Particle Agglomeration of Acid-Modified Tapioca Starches: Characterization and Use as Direct Compression Fillers in Tablets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of TS and AMTS Agglomerates
2.3. Characterization of TS and AMTS Agglomerates
2.3.1. Particle Morphology Studies
2.3.2. Differential Scanning Calorimetry (DSC)
2.3.3. Fourier Transform Infrared (FTIR) Spectroscopy
2.3.4. Particle Flowability Measurement
2.3.5. Particle Strength Determination
2.4. Tablet Preparations
2.5. Stability Study of Tablets
2.6. Tablet Evaluations
3. Results and Discussion
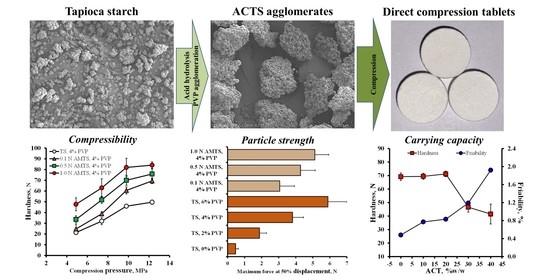
3.1. Appearance, Morphology and Strength of the Agglomerates
3.2. Molecular Interaction and Thermal Behavior of the Agglomerates
3.3. Flowability and Compressibility of the Agglomerates
3.4. Characteristics of Drug-Loaded Tablets
3.5. Use of the AMTS Agglomerates as Direct Compression Fillers
3.6. Stability Study of the Tablets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. Today 2000, 3, 58–63. [Google Scholar] [CrossRef]
- Chen, H.; Aburub, A.; Sun, C.C. Direct compression tablet containing 99% active ingredient—A tale of spherical crystallization. J. Pharm. Sci. 2019, 108, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Capece, M.; Huang, Z.; Dave, R. Insight into a novel strategy for the design of tablet formulations intended for direct compression. J. Pharm. Sci. 2017, 106, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Mitrevej, A.; Sinchaipanid, N.; Faroongsarng, D. Spray-dried rice starch: Comparative evaluation of direct compression fillers. Drug Dev. Ind. Pharm. 1996, 22, 587–594. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Pongjanyakul, T. Particle agglomeration of chitosan–magnesium aluminum silicate nanocomposites for direct compression tablets. Int. J. Pharm. 2018, 535, 410–419. [Google Scholar] [CrossRef]
- Emmanuel, O.; Ewomazino, O.; Tizazu, M. Advanced material applications of starch and its derivatives. Eur. Polym. J. 2018, 108, 570–581. [Google Scholar]
- Punia, S. Barley starch modifications: Physical, chemical and enzymatic—A review. Int. J. Biol. Macromol. 2020, 144, 578–585. [Google Scholar] [CrossRef]
- Muphy, P. Starch. In Handbook of Hydrocolloids; Philips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2000; pp. 41–65. [Google Scholar]
- Charoenthai, N.; Sanga-ngam, T.; Puttipipatkhachorn, S. Use of modified tapioca starches as pharmaceutical excipients. Pharm. Sci. Asia 2018, 45, 195–204. [Google Scholar] [CrossRef]
- Nair, S.B.; Jyothi, A.N.; Sajeev, M.S.; Misra, R. Rheological, mechanical and moisture sorption characteristics of cassava starch-konjac glucomannan blend films. Starch Stärke 2011, 63, 728–739. [Google Scholar] [CrossRef]
- Lawal, M.V. Modified starches as direct compression excipients—Effect of physical and chemical modifications on tablet properties: A review. Starch Stärke 2019, 71, 1800040. [Google Scholar] [CrossRef]
- Lemos, P.V.F.; Marcelino, H.R.; Cardoso, L.G.; de Souza, C.O.; Druzian, J.I. Starch chemical modifications applied to drug delivery systems: From fundamentals to FDA-approved raw materials. Int. J. Biol. Macromol. 2021, 184, 218–234. [Google Scholar] [CrossRef] [PubMed]
- Atichokudomchai, N.; Shobsngob, S.; Varavinit, S. Morphological properties of acid-modified tapioca starch. Starch Stärke 2000, 52, 283–289. [Google Scholar] [CrossRef]
- Atichokudomchai, N.; Shobsngob, S.; Chinachoti, P.; Varavinit, S. A study of some physicochemical properties of high-crystalline tapioca starch. Starch Stärke 2001, 53, 577–581. [Google Scholar] [CrossRef]
- Jyothi, A.N.; Sasikiran, K.; Sajeev, M.S.; Revamma, R.; Moorthy, S.N. Gelatinisation properties of cassava starch in the presence of salts, acids and oxidising agents. Starch Stärke 2005, 57, 547–555. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, D.; Xue, J.; Yanniotis, S.; Mandala, I. The effect of salt concentration on swelling power, rheological properties and saltiness perception of waxy, normal and high amylose maize starch. Food Funct. 2017, 8, 3792–3802. [Google Scholar] [CrossRef]
- Assen, A.; Belete, A.; Gebre-Mariam, T. Preparation and physicochemical characterization of acid modified cassava starch and its evaluation as directly compressible tablet excipient. Ethiop. Pharm. J. 2011, 29, 27–42. [Google Scholar] [CrossRef]
- Dos Santos, T.P.R.; Franco, C.M.L.; Demiate, I.M.; Li, X.H.; Garcia, E.L.; Jane, J.L.; Leonel, M. Spray-drying and extrusion processes: Effects on morphology and physicochemical characteristics of starches isolated from Peruvian carrot and cassava. Int. J. Biol. Macromol. 2018, 118, 1346–1353. [Google Scholar] [CrossRef]
- Siriwachirachai, C.; Pongjanyakul, T. Acid and alkali modifications of tapioca starches: Physicochemical characterizations and evaluations for use in tablets. J. Drug Deliv. Sci. Technol. 2022, 68, 103068. [Google Scholar] [CrossRef]
