivitis. Pak. J. Pharm. Sci. 2018, 31, 1903–1910. [Google Scholar] [PubMed]
- Chen, X.; Yan, J.; Yu, S.; Wang, P. Formulation and in vitro release kinetics of Mucoadhesive blend gels containing matrine for buccal administration. AAPS PharmSciTech 2018, 19, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Baus, R.A.; Zahir-Jouzdani, F.; Dünnhaupt, S.; Atyabi, F.; Bernkop-Schnürch, A. Mucoadhesive hydrogels for buccal drug delivery: In vitro-in vivo correlation study. Eur. J. Pharm. Biopharm. 2019, 142, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Nastri, L.; De Rosa, A.; De Gregorio, V.; Grassia, V.; Donnarumma, G. A new controlled-release material containing metronidazole and doxycycline for the treatment of periodontal and peri-implant diseases: Formulation and in vitro testing. Int. J. Dent. 2019, 2019, 9374607. [Google Scholar] [CrossRef] [Green Version]
- Syed, M.A.; Khan, I.U.; Iqbal, M.S.; Syed, H.K.; Irfan, M. Development of a Novel, Fast, Simple, Non-derived RP-HPLC Method for simultaneous Estimation of Benzocaine and Tibezonium Iodide from Mucoadhesive Dosage Form as well as Human Saliva and its Validation. Lat. Am. J. Pharm. 2021, 40, 1281–1287. [Google Scholar]
- Razzaq, S.; Syed, M.A.; Irfan, M.; Khan, I.; Sarfraz, R.M.; Shakir, R.; Ali, S.; Iqbal, Z.; Niaz, Y.; Mujtaba, S.H. Optimization of metronidazole SR buccal tablet for gingivitis using genetic algorithm. Pak. J. Pharm. Sci. 2021, 34, 2149–2158. [Google Scholar]
- Abu-Huwaij, R.; Obaidat, R.M.; Sweidan, K.; Al-Hiari, Y. Formulation and in vitro evaluation of xanthan gum or carbopol 934-based mucoadhesive patches, loaded with nicotine. Aaps Pharmscitech 2011, 12, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Freag, M.S.; Saleh, W.M.; Abdallah, O.Y. Exploiting polymer blending approach for fabrication of buccal chitosan-based composite sponges with augmented mucoadhesive characteristics. Eur. J. Pharm. Sci. 2018, 120, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Dabashis, R.; Kumar, M.; Saharan, R.; Malik, A.; Saini, V. Development and Validation of In Vitro Discriminatory Dissolution Testing Method for Fast Dispersible Tablets of BCS Class II Drug. Turk. J. Pharm. Sci. 2020, 17, 74. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh-Hashjin, A.; Shabani, M.; Monajjemzadeh, F. Evaluation of pharmaceutical compatibility between Acarbose and common excipients used in the development of controlled release formulations. Pharm. Sci. 2020, 27, 399–406. [Google Scholar] [CrossRef]
- Sahoo, S.; Chakraborti, C.K.; Mishra, S.C. Qualitative analysis of controlled release ciprofloxacin/carbopol 934 mucoadhesive suspension. J. Adv. Pharm. Technol. Res. 2011, 2, 195. [Google Scholar] [CrossRef]
- Hussain, A.; Syed, M.A.; Abbas, N.; Hanif, S.; Arshad, M.S.; Bukhari, N.I.; Hussain, K.; Akhlaq, M.; Ahmad, Z. Development of an ANN optimized mucoadhesive buccal tablet containing flurbiprofen and lidocaine for dental pain. Acta Pharm. 2016, 66, 245–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Marzouqi, A.H.; Jobe, B.; Dowaidar, A.; Maestrelli, F.; Mura, P. Evaluation of supercritical fluid technology as preparative technique of benzocaine–cyclodextrin complexes—Comparison with conventional methods. J. Pharm. Biomed. Anal. 2007, 43, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Ige, P.P.; Mahajan, D.R.; Sonawane, R.O. Development of mucoadhesive sustained release matrix tablets of methimazole for oral delivery. Thyroid 2015, 17, 20. [Google Scholar]
