Biopolymers in Mucoadhesive Eye Drops for Treatment of Dry Eye and Allergic Conditions: Application and Perspectives
Abstract
:1. Introduction
2. Ocular Anatomy and Physiology
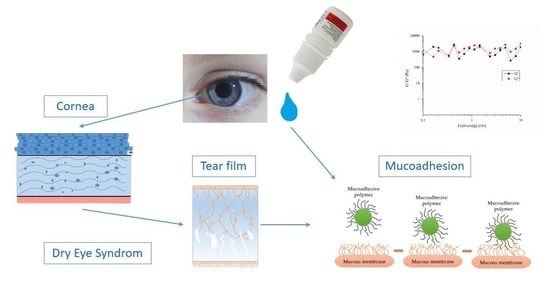
2.1. Cornea
2.2. Conjunctiva
2.3. Ocular Bariers
2.4. Tears and Lacrimal Drainage System
2.5. Mucin and Mucoadhesion
2.6. Pharmacokinetics of Locally Applied Drugs
3. Dry Eye Syndrome and Allergic Conjunctivitis
“Dry eye is a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”
| Type of Formulation/Drug Carrier | Active Substance(s) | Mucoadhesive Component | Assessment of Mucoadhesion | Reference |
|---|---|---|---|---|
| Nanoparticle | Epigallocatechin gallate | Hyaluronic acid | - | [65] |
| Nanoemulsion | Cyclosporine A | Chitosan | Mucoadhesive strength—texture analyzer, goat cornea | [66] |
| Nanoparticle | Cyclosporin A | Phenylboronic acid (PBA) | Assessment of covalent interaction between PBA and sialic acid using a spectrofluorometer | [67] |
| Nanostructured lipid carrier | Curcumin | Thiolated chitosan | - | [68] |
| Nanomicelles | Cyclosporine A | Hyaluronic acid | - | [69] |
| Niosomes | Tacrolimus | Hyaluronic acid | Surface plasmon resonance | [70] |
| Liposome | Hyaluronic acid, Crocin | Hyaluronic acid, Galactoxyloglucan | - | [71] |
| Nanoparticles | Tacrolimus | Gellan gum | - | [72] |
| Nanogels | Lysine-carbonized | Cationic lysine-carbonized nanogels | - | [73] |
| In situ gel | Vitamin B12 | Pluronic F127 and hydroxypropyl methyl cellulose | In vitro assessment adhesion | [74] |
| Solution | Hyaluronic acid | Hyaluronic acid | Rheological method | [75] |
| In situ gel | Cyclosporine A | Chitosan | Ex vivo mucoadhesion study, bovine cornea | [76] |
| Solution | 5-oxo-2-pyrrolidinecarboxylic acid | Hyaluronic acid | - | [77] |
| Nanogels | Poly(acrylic acid)/Polyvinylpyrrolidone | Poly(acrylic acid) | Rheological method; zeta potential measurements | [78] |
| Nanoemulsion | Ibuprofen | Chitosan | Rheological method | [61] |
| Cyclodextrin (CD)-based aggregate | Nepafenac | Sodium hyaluronate, sodium alginate | Mucoadhesive strength—texture analyzer, bovine cornea | [79] |
4. Polymers as Functional Excipients for Improvement of Ocular Availability
4.1. Mucoadhesive Biopolymers in the Ophthalmic Formulation
4.2. Use of Penetration Enhancers
5. Characterization of Mucoadhesive Ophthalmic Preparations
5.1. Assessment of Mucoadhesive Properties
- Sample (a): mucin dispersion 10% (m/m);
- Sample (b): tested polymeric solution;
- Sample (c): mixture of mucin dispersion 10% (m/m) and tested solution.
5.2. Consideration of Surface Tension
5.3. In Vitro and In Vivo Models Developed for Biopharmaceutical, Biocopmatibility, and Efficiency Assessment of Polymeric Eye Drops
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addo, R.T. Ocular Drug Delivery: Advances, Challenges and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Bouwman-Boer, Y.; Fenton-May, V.; Le Brun, P. Practical pharmaceutics. In An international Guideline for the Preparation, Care and Use of Medicinal Products; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Dubald, M.; Bourgeois, S.; Andrieu, V.; Fessi, H. Ophthalmic drug delivery systems for antibiotherapy—A review. Pharmaceutics 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanier, O.L.; Manfre, M.G.; Bailey, C.; Liu, Z.; Sparks, Z.; Kulkarni, S.; Chauhan, A. Review of Approaches for Increasing Ophthalmic Bioavailability for Eye Drop Formulations. AAPS PharmSciTech 2021, 22, 107. [Google Scholar] [CrossRef] [PubMed]
- Di Colo, G.; Zambito, Y.; Zaino, C.; Sanso, M. Selected polysaccharides at comparison for their mucoadhesiveness and effect on precorneal residence of different drugs in the rabbit model. Drug Dev. Ind. Pharm. 2009, 35, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Grassiri, B.; Zambito, Y.; Bernkop-Schnürch, A. Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv. Colloid Interface Sci. 2020, 288, 102342. [Google Scholar] [CrossRef]
- Ludwig, A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005, 57, 1595–1639. [Google Scholar] [CrossRef]
- Behar-Cohen, F. Drug delivery to the eye: Current trends and future perspectives. Ther. Deliv. 2012, 3, 1135–1137. [Google Scholar] [CrossRef] [Green Version]
- Galloway, N.R.; Amoaku, W.M.; Galloway, P.H.; Browning, A.C. Basic anatomy and physiology of the eye. In Common Eye Diseases and Their Management; Springer: Berlin/Heidelberg, Germany, 2022; pp. 7–18. [Google Scholar]
- Bye, L.; Modi, N.; Stanford, M. Basic Sciences for Ophthalmology; OUP Oxford: Oxford, UK, 2013. [Google Scholar]
- Frutos-Rincon, L.; Gomez-Sanchez, J.A.; Inigo-Portugues, A.; Acosta, M.C.; Gallar, J. An Experimental Model of Neuro-Immune Interactions in the Eye: Corneal Sensory Nerves and Resident Dendritic Cells. Int. J. Mol. Sci. 2022, 23, 2997. [Google Scholar] [CrossRef]
- Downie, L.E.; Bandlitz, S.; Bergmanson, J.P.; Craig, J.P.; Dutta, D.; Maldonado-Codina, C.; Ngo, W.; Siddireddy, J.S.; Wolffsohn, J.S. BCLA CLEAR-Anatomy and physiology of the anterior eye. Contact Lens Anterior Eye 2021, 44, 132–156. [Google Scholar] [CrossRef]
