Stability Study of mRNA-Lipid Nanoparticles Exposed to Various Conditions Based on the Evaluation between Physicochemical Properties and Their Relation with Protein Expression Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Mice
2.3. mRNA In Vitro Transcription
2.4. Preparation of mRNA Encapsulating LNP
2.5. Measurement of LNP Characterization
2.6. In Vitro Luciferase Assay
2.7. mRNA Extraction from mRNA-LNPs and Agarose Gel Electrophoresis
2.8. In Vivo Imaging Luciferase Activity
2.9. In Vivo Luciferase Assay
2.10. Statistical Analysis
3. Results
3.1. Time-Dependent Physical Stability of mRNA-LNP
3.2. Temperature Affected the Stability of LNPs
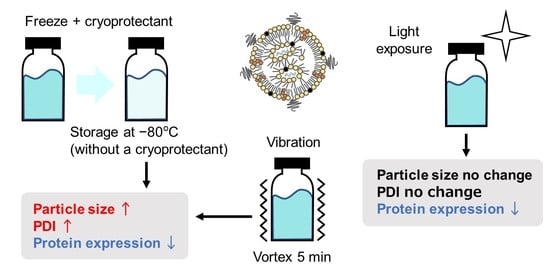
3.3. Cryoprotectants Increased the Ultra-Raw Storage Stability of LNPs
3.4. Vibration Affected the Stability of mRNA-LNPs
3.5. Light Exposure Decreased the Stability of mRNA-LNPs
3.6. The Suction Pressure from Vials Did Not Affect the Stability of mRNA-LNPs
3.7. mRNA Degradation into mRNA-LNPs
3.8. In Vivo Luciferase Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 382, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef] [PubMed]
- Domazet-Lošo, T. mRNA Vaccines: Why Is the Biology of Retroposition Ignored? Genes 2022, 13, 719. [Google Scholar] [CrossRef]
- Linares-Fernández, S.; Moreno, J.; Lambert, E.; Mercier-Gouy, P.; Vachez, L.; Verrier, B.; Exposito, J.Y. Combining an optimized mRNA template with a double purification process allows strong expression of in vitro transcribed mRNA. Mol. Ther. Nucleic Acids 2021, 26, 945–956. [Google Scholar] [CrossRef]
- Nakanishi, H.; Itaka, K. Synthetic mRNA for ex vivo therapeutic applications. Drug Metab. Pharmacokinet. 2022, 44, 10044. [Google Scholar]
- Ball, P. The lightning-fast quest for COVID vaccines-and what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef]
- Mukai, H.; Ogawa, K.; Kato, N.; Kawakami, S. Recent advances in lipid nanoparticles for delivery of nucleic acid, mRNA, and gene editing-based therapeutics. Drug Metab. Pharmacokinet. 2022, 44, 100450. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154, 37–63. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Ramachandran, S.; Satapathy, S.R.; Dutta, T. Delivery Strategies for mRNA Vaccines. Pharmaceut. Med. 2022, 36, 11–20. [Google Scholar] [CrossRef]
- FDA. Comirnaty Package Insert, Summary Basis for Regulatory Action [www Document]. 2021. Available online: https://www.fda.gov/media/151707/download (accessed on 12 April 2022).
- FDA. Spikevax Package Insert, Summary Basis for Regulatory Action [www Document]. 2021. Available online: https://www.fda.gov/media/155675/download (accessed on 12 April 2022).
