Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
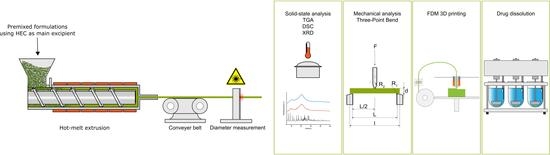
2.2. HME for Filament Production and Diameter Determination
2.3. 3D Printing for Tablet Production
2.4. Thermogravimetric Analysis of Raw Excipients
2.5. Differential Scanning Calorimetry
2.6. X-ray Diffraction
2.7. Mechanical Analysis: Three-Point-Bend Test
- is the stress in N·mm−².
- F is the applied force in N.
- L is the support pin gap in mm.
- d is the diameter of the filament in mm.
2.8. Drug Content, Drug Dissolution, and Drug Release Mechanism
3. Results and Discussion
3.1. Thermal Stability of Raw Materials and Evaluation of Extruded Filaments
3.2. Mechanical Stability, Feedability, and 3D-Printing Assessment of HEC-Based Filaments
3.3. Characterization of Solid Dosage Forms
3.4. Solid-State Analysis Using XRD and DSC
3.5. Drug Content in Filaments and Drug Release of Oral Solid Dosage Forms
3.6. Drug Release Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andreadis, I.I.; Gioumouxouzis, C.I.; Eleftheriadis, G.K.; Fatouros, D.G. The Advent of a New Era in Digital Healthcare: A Role for 3D Printing Technologies in Drug Manufacturing? Pharmaceutics 2022, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Roberts, C.J. Desktop 3D printing of controlled release pharmaceutical bilayer tablets. Int. J. Pharm. 2014, 461, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Figueiredo, S.; Fernandes, A.I.; Pinto, J.F. Polymer Selection for Hot-Melt Extrusion Coupled to Fused Deposition Modelling in Pharmaceutics. Pharmaceutics 2020, 12, 795. [Google Scholar] [CrossRef]
- Melnyk, L.A.; Oyewumi, M.O. Integration of 3D printing technology in pharmaceutical compounding: Progress, prospects, and challenges. Ann. 3D Print. Med. 2021, 4, 100035. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials-Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.J.N.; Yong, W.P.; Kochhar, J.S.; Khanolkar, J.; Yao, X.; Sun, Y.; Ao, C.K.; Soh, S. On-demand fully customizable drug tablets via 3D printing technology for personalized medicine. J. Control. Release 2020, 322, 42–52. [Google Scholar] [CrossRef]
- Simões, M.F.; Pinto, R.M.A.; Simões, S. Hot-melt extrusion in the pharmaceutical industry: Toward filing a new drug application. Drug Discov. Today 2019, 24, 1749–1768. [Google Scholar] [CrossRef]
- Gottschalk, N.; Bogdahn, M.; Harms, M.; Quodbach, J. Brittle polymers in Fused Deposition Modeling: An improved feeding approach to enable the printing of highly drug loaded filament. Int. J. Pharm. 2021, 597, 120216. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced Pharmaceutical Applications of Hot-Melt Extrusion Coupled with Fused Deposition Modelling (FDM) 3D Printing for Personalised Drug Delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Repka, M.A.; Shah, S.; Lu, J.; Maddineni, S.; Morott, J.; Patwardhan, K.; Mohammed, N.N. Melt extrusion: Process to product. Expert Opin. Drug Deliv. 2012, 9, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Keating, A.V.; Soto, J.; Tuleu, C.; Forbes, C.; Zhao, M.; Craig, D.Q.M. Solid state characterisation and taste masking efficiency evaluation of polymer based extrudates of isoniazid for paediatric administration. Int. J. Pharm. 2018, 536, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Malaquias, L.F.B.; Sá-Barreto, L.C.L.; Freire, D.O.; Silva, I.C.R.; Karan, K.; Durig, T.; Lima, E.M.; Marreto, R.N.; Gelfuso, G.M.; Gratieri, T.; et al. Taste masking and rheology improvement of drug complexed with beta-cyclodextrin and hydroxypropyl-β-cyclodextrin by hot-melt extrusion. Carbohydr. Polym. 2018, 185, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.Q.; Feng, X.; Morott, J.T.; Pimparade, M.B.; Tiwari, R.V.; Zhang, F.; Repka, M.A. A novel floating controlled release drug delivery system prepared by hot-melt extrusion. Eur. J. Pharm. Biopharm. 2016, 98, 108–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawant, K.P.; Fule, R.; Maniruzzaman, M.; Amin, P.D. Extended release delivery system of metoprolol succinate using hot-melt extrusion: Effect of release modifier on methacrylic acid copolymer. Drug Deliv. Transl. Res. 2018, 8, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Obara, S.; Liu, F.; Fu, W.; Zhang, W.; Kikuchi, S. Melt Extrusion for a High Melting Point Compound with Improved Solubility and Sustained Release. AAPS PharmSciTech 2018, 19, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Govender, R.; Kissi, E.O.; Larsson, A.; Tho, I. Polymers in pharmaceutical additive manufacturing: A balancing act between printability and product performance. Adv. Drug Deliv. Rev. 2021, 177, 113923. [Google Scholar] [CrossRef]