- Pathak, Y.; Sutariya, V.; Hirani, A.A. Nano-Biomaterials for Ophthalmic Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Sridhar, M.S. Anatomy of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 190. [Google Scholar] [CrossRef]
- Addo, E.; Bamiro, O.A.; Siwale, R. Anatomy of the eye and common diseases affecting the eye. In Ocular Drug Delivery: Advances, Challenges and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 11–25. [Google Scholar]
- Dave, V.S. Formulation approaches for ocular drug delivery. In Nano-Biomaterials for Ophthalmic Drug Delivery; Pathak, Y., Sutariya, V., Hirani, A.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 147–175. [Google Scholar]
- Irimia, T.; Ghica, M.V.; Popa, L.; Anuta, V.; Arsene, A.L.; Dinu-Pirvu, C.E. Strategies for Improving Ocular Drug Bioavailability and Corneal Wound Healing with Chitosan-Based Delivery Systems. Polymers 2018, 10, 1221. [Google Scholar] [CrossRef]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef] [PubMed]
- Gibson, M. Pharmaceutical Preformulation and Formulation, 2nd ed.; Gibson, M., Ed.; CRC Press: Boca Raton, FL, USA, 2009; Volume Ophthalmic Dosage Forms. [Google Scholar]
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Kim, D.H.; Park, C.K.; Kim, Y.H. Experimental Models, Induction Protocols, and Measured Parameters in Dry Eye Disease: Focusing on Practical Implications for Experimental Research. Int. J. Mol. Sci. 2021, 22, 12102. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, D.F.; Millar, T.J.; Raju, S.R. Tear film stability: A review. Exp. Eye Res. 2013, 117, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Van Santvliet, L.; Ludwig, A. Determinants of eye drop size. Surv. Ophthalmol. 2004, 49, 197–213. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Moncada, D.M.; Kammanadiminti, S.J.; Chadee, K. Mucin and Toll-like receptors in host defense against intestinal parasites. Trends Parasitol. 2003, 19, 305–311. [Google Scholar] [CrossRef]
- Baudouin, C.; Rolando, M.; Del Castillo, J.M.B.; Messmer, E.M.; Figueiredo, F.C.; Irkec, M.; Van Setten, G.; Labetoulle, M. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog. Retin. Eye Res. 2019, 71, 68–87. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. Macromol. Biosci. 2019, 19, 1900194. [Google Scholar] [CrossRef]
- Lehr, C.-M.; Haas, J. Developments in the area of bioadhesive drug delivery systems. Expert Opin. Biol. Ther. 2002, 2, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Öblom, H.; Hansen, A.C.N.; Jacobsen, J.; Chronakis, I.S.; Rantanen, J.; Nielsen, H.M.; Genina, N. Mucoadhesive chitosan-and cellulose derivative-based nanofiber-on-foam-on-film system for non-invasive peptide delivery. Carbohydr. Polym. 2022, 303, 120429. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, K.P.; Nalinratana, N.; Chutiwitoonchai, N.; Castillo, A.L.; Banlunara, W.; Vajragupta, O.; Rojsitthisak, P.; Rojsitthisak, P. Enhanced Nasal Deposition and Anti-Coronavirus Effect of Favipiravir-Loaded Mucoadhesive Chitosan–Alginate Nanoparticles. Pharmaceutics 2022, 14, 2680. [Google Scholar] [CrossRef]
- Sanap, S.N.; Bisen, A.C.; Kedar, A.; Yadav, K.S.; Krishna, A.; Akhir, A.; Chopra, S.; Mugale, M.N.; Bhatta, R.S. Chitosan/HPMC-based mucoadhesive film co-loaded with fluconazole and ofloxacin for management of polymicrobial keratitis. Int. J. Biol. Macromol. 2022, 222, 2785–2795. [Google Scholar] [CrossRef]
- Syed, M.A.; Aziz, G.; Jehangir, M.B.; Tabish, T.A.; Zahoor, A.F.; Khalid, S.H.; Khan, I.U.; Hosny, K.M.; Rizg, W.Y.; Hanif, S. Evaluating Novel Agarose-Based Buccal Gels Scaffold: Mucoadhesive and Pharmacokinetic Profiling in Healthy Volunteers. Pharmaceutics 2022, 14, 1592. [Google Scholar] [CrossRef]
- Ameeduzzafar; Imam, S.S.; Abbas Bukhari, S.N.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef]
- Kakkar, S.; Singh, M.; Mohan Karuppayil, S.; Raut, J.S.; Giansanti, F.; Papucci, L.; Schiavone, N.; Nag, T.C.; Gao, N.; Yu, F.X.; et al. Lipo-PEG nano-ocular formulation successfully encapsulates hydrophilic fluconazole and traverses corneal and non-corneal path to reach posterior eye segment. J. Drug Target 2021, 29, 631–650. [Google Scholar] [CrossRef]
- Zhang, W.; Prausnitz, M.R.; Edwards, A. Model of transient drug diffusion across cornea. J. Control. Release 2004, 99, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Velpandian, T. Pharmacology of Ocular Therapeutics; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kaur, I.P.; Smitha, R. Penetration enhancers and ocular bioadhesives: Two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002, 28, 353–369. [Google Scholar] [CrossRef]
- Kumar, S.; Karki, R.; Meena, M.; Prakash, T.; Rajeswari, T.; Goli, D. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J. Adv. Pharm. Technol. Res. 2011, 2, 192. [Google Scholar]
- Farkouh, A.; Frigo, P.; Czejka, M. Systemic side effects of eye drops: A pharmacokinetic perspective. Clin. Ophthalmol. 2016, 10, 2433. [Google Scholar] [CrossRef]