- El Sayeh, F.A.; El Khatib, M.M. Formulation and evaluation of new long acting metoprolol tartrate ophthalmic gels. Saudi Pharm. J. 2014, 22, 555–563. [Google Scholar]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A novel electroactive agarose-aniline pentamer platform as a potential candidate for neural tissue engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, L.H.; de Carvalho, M.Z.; da Silva Melo, P.; de Paula, E.; Saczk, A.A.; de Matos Alves Pinto, L. Characterization and cytotoxicity of a benzocaine inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 9–15. [Google Scholar] [CrossRef]
- Basha, M.; El-Alim, S.H.A.; Alaa Kassem, A.; El Awdan, S.; Awad, G. Benzocaine loaded solid lipid nanoparticles: Formulation design, in vitro and in vivo evaluation of local anesthetic effect. Curr. Drug Deliv. 2015, 12, 680–692. [Google Scholar] [CrossRef] [PubMed]
- Bertula, K.; Martikainen, L.; Munne, P.; Hietala, S.; Klefström, J.; Ikkala, O.; Nonappa. Strain-stiffening of agarose gels. ACS Macro Lett. 2019, 8, 670–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelbary, G.A.; Aburahma, M.H. Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J. Liposome Res. 2015, 25, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Gutowski, I.A. The Effects of pH and Concentration on the Rheology of Carbopol Gels. Master’s Thesis, Simon Fraser University, Burnaby, BC, Canada, 2010. [Google Scholar]
- Samanta, H.S.; Ray, S.K. Synthesis, characterization, swelling and drug release behavior of semi-interpenetrating network hydrogels of sodium alginate and polyacrylamide. Carbohydr. Polym. 2014, 99, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Manouchehri, S.; Ahmadi, Z.; Saeb, M.R.; Urbanska, A.M.; Kaplan, D.L.; Mozafari, M. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018, 187, 66–84. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Santoro, M.; Casalini, T.; Veglianese, P.; Masi, M.; Perale, G. Characterization and degradation behavior of agar–carbomer based hydrogels for drug delivery applications: Solute effect. Int. J. Mol. Sci. 2011, 12, 3394–3408. [Google Scholar] [CrossRef] [Green Version]
- Hanif, S.; Irfan, N.; Danish, Z.; Hussain, N.; Ali, M.; Nasir, B.; Iqbal, J.; Saeed, H.; Ali, R.; Saleem, Z. Computer Aided Formulation and Characterization of Propranolol Hcl Buccal Tablet Using Polymeric Blend. Open Conf. Proc. J. 2017, 8, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Builders, P.F.; Kunle, O.O.; Adikwu, M.U. Preparation and characterization of mucinated agarose: A mucin–agarose physical crosslink. Int. J. Pharm. 2008, 356, 174–180. [Google Scholar] [CrossRef]
- Bertasa, M.; Dodero, A.; Alloisio, M.; Vicini, S.; Riedo, C.; Sansonetti, A.; Scalarone, D.; Castellano, M. Agar gel strength: A correlation study between chemical composition and rheological properties. Eur. Polym. J. 2020, 123, 109442. [Google Scholar] [CrossRef]
- Daneshi, M.; Pourzahedi, A.; Martinez, D.; Grecov, D. Characterising wall-slip behaviour of Carbopol gels in a fully-developed Poiseuille flow. J. Non-Newton. Fluid Mech. 2019, 269, 65–72. [Google Scholar] [CrossRef]
- Ni, X.; Guo, Q.; Zou, Y.; Xuan, Y.; Mohammad, I.S.; Ding, Q.; Hu, H. Preparation and characterization of bear bile-loaded pH sensitive in-situ gel eye drops for ocular drug delivery. Iran. J. Basic Med. Sci. 2020, 23, 922. [Google Scholar]
- Lee, K.J.; Yun, S.I. Nanocomposite hydrogels based on agarose and diphenylalanine. Polymer 2018, 139, 86–97. [Google Scholar] [CrossRef]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; Di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]













 ) and after (
) and after (  ) stability conditions.
) stability conditions.
 ) and after (
) and after (  ) stability conditions.
) stability conditions.
 ) and after (
) and after (  ) stability conditions.
) stability conditions.
 ) and after (
) and after (  ) stability conditions.
) stability conditions.