- Przybytek, A.; Sienkiewicz, M.; Kucińska-Lipka, J.; Janik, H. Preparation and characterization of biodegradable and compostable PLA/TPS/ESO compositions. Ind. Crops Prod. 2018, 122, 375–383. [Google Scholar] [CrossRef]
- Haryńska, A.; Janik, H.; Sienkiewicz, M.; Mikolaszek, B.; Kucińska-Lipka, J. PLA–Potato Thermoplastic Starch Filament as a Sustainable Alternative to the Conventional PLA Filament: Processing, Characterization, and FFF 3D Printing. ACS Sustain. Chem. Eng. 2021, 9, 6923–6938. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A review of hot-melt extrusion: Process technology to pharmaceutical products. ISRN Pharm. 2012, 2012, 436763. [Google Scholar] [CrossRef] [Green Version]
- Aho, J.; Bøtker, J.P.; Genina, N.; Edinger, M.; Arnfast, L.; Rantanen, J. Roadmap to 3D-Printed Oral Pharmaceutical Dosage Forms: Feedstock Filament Properties and Characterization for Fused Deposition Modeling. J. Pharm. Sci. 2019, 108, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora-Castaño, G.; Millán-Jiménez, M.; Linares, V.; Caraballo, I. Assessment of the Extrusion Process and Printability of Suspension-Type Drug-Loaded AffinisolTM Filaments for 3D Printing. Pharmaceutics 2022, 14, 871. [Google Scholar] [CrossRef] [PubMed]
- Akbari, J.; Enayatifard, R.; Saeedi, M.; Saghafi, M. Influence of Hydroxypropyl Methylcellulose Molecular Weight Grade on Water Uptake, Erosion and Drug Release Properties of Diclofenac Sodium Matrix Tablets. Trop. J. Pharm. Res. 2011, 10, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Giri, B.R.; Poudel, S.; Kim, D.W. Cellulose and its derivatives for application in 3D printing of pharmaceuticals. J. Pharm. Investig. 2021, 51, 1–22. [Google Scholar] [CrossRef]
- Picker-Freyer, K.M.; Dürig, T. Physical mechanical and tablet formation properties of hydroxypropylcellulose: In pure form and in mixtures. AAPS PharmSciTech 2007, 8, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Sarode, A.; Wang, P.; Cote, C.; Worthen, D.R. Low-viscosity hydroxypropylcellulose (HPC) grades SL and SSL: Versatile pharmaceutical polymers for dissolution enhancement, controlled release, and pharmaceutical processing. AAPS PharmSciTech 2013, 14, 151–159. [Google Scholar] [CrossRef] [Green Version]
- Reddy Dumpa, N.; Bandari, S.; A Repka, M. Novel Gastroretentive Floating Pulsatile Drug Delivery System Produced via Hot-Melt Extrusion and Fused Deposition Modeling 3D Printing. Pharmaceutics 2020, 12, 52. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, D.; Maheshwari, R.; Verma, K.; Sharma, S.; Ghode, P.; Tekade, R.K. Coating technologies in pharmaceutical product development. In Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 665–719. ISBN 9780128144879. [Google Scholar]
- Arca, H.C.; Mosquera-Giraldo, L.I.; Bi, V.; Xu, D.; Taylor, L.S.; Edgar, K.J. Pharmaceutical Applications of Cellulose Ethers and Cellulose Ether Esters. Biomacromolecules 2018, 19, 2351–2376. [Google Scholar] [CrossRef]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; W Basit, A. 3D Printing of Tunable Zero-Order Release Printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef]
- Sanoufi, M.R.; Aljaberi, A.; Hamdan, I.; Al-Zoubi, N. The use of design of experiments to develop hot melt extrudates for extended release of diclofenac sodium. Pharm. Dev. Technol. 2020, 25, 187–196. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef] [PubMed]
- DIN EN ISO 11358-1:2020-12; Plastics—Thermogravimetry (TG) of Polymers—Part 1: General Principles (ISO/DIS 11358-1:2020). Beuth Verlag GmbH: Berlin, Germany, 2020.