- Ranch, K.M.; Maulvi, F.A.; Naik, M.J.; Koli, A.R.; Parikh, R.K.; Shah, D.O. Optimization of a novel in situ gel for sustained ocular drug delivery using Box-Behnken design: In vitro, ex vivo, in vivo and human studies. Int. J. Pharm. 2019, 554, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Badran, M.M.; Alomrani, A.H.; Almomen, A.; Bin Jardan, Y.A.; Abou El Ela, A.E.S. Novel Metoprolol-Loaded Chitosan-Coated Deformable Liposomes in Thermosensitive In Situ Gels for the Management of Glaucoma: A Repurposing Approach. Gels 2022, 8, 635. [Google Scholar] [CrossRef]
- Szalai, B.; Jójárt-Laczkovich, O.; Kovács, A.; Berkó, S.; Balogh, G.T.; Katona, G.; Budai-Szűcs, M. Design and Optimization of In Situ Gelling Mucoadhesive Eye Drops Containing Dexamethasone. Gels 2022, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Hom, M.M.; Nguyen, A.L.; Bielory, L. Allergic conjunctivitis and dry eye syndrome. Ann. Allergy Asthma Immunol. 2012, 108, 163–166. [Google Scholar] [CrossRef]
- Leonardi, A.; Modugno, R.L.; Salami, E. Allergy and Dry Eye Disease. Ocul. Immunol. Inflamm. 2021, 29, 1168–1176. [Google Scholar] [CrossRef]
- Villani, E.; Rabbiolo, G.; Nucci, P. Ocular allergy as a risk factor for dry eye in adults and children. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A.; Foulks, G.N. The definition and classification of dry eye disease. Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K. TFOS DEWS II definition and classification report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V. Tfos dews ii pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar]
- Galor, A.; Moein, H.-R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Mantelli, F.; Massaro-Giordano, M.; Macchi, I.; Lambiase, A.; Bonini, S. The cellular mechanisms of dry eye: From pathogenesis to treatment. J. Cell. Physiol. 2013, 228, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Nye, M.; Rudner, S.; Bielory, L. Emerging therapies in allergic conjunctivitis and dry eye syndrome. Expert Opin. Pharmacother. 2013, 14, 1449–1465. [Google Scholar] [CrossRef]
- Jones, L.; Downie, L.E.; Korb, D.; Benitez-del-Castillo, J.M.; Dana, R.; Deng, S.X.; Dong, P.N.; Geerling, G.; Hida, R.Y.; Liu, Y. TFOS DEWS II management and therapy report. Ocul. Surf. 2017, 15, 575–628. [Google Scholar] [PubMed]
- Agarwal, P.; Craig, J.P.; Rupenthal, I.D. Formulation considerations for the management of dry eye disease. Pharmaceutics 2021, 13, 207. [Google Scholar] [CrossRef]
- Sheskey, P.J.; Cook, W.G.; Cable, C.G. Handbook of Pharmaceutical Excipients, 8th ed.; Pharmaceutical Press: London, UK, 2017. [Google Scholar]
- Benelli, U. Systane lubricant eye drops in the management of ocular dryness. Clin. Ophthalmol. 2011, 5, 783–790. [Google Scholar] [CrossRef] [Green Version]
- Chowhan, M.; Lang, J.C.; Missel, P. Ophthalmic preparations. In Remington: The Science and Practice of Pharmacy; Felton, L., Ed.; Pharmaceutical Press: London, UK, 2012. [Google Scholar]
- Tong, L.; Petznick, A.; Lee, S.; Tan, J. Choice of artificial tear formulation for patients with dry eye: Where do we start? Cornea 2012, 31, S32–S36. [Google Scholar] [CrossRef]
- Laddha, U.D.; Kshirsagar, S.J. Formulation of nanoparticles loaded in situ gel for treatment of dry eye disease: In vitro, ex vivo and in vivo evidences. J. Drug Deliv. Sci. Technol. 2021, 61, 102112. [Google Scholar] [CrossRef]
- Luo, L.-J.; Nguyen, D.D.; Lai, J.-Y. Long-acting mucoadhesive thermogels for improving topical treatments of dry eye disease. Mater. Sci. Eng. C 2020, 115, 111095. [Google Scholar] [CrossRef]
- Dukovski, B.J.; Juretić, M.; Bračko, D.; Randjelović, D.; Savić, S.; Moral, M.C.; Diebold, Y.; Filipović-Grčić, J.; Pepić, I.; Lovrić, J. Functional ibuprofen-loaded cationic nanoemulsion: Development and optimization for dry eye disease treatment. Int. J. Pharm. 2020, 576, 118979. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Rambasek, T.; Bielory, L. Clinical implications of mast cell involvement in allergic conjunctivitis. Allergy 2018, 73, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Rietveld, R.P.; ter Riet, G.; Bindels, P.J.; Sloos, J.H.; van Weert, H.C. Predicting bacterial cause in infectious conjunctivitis: Cohort study on informativeness of combinations of signs and symptoms. Bmj 2004, 329, 206–210. [Google Scholar] [CrossRef] [Green Version]
- Gumus, K.; Cavanagh, D.H. The role of inflammation and antiinflammation therapies in keratoconjunctivitis sicca. Clin. Ophthalmol. 2009, 3, 57. [Google Scholar] [PubMed]
- Huang, H.-Y.; Wang, M.-C.; Chen, Z.-Y.; Chiu, W.-Y.; Chen, K.-H.; Lin, I.-C.; Yang, W.-C.V.; Wu, C.-C.; Tseng, C.-L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251. [Google Scholar] [CrossRef] [Green Version]
- Akhter, S.; Anwar, M.; Siddiqui, M.A.; Ahmad, I.; Ahmad, J.; Ahmad, M.Z.; Bhatnagar, A.; Ahmad, F.J. Improving the topical ocular pharmacokinetics of an immunosuppressant agent with mucoadhesive nanoemulsions: Formulation development, in-vitro and in-vivo studies. Colloids Surf. B Biointerfaces 2016, 148, 19–29. [Google Scholar] [CrossRef]
- Liu, S.; Dozois, M.D.; Chang, C.N.; Ahmad, A.; Ng, D.L.; Hileeto, D.; Liang, H.; Reyad, M.-M.; Boyd, S.; Jones, L.W. Prolonged ocular retention of mucoadhesive nanoparticle eye drop formulation enables treatment of eye diseases using significantly reduced dosage. Mol. Pharm. 2016, 13, 2897–2905. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Tan, G.; Zhao, Z.; Yang, X.; Pan, W. A comparative study on the efficiency of chitosan-N-acetylcysteine, chitosan oligosaccharides or carboxymethyl chitosan surface modified nanostructured lipid carrier for ophthalmic delivery of curcumin. Carbohydr. Polym. 2016, 146, 435–444. [Google Scholar] [CrossRef]