| Codes | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AG (% w/v) | 0.5 | 0.75 | 1.0 | 1.25 | - | - | - | - | 0.5 | 0.75 | 1.0 | 1.25 |
| CP (% w/v) | - | - | - | - | 0.5 | 0.75 | 1.0 | 1.25 | 0.5 | 0.75 | 1.0 | 1.25 |
| Code | SP (%) | ME (%) |
|---|---|---|
| F1 | 140.2 | 90.0 |
| F2 | 101.9 | 89.9 |
| F3 | 88.7 | 91.2 |
| F4 | 75.7 | 89.8 |
| F5 | 165 | 93.7 |
| F6 | 159.4 | 92.3 |
| F7 | 144.7 | 91.6 |
| F8 | 139.3 | 91.3 |
| F9 | 156.1 | 90.9 |
| F10 | 134.37 | 93.4 |
| F11 | 115.2 | 93.3 |
| F12 | 95.56 | 93.7 |
| TIB | BZN | |||
|---|---|---|---|---|
| Coefficient Value (r2) | ||||
| In Vitro | Salivary | In Vitro | Salivary | |
| Zero-order (k0) | 0.8958 | 0.7755 | 0.9801 | 0.2800 |
| First order(k1st) | 0.8958 | 0.8509 | 0.9700 | 0.0315 |
| Higuchi (kH) | 0.8034 | 0.8758 | 0.9230 | 0.3777 |
| Korsmeyer-Peppas (kKP) (n) | 0.9892 (1.212) | 0.8851 (0.591) | 0.9960 (0.811) | 0.8337 (0.490) |
| Hixson Crowell (kHC) | 0.9255 | 0.8298 | 0.9881 | 0.1154 |
| Parameters | Response (%) | Parameters | Response (%) |
|---|---|---|---|
| 1-Mucosal irritation during adhesion | 5-Numbness post 4 h administration | ||
| Yes | - | Yes | 100 |
| No | 100 | No | - |
| 2-Dosage form displacement from point of administration | 6-Post removal taste disturbance till 10 h | ||
| severe | - | ||
| Yes | - | slight | 6.67 |
| No | 100 | none | 93.33 |
| 3-Saliva production response | 7-Mucosal soreness | ||
| moderate | 13.3 | severe | - |
| slight | 40.0 | slight | - |
| none | 46.7 | c- none | 100 |
| 4-Numbness post 0.5 h administration | 8-Mucosal dryness | ||
| Yes | 93.33 | Yes | - |
| No | 6.67 | No | 100 |
| Parameters | BZN | TIB |
|---|---|---|
| Dose (mg) | 15 | 15 |
| Cmax (µg/mL) | 9.97 | 8.69 |
| tmax (h) | 2 | 6 |
| Kel (h−1) | −0.24 | −0.71 |
| AUC0–t (µG·h/mL) | 59.28 | 55.75 |
| AUCt–∞ (µg·h/mL) | 26.89 | 10.12 |
| AUC0–∞ (µg·h/mL) | 86.17 | 65.88 |
| AUCt–∞ (%) | 31.21 | 17.88 |
| Contribution AUCt-∞ | significant | insignificant |
| Month | pH | MS (g ± SD) | DF (g ± SD) | ET (h ± SD) | FT (h ± SD) | Contents (% ± SD) n = 3 | |
|---|---|---|---|---|---|---|---|
| BZN | TIB | ||||||
| 0 | 6.75 | 32.19 ± 3.28 | 38.37 ± 2.63 | 30.04 ± 0.52 | >20.0 | 99.62 ± 0.14 | 98.10 ± 0.27 |
| 0.5 | 6.73 | 34.61 ± 4.02 | 39.13 ± 3.62 | >30 h | >20.0 | 99.54 ± 1.33 | 99.92 ± 1.66 |
| 1 | 6.78 | 33.86 ± 2.19 | 39.58 ± 1.09 | >30 h | >20.0 | 99.87 ± 0.86 | 99.00 ± 0.98 |
| 3 | 6.78 | 33. 82 ± 3.67 | 39.33 ± 1.11 | >30 h | >20.0 | 98.67 ± 0.69 | 98.28 ± 1.79 |
| 6 | 6.78 | 33.05 ± 2.33 | 39.98 ± 2.23 | >30 h | >20.0 | 99.19 ± 0.83 | 99.48 ± 0.17 |
| Release profile comparison after stability conditions | |||||||
| Similarity factor (f2) | 70.72 | 66.19 | |||||
| Dissimilarity factor (f1) | 7.00 | 10.79 | |||||
| Before–after Stability | Mean | Standard Deviation | Standard Error Mean | 95% Confidence Interval of the Difference | t Value | df | Sig. (2-Tailed) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| BZN | −2.617 | 2.853 | 1.078 | −5.256 | 0.022 | −2.426 | 6 | 0.051 |
| TIB | −3.442 | 3.355 | 1.268 | −6.545 | −0.339 | −2.715 | 6 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, M.A.; Aziz, G.; Jehangir, M.B.; Tabish, T.A.; Zahoor, A.F.; Khalid, S.H.; Khan, I.U.; Hosny, K.M.; Rizg, W.Y.; Hanif, S.; et al. Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 1592. https://doi.org/10.3390/pharmaceutics14081592
Syed MA, Aziz G, Jehangir MB, Tabish TA, Zahoor AF, Khalid SH, Khan IU, Hosny KM, Rizg WY, Hanif S, et al. Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers. Pharmaceutics. 2022; 14(8):1592. https://doi.org/10.3390/pharmaceutics14081592
Chicago/Turabian StyleSyed, Muhammad Ali, Ghiyyas Aziz, Muhammad Bilal Jehangir, Tanveer A. Tabish, Ameer Fawad Zahoor, Syed Haroon Khalid, Ikram Ullah Khan, Khaled Mohamed Hosny, Waleed Yousof Rizg, Sana Hanif, and et al. 2022. "Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers" Pharmaceutics 14, no. 8: 1592. https://doi.org/10.3390/pharmaceutics14081592