- Đuranović, M.; Obeid, S.; Madžarević, M.; Cvijić, S.; Ibrić, S. Paracetamol extended release FDM 3D printlets: Evaluation of formulation variables on printability and drug release. Int. J. Pharm. 2021, 592, 120053. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Bruschi, M.L. (Ed.) Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier Science: Burlington, MA, USA, 2015; pp. 63–86. ISBN 9780081000922. [Google Scholar]
- Li, X.-G.; Huang, M.-R.; Bai, H. Thermal decomposition of cellulose ethers. J. Appl. Polym. Sci. 1999, 73, 2927–2936. [Google Scholar] [CrossRef]
- Brydson, J.A. Relation of Structure to Thermal and Mechanical Properties. In Plastics Materials; Elsevier: Amsterdam, The Netherlands, 1999; pp. 59–75. ISBN 9780750641326. [Google Scholar]
- Zhang, J.; Feng, X.; Patil, H.; Tiwari, R.V.; Repka, M.A. Coupling 3D printing with hot-melt extrusion to produce controlled-release tablets. Int. J. Pharm. 2017, 519, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Korte, C.; Quodbach, J. Formulation development and process analysis of drug-loaded filaments manufactured via hot-melt extrusion for 3D-printing of medicines. Pharm. Dev. Technol. 2018, 23, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D.; et al. Quality of FDM 3D Printed Medicines for Pediatrics: Considerations for Formulation Development, Filament Extrusion, Printing Process and Printer Design. Ther. Innov. Regul. Sci. 2021, 56, 910–928. [Google Scholar] [CrossRef]
- Seymour, R.B.; Carraher, C.E. Mechanical Properties of Polymers. In Structure—Property Relationships in Polymers; Seymour, R.B., Carraher, C.E., Eds.; Springer US: Boston, MA, USA, 1984; pp. 57–72. ISBN 978-1-4684-4750-7. [Google Scholar]
- Physical, Thermal, and Mechanical Properties of Polymers. In Biosurfaces; Balani, K.; Verma, V.; Agarwal, A.; Narayan, R. (Eds.) John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 329–344. ISBN 9781118950623. [Google Scholar]
- Di Giuseppe, E. Analogue Materials in Experimental Tectonics. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780124095489. [Google Scholar]
- Prasad, E.; Islam, M.T.; Goodwin, D.J.; Megarry, A.J.; Halbert, G.W.; Florence, A.J.; Robertson, J. Development of a hot-melt extrusion (HME) process to produce drug loaded Affinisol™ 15LV filaments for fused filament fabrication (FFF) 3D printing. Addit. Manuf. 2019, 29, 100776. [Google Scholar] [CrossRef]
- Bruère, V.M.; Lion, A.; Holtmannspötter, J.; Johlitz, M. Under-extrusion challenges for elastic filaments: The influence of moisture on additive manufacturing. Prog. Addit. Manuf. 2022, 7, 445–452. [Google Scholar] [CrossRef]
- Homaee Borujeni, S.; Mirdamadian, S.Z.; Varshosaz, J.; Taheri, A. Three-dimensional (3D) printed tablets using ethyl cellulose and hydroxypropyl cellulose to achieve zero order sustained release profile. Cellulose 2020, 27, 1573–1589. [Google Scholar] [CrossRef]
- Nashed, N.; Lam, M.; Ghafourian, T.; Pausas, L.; Jiri, M.; Majumder, M.; Nokhodchi, A. An Insight into the Impact of Thermal Process on Dissolution Profile and Physical Characteristics of Theophylline Tablets Made through 3D Printing Compared to Conventional Methods. Biomedicines 2022, 10, 1335. [Google Scholar] [CrossRef]
- Castellari, C.; Ottani, S. Two Monoclinic Forms of Diclofenac Acid. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1997, 53, 794–797. [Google Scholar] [CrossRef]
- Jaiboon, N.; Yos-In, K.; Ruangchaithaweesuk, S.; Chaichit, N.; Thutivoranath, R.; Siritaedmukul, K.; Hannongbua, S. New orthorhombic form of 2-(2,6-dichlorophenyl)aminobenzeneacetic acid (diclofenac acid). Anal. Sci. 2001, 17, 1465–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohapatra, S.; Samanta, S.; Kothari, K.; Mistry, P.; Suryanarayanan, R. Effect of Polymer Molecular Weight on the Crystallization Behavior of Indomethacin Amorphous Solid Dispersions. Cryst. Growth Des. 2017, 17, 3142–3150. [Google Scholar] [CrossRef]