- Terreni, E.; Chetoni, P.; Tampucci, S.; Burgalassi, S.; Al-Kinani, A.A.; Alany, R.G.; Monti, D. Assembling surfactants-mucoadhesive polymer nanomicelles (ASMP-nano) for ocular delivery of cyclosporine-A. Pharmaceutics 2020, 12, 253. [Google Scholar] [CrossRef] [Green Version]
- Zeng, W.; Li, Q.; Wan, T.; Liu, C.; Pan, W.; Wu, Z.; Zhang, G.; Pan, J.; Qin, M.; Lin, Y. Hyaluronic acid-coated niosomes facilitate tacrolimus ocular delivery: Mucoadhesion, precorneal retention, aqueous humor pharmacokinetics, and transcorneal permeability. Colloids Surf. B Biointerfaces 2016, 141, 28–35. [Google Scholar] [CrossRef]
- Sánchez-González, J.-M.; De-Hita-Cantalejo, C.; Sánchez-González, M.C. Crosslinked hyaluronic acid with liposomes and crocin for management symptoms of dry eye disease caused by moderate meibomian gland dysfunction. Int. J. Ophthalmol. 2020, 13, 1368. [Google Scholar] [CrossRef]
- Modi, D.; Nirmal, J.; Warsi, M.H.; Bhatia, M.; Hasan, N.; Kesharwani, P.; Jain, G.K. Formulation and development of tacrolimus-gellan gum nanoformulation for treatment of dry eye disease. Colloids Surf. B Biointerfaces 2022, 211, 112255. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Jian, H.-J.; Li, Y.-J.; Huang, Y.-F.; Anand, A.; Huang, C.-C.; Lin, H.-J.; Lai, J.-Y. Alleviation of dry eye syndrome with one dose of antioxidant, anti-inflammatory, and mucoadhesive lysine-carbonized nanogels. Acta Biomater. 2022, 141, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.A.; Alaaeldin, E.; Abdallah, R.; Mansour, H.F. A New Approach for Dry Eye Management By Mucoadhesive In situ Gel of Vitamin B12: Formulation, In vitro and In vivo Assessment. AAPS PharmSciTech 2021, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Ocular lubricants efficacy: Mucoadhesive evaluation using rheological methods. In Proceedings of the Iberian Meeting on Rheology; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 30–34. [Google Scholar]
- Eldesouky, L.M.; El-Moslemany, R.M.; Ramadan, A.A.; Morsi, M.H.; Khalafallah, N.M. Cyclosporine lipid nanocapsules as thermoresponsive gel for dry eye management: Promising corneal mucoadhesion, biodistribution and preclinical efficacy in rabbits. Pharmaceutics 2021, 13, 360. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Monti, D.; Burgalassi, S.; Terreni, E.; Zucchetti, E.; Baldacci, F.; Chetoni, P. Effect of 5-oxo-2-pyrrolidinecarboxylic acid (PCA) as a new topically applied agent for dry eye syndrome treatment. Pharmaceutics 2018, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swilem, A.E.; Elshazly, A.H.; Hamed, A.A.; Hegazy, E.-S.A.; Abd El-Rehim, H.A. Nanoscale poly (acrylic acid)-based hydrogels prepared via a green single-step approach for application as low-viscosity biomimetic fluid tears. Mater. Sci. Eng. C 2020, 110, 110726. [Google Scholar] [CrossRef]
- Lorenzo-Veiga, B.; Diaz-Rodriguez, P.; Alvarez-Lorenzo, C.; Loftsson, T.; Sigurdsson, H.H. In vitro and ex vivo evaluation of Nepafenac-based cyclodextrin microparticles for treatment of eye inflammation. Nanomaterials 2020, 10, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef]
- Dave, R.S.; Goostrey, T.C.; Ziolkowska, M.; Czerny-Holownia, S.; Hoare, T.; Sheardown, H. Ocular drug delivery to the anterior segment using nanocarriers: A mucoadhesive/mucopenetrative perspective. J. Control. Release 2021, 336, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Kanwar, M. Ocular preparations: The formulation approach. Drug Dev. Ind. Pharm. 2002, 28, 473–493. [Google Scholar] [CrossRef]
- Rodríguez, I.; Vázquez, J.A.; Pastrana, L.; Khutoryanskiy, V.V. Enhancement and inhibition effects on the corneal permeability of timolol maleate: Polymers, cyclodextrins and chelating agents. Int. J. Pharm. 2017, 529, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Grover, K.; Pawar, P.; Singh, I. Mucoadhesive Polymers for Enhancing Retention in Ocular Drug Delivery: A Critical Review. Rev. Adhes. Adhes. 2014, 2, 467–502. [Google Scholar] [CrossRef]
- Dubashynskaya, N.; Poshina, D.; Raik, S.; Urtti, A.; Skorik, Y.A. Polysaccharides in ocular drug delivery. Pharmaceutics 2020, 12, 22. [Google Scholar] [CrossRef]
- Jarho, P.; Järvinen, K.; Urtti, A.; Stella, V.J.; Järvinen, T. Modified β-cyclodextrin (SBE7-β-CyD) with viscous vehicle improves the ocular delivery and tolerability of pilocarpine prodrug in rabbits. J. Pharm. Pharmacol. 1996, 48, 263–269. [Google Scholar] [CrossRef]
- Al Khateb, K.; Ozhmukhametova, E.K.; Mussin, M.N.; Seilkhanov, S.K.; Rakhypbekov, T.K.; Lau, W.M.; Khutoryanskiy, V.V. In situ gelling systems based on Pluronic F127/Pluronic F68 formulations for ocular drug delivery. Int. J. Pharm. 2016, 502, 70–79. [Google Scholar] [CrossRef]