- Roesler, E.; Weiss, R.; Weinberger, E.E.; Fruehwirth, A.; Stoecklinger, A.; Mostböck, S.; Ferreira, F.; Thalhamer, J.; Scheiblhofer, S. Immunize and disappear-safety-optimized mRNA vaccination with a panel of 29 allergens. J. Allergy Clin. Immunol. 2009, 124, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Kudsiova, L.; Lansley, A.; Scutt, G.; Allen, M.; Bowler, L.; Williams, S.; Lippett, S.; Stafford, S.; Tarzi, M.; Cross, M.; et al. Stability testing of the Pfizer-BioNTech BNT162b2 COVID-19 vaccine: A translational study in UK vaccination centres. BMJ Open Sci. 2021, 12, e100203. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 1, 1084–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, Y.; Li, A.; Lin, J.; Hsieh, K.; Schneiderman, Z.; Zhang, P.; Zhu, Y.; Qiu, C.; Kokkoli, E.; et al. Payload distribution and capacity of mRNA lipid nanoparticles. Nat. Commun. 2022, 13, 5561. [Google Scholar] [CrossRef]
- Kis, Z. Stability Modelling of mRNA Vaccine Quality Based on Temperature Monitoring throughout the Distribution Chain. Pharmaceutics 2022, 14, 430. [Google Scholar] [CrossRef]
- Ogawa, K.; Kato, N.; Yoshida, M.; Hiu, T.; Matsuo, T.; Mizukami, S.; Omata, D.; Suzuki, R.; Maruyama, K.; Mukai, H.; et al. Focused ultrasound/microbubbles-assisted BBB opening enhances LNP-mediated mRNA delivery to brain. J. Control. Release 2022, 348, 34–41. [Google Scholar] [CrossRef]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hobo, W.; Novobrantseva, T.I.; Fredrix, H.; Wong, J.; Milstein, S.; Epstein-Barash, H.; Liu, J.; Schaap, N.; van der Voort, R.; Dolstra, H. Improving dendritic cell vaccine immunogenicity by silencing PD-1 ligands using siRNA-lipid nanoparticles combined with antigen mRNA electroporation. Cancer Immunol. Immunother. 2013, 62, 285–297. [Google Scholar] [CrossRef]
- Kawakami, S.; Fumoto, S.; Nishikawa, M.; Yamashita, F.; Hashida, M. In vivo gene delivery to the liver using novel galactosylated cationic liposomes. Pharm. Res. 2000, 17, 306–313. [Google Scholar] [CrossRef]
- Oyama, N.; Kawaguchi, M.; Itaka, K.; Kawakami, S. Efficient Messenger RNA Delivery to the Kidney Using Renal Pelvis Injection in Mice. Pharmaceutics 2021, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Ayat, N.R.; Sun, Z.; Sun, D.; Yin, M.; Hall, R.C.; Vaidya, A.M.; Liu, X.; Schilb, A.L.; Scheidt, J.H.; Lu, Z.R. Formulation of Bio-compatible Targeted ECO/siRNA Nanoparticles with Long-Term Stability for Clinical Translation of RNAi. Nucleic Acid Ther. 2019, 29, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Selmin, F.; Musazzi, U.M.; Franzè, S.; Scarpa, E.; Rizzello, L.; Procacci, P.; Minghetti, P. Pre-Drawn Syringes of Comirnaty for an Efficient COVID-19 Mass Vaccination: Demonstration of Stability. Pharmaceutics 2021, 13, 1029. [Google Scholar] [CrossRef]
- Démoulins, T.; Ebensen, T.; Schulze, K.; Englezou, P.C.; Pelliccia, M.; Guzmán, C.A.; Ruggli, N.; McCullough, K.C. Self-replicating RNA vaccine functionality modulated by fine-tuning of polyplex delivery vehicle structure. J. Control. Release 2017, 266, 256–271. [Google Scholar] [CrossRef]