- Ramkissoon-Ganorkar, C.; Liu, F.; Baudys, M.; Kim, S.W. Effect of molecular weight and polydispersity on kinetics of dissolution and release from ph/temperature-sensitive polymers. J. Biomater. Sci. Polym. Ed. 1999, 10, 1149–1161. [Google Scholar] [CrossRef] [PubMed]
- Omelczuk, M.O.; McGinity, J.W. The influence of polymer glass transition temperature and molecular weight on drug release from tablets containing poly(DL-lactic acid). Pharm. Res. 1992, 9, 26–32. [Google Scholar] [CrossRef]
- Cantin, O.; Siepmann, F.; Danede, F.; Willart, J.F.; Karrout, Y.; Siepmann, J. PEO hot melt extrudates for controlled drug delivery: Importance of the molecular weight. J. Drug Deliv. Sci. Technol. 2016, 36, 130–140. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Sinha Roy, D.; Rohera, B.D. Comparative evaluation of rate of hydration and matrix erosion of HEC and HPC and study of drug release from their matrices. Eur. J. Pharm. Sci. 2002, 16, 193–199. [Google Scholar] [CrossRef]
- Sujja-areevath, J.; Munday, D.L.; Cox, P.J.; Khan, K. Relationship between swelling, erosion and drug release in hydrophillic natural gum mini-matrix formulations. Eur. J. Pharm. Sci. 1998, 6, 207–217. [Google Scholar] [CrossRef]





| Formulation (F) | HEC L [%w/w] | HEC G [%w/w] | HEC M [%w/w] | HEC HX [%w/w] | HPC SSL [%w/w] | Diclofenac Sodium [%w/w] |
|---|---|---|---|---|---|---|
| F-L | 75 | - | - | - | 20 | 5 |
| F-G | - | 75 | - | - | 20 | 5 |
| F-M | - | - | 75 | - | 20 | 5 |
| F-HX | - | - | - | 75 | 20 | 5 |
| Model | Equation | |
|---|---|---|
| Zero order | (2) | |
| First order | (3) | |
| Higuchi | (4) | |
| Korsmeyer–Peppas | (5) |
| Formulation | Extrusion Temperature [°C] | Screw Speed [rpm] | Max. Torque [Nm] | Filament Diameter [mm] Measure Points > 150 |
|---|---|---|---|---|
| F-L | 135 | 25 | 11 | 2.90 ± 0.04 |
| F-G | 135 | 25 | 12 | 2.92 ± 0.03 |
| F-M | 135 | 20 | 11 | 2.85 ± 0.02 |
| F-HX | 135 | 15 | 12 | 2.83 ± 0.02 |
| Formulation | Nozzle Size [mm] | Temperature [°C] | Print Speed [mm·s−1] | Layer Height [mm] | Feedability | Printability | |
|---|---|---|---|---|---|---|---|
| Nozzle | Print Bed | ||||||
| F-L | 0.8 | 200 | 60 | 25 | 0.3 | 🗸 | 🗸 |
| F-G | 0.8 | 195 | 60 | 35 | 0.3 | ✗ | 🗸 |
| F-M | 0.8 | 195 | 60 | 35 | 0.3 | ✗ | 🗸 |
| F-HX | 0.8 | 195 | 60 | 35 | 0.3 | ✗ | 🗸 |
| Formulation | Tablet Weight [mg] | Tablet Diameter [mm] | Tablet Height [mm] | Filament Drug Load [%w/w] |
|---|---|---|---|---|
| F-L | 358.37 ± 5.07 | 10.33 ± 0.04 | 5.01 ± 0.03 | 5.65 ± 0.46 |
| F-G | 354.54 ± 12.42 | 10.33 ± 0.06 | 4.97 ± 0.07 | 5.87 ± 0.11 |
| F-M | 360.58 ± 9.44 | 10.28 ± 0.04 | 5.00 ± 0.02 | 5.71 ± 0.07 |
| F-HX | 347.85 ± 11.16 | 10.32 ± 0.04 | 4.97 ± 0.02 | 5.45 ± 0.27 |
| Model | Statistic | F-L | F-G | F-M | F-HX |
|---|---|---|---|---|---|
| Zero order | adj. R² | 0.938 | 0.926 | 0.942 | 0.945 |
| First order | adj. R² | 0.784 | 0.988 | 0.983 | 0.981 |
| Higuchi | adj. R² | 0.930 | 0.964 | 0.976 | 0.974 |
| Korsmeyer-Peppas | adj. R² | 0.996 | 0.999 | 0.999 | 0.999 |
| k | 0.485 | 0.270 | 0.479 | 0.359 | |
| n | 0.866 | 0.790 | 0.655 | 0.683 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartzke, D.; Pössl, A.; Schlupp, P.; Runkel, F.E. Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms. Pharmaceutics 2022, 14, 2103. https://doi.org/10.3390/pharmaceutics14102103
Hartzke D, Pössl A, Schlupp P, Runkel FE. Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms. Pharmaceutics. 2022; 14(10):2103. https://doi.org/10.3390/pharmaceutics14102103
Chicago/Turabian StyleHartzke, David, Axel Pössl, Peggy Schlupp, and Frank E. Runkel. 2022. "Evaluation of Hydroxyethyl Cellulose Grades as the Main Matrix Former to Produce 3D-Printed Controlled-Release Dosage Forms" Pharmaceutics 14, no. 10: 2103. https://doi.org/10.3390/pharmaceutics14102103