- Mandal, S.; Thimmasetty, M.K.; Prabhushankar, G.; Geetha, M. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzillo, R.; Schiraldi, C.; Corsuto, L.; D’Agostino, A.; Filosa, R.; De Rosa, M.; La Gatta, A. Optimization of hyaluronan-based eye drop formulations. Carbohydr. Polym. 2016, 153, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceulemans, J.; Ludwig, A. Optimisation of carbomer viscous eye drops: An in vitro experimental design approach using rheological techniques. Eur. J. Pharm. Biopharm. 2002, 54, 41–50. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, Z. A novel ocular delivery of brinzolamide based on gellan gum: In vitro and in vivo evaluation. Drug Des. Dev. Ther. 2018, 12, 383–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalam, M.A.; Alshamsan, A.; Aljuffali, I.A.; Mishra, A.K.; Sultana, Y. Delivery of gatifloxacin using microemulsion as vehicle: Formulation, evaluation, transcorneal permeation and aqueous humor drug determination. Drug Deliv. 2016, 23, 886–897. [Google Scholar] [CrossRef]
- Yermak, I.M.; Davydova, V.N.; Volod’ko, A.V. Mucoadhesive Marine Polysaccharides. Mar. Drugs 2022, 20, 522. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications–A review. J. Drug Deliv.Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcantara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Graça, A.; Gonçalves, L.M.; Raposo, S.; Ribeiro, H.M.; Marto, J. Useful in vitro techniques to evaluate the mucoadhesive properties of hyaluronic acid-based ocular delivery systems. Pharmaceutics 2018, 10, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, B.; Mishra, V.; Gharat, S.; Momin, M.; Omri, A. Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems. Pharmaceuticals 2021, 14, 1201. [Google Scholar] [CrossRef]
- Al-Saedi, Z.H.; Alzhrani, R.M.; Boddu, S.H. Formulation and In Vitro Evaluation of Cyclosporine-A Inserts Prepared Using Hydroxypropyl Methylcellulose for Treating Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2016, 32, 451–462. [Google Scholar] [CrossRef]
- Ubels, J.; Clousing, D.; Van Haitsma, T.; Hong, B.-S.; Stauffer, P.; Asgharian, B.; Meadows, D. Pre-clinical investigation of the efficacy of an artificial tear solution containing hydroxypropyl-guar as a gelling agent. Curr. Eye Res. 2004, 28, 437–444. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Advances in mucoadhesion and mucoadhesive polymers. Macromol. Biosci. 2011, 11, 748–764. [Google Scholar] [CrossRef]
- Račić, A.; Čalija, B.; Milić, J.; Milašinović, N.; Krajišnik, D. Development of polysaccharide-based mucoadhesive ophthalmic lubricating vehicles: The effect of different polymers on physicochemical properties and functionality. J. Drug Deliv. Sci. Technol. 2019, 49, 50–57. [Google Scholar] [CrossRef]
- Efiana, N.A.; Kali, G.; Fürst, A.; Dizdarević, A.; Bernkop-Schnürch, A. Betaine-modified hydroxyethyl cellulose (HEC): A biodegradable mucoadhesive polysaccharide exhibiting quaternary ammonium substructures. Eur. J. Pharm. Sci. 2023, 180, 106313. [Google Scholar] [CrossRef]
- Irimia, T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Lupuleasa, D.; Muntean, D.-L.; Udeanu, D.I.; Popa, L. Chitosan-based in situ gels for ocular delivery of therapeutics: A state-of-the-art Review. Mar. Drugs 2018, 16, 373. [Google Scholar] [CrossRef] [Green Version]
- de la Fuente, M.; Ravina, M.; Paolicelli, P.; Sanchez, A.; Seijo, B.; Alonso, M.J. Chitosan-based nanostructures: A delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev. 2010, 62, 100–117. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A review on chitin and chitosan polymers: Structure, chemistry, solubility, derivatives, and applications. Chem. Bio. Eng. Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Pahuja, P.; Arora, S.; Pawar, P. Ocular drug delivery system: A reference to natural polymers. Expert Opin. Drug Deliv. 2012, 9, 837–861. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.; Gui, Q.; Yang, H. Drug-loaded chitosan film prepared via facile solution casting and air-drying of plain water-based chitosan solution for ocular drug delivery. Bioact. Mater. 2020, 5, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Drug transport mechanisms and in vitro release kinetics of vancomycin encapsulated chitosan-alginate polyelectrolyte microparticles as a controlled drug delivery system. Eur. J. Pharm. Sci. 2018, 114, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Fiqri, M.; Athiyyah, U.; Layadi, P.; Fadjar, T.G.A.; Permana, A.D. Enhanced localization of cefazoline sodium in the ocular tissue using thermosensitive-mucoadhesive hydrogels: Formulation development, hemocompatibility and in vivo irritation studies. J. Drug Deliv. Sci. Technol. 2022, 76, 103763. [Google Scholar] [CrossRef]
- Tighsazzadeh, M.; Mitchell, J.C.; Boateng, J.S. Development and evaluation of performance characteristics of timolol-loaded composite ocular films as potential delivery platforms for treatment of glaucoma. Int. J. Pharm. 2019, 566, 111–125. [Google Scholar] [CrossRef]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, A.D.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89. [Google Scholar] [PubMed]
- Morrison, P.W.; Khutoryanskiy, V.V. Advances in ophthalmic drug delivery. Ther. Deliv. 2014, 5, 1297–1315. [Google Scholar] [CrossRef] [Green Version]