- Gerhardt, A.; Voigt, E.; Archer, M.; Reed, S.; Larson, E.; Van Hoeven, N.; Kramer, R.; Fox, C.; Casper, C. A flexible, thermostable nanostructured lipid carrier platform for RNA vaccine delivery. Mol. Ther. Methods Clin. Dev. 2022, 25, 205–214. [Google Scholar]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinegra, A.J.; Evangelopoulos, M.; Park, J.; Huang, Z.; Mirkin, C.A. Lipid Nanoparticle Spherical Nucleic Acids for Intracellular DNA and RNA Delivery. Nano Lett. 2021, 21, 6584–6659. [Google Scholar] [CrossRef]
- Carrasco, M.J.; Alishetty, S.; Alameh, M.G.; Said, H.; Wright, L.; Paige, M.; Soliman, O.; Weissman, D.; Cleveland, T.E., 4th; Grishaev, A.; et al. Ionization and structural properties of mRNA lipid nanoparticles influence expression in intramuscular and intravascular administration. Commun. Biol. 2021, 4, 956–971. [Google Scholar]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Achieving long-term stability of lipid nanoparticles: Examining the effect of pH, temperature, and lyophilization. Int. J. Nanomed. 2017, 12, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Trenkenschuh, E.; Friess, W. Freeze-drying of nanoparticles: How to overcome colloidal instability by formulation and process optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Pogocki, D.; Schöneich, C. Chemical stability of nucleic acid-derived drugs. J. Pharm. Sci. 2000, 89, 443–456. [Google Scholar] [CrossRef]
- Mauger, D.M.; Joseph Cabral, B.; Presnyak, V.; Su, S.V.; Reid, D.W.; Goodman, B.; Link, K.; Khatwani, N.; Reynders, J.; Moore, M.J.; et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. USA 2019, 116, 24075–24083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M.; Gyawali, D.; Yerabolu, R.; Schariter, J.; White, P.A. A novel mechanism for the loss of mRNA activity in lipid nanoparticle delivery systems. Nat. Commun. 2021, 12, 6777–6788. [Google Scholar] [CrossRef] [PubMed]







| Time (Days) | Size (nm) | PDI | Zeta-Potential (mV) | mRNA Retention Rate (%) |
|---|---|---|---|---|
| 0 | 112.2 ± 1.3 | 0.081 ± 0.022 | −5.72 ± 1.41 | 93.96 ± 1.19 |
| 1 | 114.9 ± 2.9 | 0.086 ± 0.039 | −6.29 ± 2.34 | 95.67 ± 5.06 |
| 3 | 113.1 ± 2.2 | 0.061 ± 0.018 | −6.13 ± 1.26 | 93.00 ± 2.50 |
| 5 | 111.6 ± 2.0 | 0.047 ± 0.043 | −4.88 ± 0.67 | 95.45 ± 0.96 |
| 7 | 112.5 ± 1.0 | 0.065 ± 0.027 | −4.15 ± 1.62 | 95.58 ± 1.46 |
| Storage Temperature | Size (nm) | PDI | Zeta-Potential (mV) | mRNA Retention Rate (%) |
|---|---|---|---|---|
| −80 °C | 904.6 ± 107.7 ** | 0.695 ± 0.055 | −20.06 ± 1.01 | 50.51 ± 10.37 ** |
| −30 °C | 170.8 ± 6.8 | 0.235 ± 0.028 | −4.20 ± 1.19 | 92.97 ± 2.48 |
| 4 °C | 112.5 ± 2.3 | 0.081 ± 0.040 | −5.46 ± 1.43 | 97.34 ± 0.73 |
| 25 °C | 113.4 ± 6.8 | 0.083 ± 0.005 | −6.61 ± 2.15 | 96.72 ± 0.40 |
| Storage Temperature and Cryoprotectants | Size (nm) | PDI | Zeta-Potential (mV) | mRNA Retention Rate (%) |
|---|---|---|---|---|
| −80 °C, Sucrose (−) | 383.2 ± 41.9 ** | 0.593 ± 0.065 | −10.65 ± 0.92 | 50.83 ± 11.24 ** |
| −80 °C, Sucrose (+) | 118.4 ± 5.9 | 0.098 ± 0.011 | −8.71 ± 0.60 | 83.66 ± 3.22 |
| 4 °C | 110.7 ± 1.5 | 0.065 ± 0.038 | −7.75 ± 2.75 | 87.25 ± 4.30 |
| Vibration | Size (nm) | PDI | Zeta-Potential (mV) | mRNA Retention Rate (%) |
|---|---|---|---|---|
| Non-treatment | 114.5 ± 2.9 | 0.076 ± 0.027 | −10.69 ± 5.29 | 89.84 ± 1.24 |
| Tapping 10 s | 120.5 ± 20.7 | 0.139 ± 0.081 | −8.73 ± 3.19 | 93.51 ± 3.09 |
| Tapping 30 s | 114.7 ± 4.5 | 0.084 ± 0.055 | −8.93 ± 1.86 | 91.85 ± 2.13 |
| Tapping 5 min | 119.6 ± 5.2 | 0.154 ± 0.067 | −11.58 ± 4.51 | 85.62 ± 4.04 |
| Vortex 10 s | 115.5 ± 6.4 | 0.100 ± 0.043 | −9.29 ± 2.21 | 88.29 ± 2.59 |
| Vortex 30 s | 116.7 ± 5.9 | 0.085 ± 0.024 | −11.47 ± 1.01 | 93.69 ± 1.39 |
| Vortex 5 min | 242.8 ± 55.3 ** | 0.427 ± 0.202 | −15.35 ± 0.17 | 41.58 ± 30.61 ** |
| Condition | Size (nm) | PDI | Zeta-Potential (mV) | mRNA Retention Rate (%) |
|---|---|---|---|---|
| No exposure | 116.8 ± 5.8 | 0.045 ± 0.016 | −4.37 ± 0.32 | 93.06 ± 2.32 |
| Light exposure (2 × 104 lx·h) | 116.1 ± 6.8 | 0.079 ± 0.060 | −4.71 ± 2.29 | 94.77 ± 1.54 |
| Light exposure (1.2 × 106 lx·h) | 114.3 ± 5.1 | 0.050 ± 0.009 | −5.24 ± 1.06 | 95.11 ± 1.30 |
| The Number of Suctions | Size (nm) | PDI | Zeta-Potential (mV) | mRNA Retention Rate (%) |
|---|---|---|---|---|
| 1 | 90.6 ± 2.2 | 0.072 ± 0.020 | −11.16 ± 0.37 | 91.47 ± 1.57 |
| 2 | 90.5 ± 1.5 | 0.062 ± 0.040 | −10.81 ± 1.55 | 89.52 ± 4.22 |
| 3 | 92.0 ± 1.8 | 0.074 ± 0.016 | −12.08 ± 1.95 | 94.44 ± 2.09 |
| 4 | 91.4 ± 1.1 | 0.071 ± 0.026 | −10.18 ± 2.13 | 92.29 ± 2.88 |
| 5 | 90.2 ± 1.8 | 0.064 ± 0.016 | −11.53 ± 1.80 | 92.81 ± 2.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiya, M.; Matsumoto, M.; Yamashita, K.; Izumi, T.; Kawaguchi, M.; Mizukami, S.; Tsurumaru, M.; Mukai, H.; Kawakami, S. Stability Study of mRNA-Lipid Nanoparticles Exposed to Various Conditions Based on the Evaluation between Physicochemical Properties and Their Relation with Protein Expression Ability. Pharmaceutics 2022, 14, 2357. https://doi.org/10.3390/pharmaceutics14112357
Kamiya M, Matsumoto M, Yamashita K, Izumi T, Kawaguchi M, Mizukami S, Tsurumaru M, Mukai H, Kawakami S. Stability Study of mRNA-Lipid Nanoparticles Exposed to Various Conditions Based on the Evaluation between Physicochemical Properties and Their Relation with Protein Expression Ability. Pharmaceutics. 2022; 14(11):2357. https://doi.org/10.3390/pharmaceutics14112357
Chicago/Turabian StyleKamiya, Mariko, Makoto Matsumoto, Kazuma Yamashita, Tatsunori Izumi, Maho Kawaguchi, Shusaku Mizukami, Masako Tsurumaru, Hidefumi Mukai, and Shigeru Kawakami. 2022. "Stability Study of mRNA-Lipid Nanoparticles Exposed to Various Conditions Based on the Evaluation between Physicochemical Properties and Their Relation with Protein Expression Ability" Pharmaceutics 14, no. 11: 2357. https://doi.org/10.3390/pharmaceutics14112357