- Uccello-Barretta, G.; Nazzi, S.; Zambito, Y.; Di Colo, G.; Balzano, F.; Sansò, M. Synergistic interaction between TS-polysaccharide and hyaluronic acid: Implications in the formulation of eye drops. Int. J. Pharm. 2010, 395, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.R.; Vyas, S. Hyaluronic acid modified chitosan nanoparticles for effective management of glaucoma: Development, characterization, and evaluation. J. Drug Target. 2010, 18, 292–302. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Chetoni, P.; Giunchedi, P.; Rossi, S.; Ferrari, F.; Burgalassi, S.; Caramella, C. Carrageenan–gelatin mucoadhesive systems for ion-exchange based ophthalmic delivery: In vitro and preliminary in vivo studies. Eur. J. Pharm. Biopharm. 2004, 57, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Sandri, G.; Bonferoni, M.C.; Chetoni, P.; Rossi, S.; Ferrari, F.; Ronchi, C.; Caramella, C. Ophthalmic delivery systems based on drug–polymer–polymer ionic ternary interaction: In vitro and in vivo characterization. Eur. J. Pharm. Biopharm. 2006, 62, 59–69. [Google Scholar] [CrossRef]
- Pinto-Bonilla, J.C.; del Olmo-Jimeno, A.; Llovet-Osuna, F.; Hernández-Galilea, E. A randomized crossover study comparing trehalose/hyaluronate eyedrops and standard treatment: Patient satisfaction in the treatment of dry eye syndrome. Ther. Clin. Risk Manag. 2015, 11, 595. [Google Scholar]
- Rangarajan, R.; Kraybill, B.; Ogundele, A.; Ketelson, H.A. Effects of a hyaluronic acid/hydroxypropyl guar artificial tear solution on protection, recovery, and lubricity in models of corneal epithelium. J. Ocul. Pharmacol. Ther. 2015, 31, 491–497. [Google Scholar] [CrossRef]
- Bhowmik, M.; Kumari, P.; Sarkar, G.; Bain, M.K.; Bhowmick, B.; Mollick, M.M.R.; Mondal, D.; Maity, D.; Rana, D.; Bhattacharjee, D. Effect of xanthan gum and guar gum on in situ gelling ophthalmic drug delivery system based on poloxamer-407. Int. J. Biol. Macromol. 2013, 62, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Moiseev, R.V.; Morrison, P.W.J.; Steele, F.; Khutoryanskiy, V.V. Penetration Enhancers in Ocular Drug Delivery. Pharmaceutics 2019, 11, 321. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Mitra, A.K. Chemical modification and formulation approaches to elevated drug transport across cell membranes. Expert Opin. Drug Deliv. 2006, 3, 511–527. [Google Scholar] [CrossRef]
- Van der Merwe, S.; Verhoef, J.; Verheijden, J.; Kotzé, A.; Junginger, H. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur. J. Pharm. Biopharm. 2004, 58, 225–235. [Google Scholar] [CrossRef]
- Pavinatto, F.J.; Pavinatto, A.; Caseli, L.; dos Santos, D.S.; Nobre, T.M.; Zaniquelli, M.E.; Oliveira, O.N. Interaction of chitosan with cell membrane models at the air− water interface. Biomacromolecules 2007, 8, 1633–1640. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Furrer, P.; Mayer, J.M.; Plazonnet, B.; Gurny, R. Ocular tolerance of absorption enhancers in ophthalmic preparations. AAPS Pharm. Sci 2002, 4, E2. [Google Scholar] [CrossRef]
- Baranowski, P.; Karolewicz, B.; Gajda, M.; Pluta, J. Ophthalmic drug dosage forms: Characterisation and research methods. Sci. World J. 2014, 2014, 861904. [Google Scholar] [CrossRef] [PubMed]
- Bassi da Silva, J.; Ferreira, S.B.d.S.; de Freitas, O.; Bruschi, M.L. A critical review about methodologies for the analysis of mucoadhesive properties of drug delivery systems. Drug Dev. Ind. Pharm. 2017, 43, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Ponchel, G.; Touchard, F.; Duchêne, D.; Peppas, N.A. Bioadhesive analysis of controlled-release systems. I. Fracture and interpenetration analysis in poly (acrylic acid)-containing systems. J. Control. Release 1987, 5, 129–141. [Google Scholar] [CrossRef]
- Smart, J.; Kellaway, I.; Worthington, H. An in-vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J. Pharm. Pharmacol. 1984, 36, 295–299. [Google Scholar] [CrossRef]
- Chandasana, H.; Prasad, Y.D.; Chhonker, Y.S.; Chaitanya, T.K.; Mishra, N.N.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Corneal targeted nanoparticles for sustained natamycin delivery and their PK/PD indices: An approach to reduce dose and dosing frequency. Int. J. Pharm. 2014, 477, 317–325. [Google Scholar] [CrossRef]
- Chhonker, Y.S.; Prasad, Y.D.; Chandasana, H.; Vishvkarma, A.; Mitra, K.; Shukla, P.K.; Bhatta, R.S. Amphotericin-B entrapped lecithin/chitosan nanoparticles for prolonged ocular application. Int. J. Biol. Macromol. 2015, 72, 1451–1458. [Google Scholar] [CrossRef]
- Takeuchi, H.; Thongborisute, J.; Matsui, Y.; Sugihara, H.; Yamamoto, H.; Kawashima, Y. Novel mucoadhesion tests for polymers and polymer-coated particles to design optimal mucoadhesive drug delivery systems. Adv. Drug Deliv. Rev. 2005, 57, 1583–1594. [Google Scholar] [CrossRef]
- Lee, C.-A.; Kim, B.-S.; Cho, C.-W. Quantitative evaluation of mucoadhesive polymers to compare the mucoadhesion. J. Pharm. Investig. 2016, 46, 189–194. [Google Scholar] [CrossRef]
- Hassan, E.E.; Gallo, J.M. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm. Res. 1990, 7, 491–495. [Google Scholar] [CrossRef]
- Rossi, S.; Vigani, B.; Bonferoni, M.C.; Sandri, G.; Caramella, C.; Ferrari, F. Rheological analysis and mucoadhesion: A 30 year-old and still active combination. J. Pharm. Biomed. Anal. 2018, 156, 232–238. [Google Scholar] [CrossRef]
- Patel, N.; Thakkar, V.; Metalia, V.; Baldaniya, L.; Gandhi, T.; Gohel, M. Formulation and development of ophthalmic in situ gel for the treatment ocular inflammation and infection using application of quality by design concept. Drug Dev. Ind. Pharm. 2016, 42, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Bonferoni, M.; D’Autilia, F.; Sandri, G.; Ferrari, F.; Caramella, C.; Mortara, E.; Giannini, V.; Gasparri, F. Associations of natural polymers to modulate mucoadhesion of vaginal rinse-off and leave-on formulations. J. Drug Deliv. Sci. Technol. 2014, 24, 435–560. [Google Scholar] [CrossRef]
- Mezger, T. The Rheology Handbook, 4th ed.; Vincentz Network: Hanover, Germany, 2014. [Google Scholar]
- Hotujac Grgurević, M.; Juretić, M.; Hafner, A.; Lovrić, J.; Pepić, I. Tear fluid-eye drops compatibility assessment using surface tension. Drug Dev. Ind. Pharm. 2017, 43, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.; Tiffany, J.; Gouveia, S.; Yokoi, N.; Voon, L. Functional aspects of the tear film lipid layer. Exp. Eye Res. 2004, 78, 347–360. [Google Scholar] [CrossRef] [PubMed]
- German, E.J.; Hurst, M.A.; Wood, D. Reliability of drop size from multi-dose eye drop bottles: Is it cause for concern? Eye 1999, 13, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Nagyova, B.; Tiffany, J. Components responsible for the surface tension of human tears. Curr. Eye Res. 1999, 19, 4–11. [Google Scholar] [CrossRef]
- Kalam, M.A. Development of chitosan nanoparticles coated with hyaluronic acid for topical ocular delivery of dexamethasone. Int. J. Biol. Macromol. 2016, 89, 127–136. [Google Scholar] [CrossRef]
- McKenzie, B.; Kay, G.; Matthews, K.H.; Knott, R.; Cairns, D. Preformulation of cysteamine gels for treatment of the ophthalmic complications in cystinosis. Int. J. Pharm. 2016, 515, 575–582. [Google Scholar] [CrossRef] [Green Version]
- Dargó, G.; Vincze, A.; Müller, J.; Kiss, H.J.; Nagy, Z.Z.; Balogh, G.T. Corneal-PAMPA: A novel, non-cell-based assay for prediction of corneal drug permeability. Eur. J. Pharm. Sci. 2019, 128, 232–239. [Google Scholar] [CrossRef]
- Hahne, M.; Reichl, S. Development of a serum-free human cornea construct for in vitro drug absorption studies: The influence of varying cultivation parameters on barrier characteristics. Int. J. Pharm. 2011, 416, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Hahne, M.; Zorn-Kruppa, M.; Guzman, G.; Brandner, J.M.; Haltner-Ukomado, E.; Wätzig, H.; Reichl, S. Prevalidation of a human cornea construct as an alternative to animal corneas for in vitro drug absorption studies. J. Pharm. Sci. 2012, 101, 2976–2988. [Google Scholar] [CrossRef] [PubMed]
- Pepić, I.; Lovrić, J.; Cetina-Čižmek, B.; Reichl, S.; Filipović-Grčić, J. Toward the practical implementation of eye-related bioavailability prediction models. Drug Discov. 2014, 19, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Rupenthal, I.D. In vitro and ex vivo corneal penetration and absorption models. Drug Deliv. Transl. Res. 2016, 6, 634–647. [Google Scholar] [CrossRef]
- Reichl, S.; Müller-Goymann, C.C. The use of a porcine organotypic cornea construct for permeation studies from formulations containing befunolol hydrochloride. Int. J. Pharm. 2003, 250, 191–201. [Google Scholar] [CrossRef]
- Toropainen, E.; Ranta, V.-P.; Talvitie, A.; Suhonen, P.; Urtti, A. Culture model of human corneal epithelium for prediction of ocular drug absorption. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2942–2948. [Google Scholar]
- Toropainen, E.; Ranta, V.-P.; Vellonen, K.-S.; Palmgrén, J.; Talvitie, A.; Laavola, M.; Suhonen, P.; Hämäläinen, K.M.; Auriola, S.; Urtti, A. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur. J. Pharm. Sci. 2003, 20, 99–106. [Google Scholar] [CrossRef]
- Becker, U.; Ehrhardt, C.; Schneider, M.; Muys, L.; Gross, D.; Eschmann, K.; Schaefer, U.F.; Lehr, C.-M. A comparative evaluation of corneal epithelial cell cultures for assessing ocular permeability. Altern. Lab. Anim. 2008, 36, 33–44. [Google Scholar] [CrossRef]
- Račić, A.; Čalija, B.; Milić, J.; Dukovski, B.J.; Lovrić, J.; Dobričić, V.; Micov, A.; Vuković, M.; Stepanović-Petrović, R.; Krajišnik, D. Formulation of olopatadine hydrochloride viscous eye drops–physicochemical, biopharmaceutical and efficacy assessment using in vitro and in vivo approaches. Eur. J. Pharm. Sci. 2021, 166, 105906. [Google Scholar] [CrossRef] [PubMed]
- Sunkara, G.; Kompella, U. Ophthalmic Drug Delivery Systems; Marcel Dekker, Inc.: New York, NY, USA, 2003. [Google Scholar]
- Barar, J.; Javadzadeh, A.R.; Omidi, Y. Ocular novel drug delivery: Impacts of membranes and barriers. Expert Opin. Drug Deliv. 2008, 5, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.L.; Ahearne, M.; Hopkinson, A. An overview of current techniques for ocular toxicity testing. Toxicology 2015, 327, 32–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draize, J.H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- Eur-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32010L0063 (accessed on 21 December 2022).
- Pauly, A.; Meloni, M.; Brignole-Baudouin, F.; Warnet, J.-M.; Baudouin, C. Multiple endpoint analysis of the 3D-reconstituted corneal epithelium after treatment with benzalkonium chloride: Early detection of toxic damage. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1644–1652. [Google Scholar] [CrossRef] [Green Version]
- Lynch, C.R.; Kondiah, P.P.; Choonara, Y.E.; Du Toit, L.C.; Ally, N.; Pillay, V. Hydrogel biomaterials for application in ocular drug delivery. Front. Bioeng. Biotechnol. 2020, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Felt, O.; Furrer, P.; Mayer, J.M.; Plazonnet, B.; Buri, P.; Gurny, R. Topical use of chitosan in ophthalmology: Tolerance assessment and evaluation of precorneal retention. Int. J. Pharm. 1999, 180, 185–193. [Google Scholar] [CrossRef]
- Minami, K.; Kamei, C. A chronic model for evaluating the itching associated with allergic conjunctivitis in rats. Int. Immunopharmacol. 2004, 4, 101–108. [Google Scholar] [CrossRef]
- Shinde, U.A.; Shete, J.N.; Nair, H.A.; Singh, K.H. Design and characterization of chitosan-alginate microspheres for ocular delivery of azelastine. Pharm. Dev. Technol. 2014, 19, 813–823. [Google Scholar] [CrossRef]
- Takada, M.; Yamada, T.; Nakahara, H.; Sugimoto, Y.; Izushi, K.; Kamei, C. Experimental allergic conjunctivitis in guinea pigs induced by Japanese cedar pollen. Biol. Pharm. Bull. 2000, 23, 566–569. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Yang, W.; Guo, C.; Jiang, H.; Li, F.; Xiao, M.; Davidson, S.; Yu, G.; Duan, B.; Huang, T. Anatomical and functional dichotomy of ocular itch and pain. Nat. Med. 2018, 24, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Nabe, T.; Mizutani, N.; Nakata, K.; Kohno, S. Multiple cedar pollen challenge diminishes involvement of histamine in allergic conjunctivitis of Guinea pigs. Biol. Pharm. Bull. 2003, 26, 1696–1700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakazawa, Y.; Oka, M.; Takehana, M. Model for studying anti-allergic drugs for allergic conjunctivitis in animals. Open Med. 2017, 12, 231–238. [Google Scholar] [CrossRef]
- Liu, F.; Xu, L.; Chen, N.; Zhou, M.; Li, C.; Yang, Q.; Xie, Y.; Huang, Y.; Ma, C. Neuronal Fc-epsilon receptor I contributes to antigen-evoked pruritus in a murine model of ocular allergy. Brain Behav. Immun. 2017, 61, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, T.; Amano, T.; Ohmori, K.; Manabe, H. The effects of olopatadine hydrochloride on the number of scratching induced by repeated application of oxazolone in mice. Eur. J. Pharmacol. 2005, 524, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Groneberg, D.; Bielory, L.; Fischer, A.; Bonini, S.; Wahn, U. Animal models of allergic and inflammatory conjunctivitis. Allergy 2003, 58, 1101–1113. [Google Scholar] [CrossRef]
- Schmelz, M.; Schmidt, R.; Weidner, C.; Hilliges, M.; Torebjork, H.; Handwerker, H.O. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003, 89, 2441–2448. [Google Scholar] [CrossRef]






| Polymer Name | Characteristics | Mucoadhesive Potential a | Role in Artificial Tears | Chemical Structure | Reference |
|---|---|---|---|---|---|
| Chitosan | Cationic, non-toxic, biodegradable, biocompatible, soluble in acetic acid | +++ | Mucoadhesive polymer |  | [95] |
| Hyaluronic acid | Anionic, biocompatible, viscoelastic, mucin-like polymer, slightly soluble in water | +++ | Active ingredient/vehicle, mucoadhesive polymer |  | [96] |
| Hypromellose | Non-ionic, biocompatible, biodegradable material, transparency, water-soluble | + | Active ingredient/tear substitution |  | [97] |
| Xanthan Gum | Anionic, low-toxic, high stability | ++ | Mucoadhesive polymer, prolonged retention time |  | [98] |
| Hydroxypropyl Guar Gum | Non-ionic, viscosity-increasing polymer, effective at low concentrations, produces clear solutions | ++ | Gelling agent |  | [99] |
| Gellan Gum | Anionic, mucoadhesive, thixotropic, pseudoplastic | ++ | Mucoadhesive polymer, prolonged retention time |  | [72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Račić, A.; Krajišnik, D. Biopolymers in Mucoadhesive Eye Drops for Treatment of Dry Eye and Allergic Conditions: Application and Perspectives. Pharmaceutics 2023, 15, 470. https://doi.org/10.3390/pharmaceutics15020470
Račić A, Krajišnik D. Biopolymers in Mucoadhesive Eye Drops for Treatment of Dry Eye and Allergic Conditions: Application and Perspectives. Pharmaceutics. 2023; 15(2):470. https://doi.org/10.3390/pharmaceutics15020470
Chicago/Turabian StyleRačić, Anđelka, and Danina Krajišnik. 2023. "Biopolymers in Mucoadhesive Eye Drops for Treatment of Dry Eye and Allergic Conditions: Application and Perspectives" Pharmaceutics 15, no. 2: 470. https://doi.org/10.3390/pharmaceutics15020470