- Fu, X.; Huck, D.; Makein, L.; Armstrong, B.; Willen, U.; Freeman, T. Effect of particle shape and size on flow properties of lactose powders. Particuology 2012, 10, 203–208. [Google Scholar] [CrossRef]
- Pietsch, W. An interdisciplinary approach to size enlargement by agglomeration. Powder Technol. 2003, 130, 8–13. [Google Scholar] [CrossRef]
- Edwards-Lévy, F.; Lévy, M.C. Serum albumin–alginate coated beads: Mechanical properties and stability. Biomaterials 1999, 20, 2069–2084. [Google Scholar] [CrossRef]
- Pongjanyakul, T.; Rongthong, T. Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohyd. Polym. 2010, 81, 409–419. [Google Scholar] [CrossRef]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Xie, F.; Ji, S.; Cheng, Z. In vitro dissolution similarity factor (f2) and in vivo bioequivalence criteria, how and when do they match? Using a BCS class II drug as a simulation example. Eur. J. Pharm. Sci. 2015, 66, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Koteswara Rao, G.S.N. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Chavalparit, O.; Ongwandee, M. Clean technology for the tapioca starch industry in Thailand. J. Clean. Prod. 2009, 17, 105–110. [Google Scholar] [CrossRef]
- Horisawa, E.; Komura, A.; Danjo, K.; Otsuka, A. Effect of binder characteristics on the strength of agglomerates prepared by the wet method II. Chem. Pharm. Bull. 1995, 43, 488–492. [Google Scholar] [CrossRef]
- Horisawa, E.; Komura, A.; Danjo, K.; Otsuka, A. Effect of granule strength on compressed tablet strength. Chem. Pharm. Bull. 1995, 43, 2261–2263. [Google Scholar] [CrossRef]
- Simons, S.J.R.; Rossetti, D.; Pagliai, P.; Ward, R.; Fitzpatrick, S. The relationship between surface properties and binder perfoermance in granulation. Chem. Eng. Sci. 2005, 60, 4055–4060. [Google Scholar] [CrossRef]
- Safo, I.A.; Werheid, M.; Dosche, C.; Oezaslan, M. The role of polyvinylpyrrolidone (PVP) as a capping and structure-directing agent in the formation of Pt nanocubes. Nanoscale Adv. 2019, 1, 3095–3106. [Google Scholar] [CrossRef]
- Zidan, H.M.; Abdelrazek, E.M.; Abdelghany, A.M.; Tarabiah, A.E. Characterization and some physical studies of PVA/PVP filled with MWCNTs. J. Mater. Res. Technol. 2019, 8, 904–913. [Google Scholar] [CrossRef]
- Carr, R.L. Evaluating flow properties of solids. Chem. Eng. 1965, 72, 163–168. [Google Scholar]
- Horio, T.; Yasuda, M.; Matsusaka, S. Effect of particle shape on powder flowability of microcrystalline cellulose as determined using the vibration shear tube method. Int. J. Pharm. 2014, 473, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Leturia, M.; Benali, M.; Lagarde, S.; Ronga, I.; Saleh, K. Characterization of flow properties of cohesive powders: A comparative study of traditional and new testing methods. Powder Technol. 2014, 253, 406–423. [Google Scholar] [CrossRef]
- Petra, S.; Jitka, M.; Pavel, O. Comparison of compressibility, compactability, and lubricant sensitivity of two partially pregelatinized starches. Starch Stärke 2021, 73, 2000166. [Google Scholar]
- Akin-Ajani, O.D.; Itiola, O.A.; Odeku, O.A. Effect of acid modification on the material and compaction properties of fonio and sweet potato starches. Starch Stärke 2014, 66, 749–759. [Google Scholar] [CrossRef]
- Nyström, C.; Glazer, M. Studies on direct compression of tablets. XIII. The effect of some dry binders on the tablet strength of compounds with different fragmentation propensity. Int. J. Pharm. 1985, 23, 255–263. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Pongjanyakul, T. Spray-dried chitosan-magnesium aluminum silicate microparticles as matrix formers in controlled release tablets. J. Drug Deliv. Sci. Technol. 2015, 30, 114–122. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, L.; Lin, X.; Shen, L.; Feng, Y. Direct compaction: An update of materials, trouble-shooting, and application. Int. J. Pharm. 2017, 529, 543–556. [Google Scholar] [CrossRef]
- Mohammed, H.; Briscoe, B.J.; Pitt, K.G. The interrelationship between the compaction behaviour and the mechanical strength of pure pharmaceutical tablets. Chem. Eng. Sci. 2005, 60, 3941–3947. [Google Scholar] [CrossRef]
- Mohammed, H.; Briscoe, B.J.; Pitt, K.G. The intrinsic nature and the coherence of compacted pure pharmaceutical tablets. Powder Technol. 2006, 165, 11–21. [Google Scholar] [CrossRef]
- EI-Obeid, H.A.; Al-Badr, A.A. Actaminophen. Anal. Profiles Drug Subst. 1985, 14, 552–596. [Google Scholar]
- Kockler, J.; Robertson, S.; Hope, D.; Haywood, A.; Glass, B. Stability of paracetamol tablets repackaged in dose administration aids for prn use: Implications for practice. J. Pharm. Pract. Res. 2013, 43, 218–220. [Google Scholar] [CrossRef]
- Veronica, N.; Hiew, T.N.; Liew, C.V.; Heng, P.W.S. Insights into the moisture scavenging properties of different types of starch in tablets containing a moisture-sensitive drug. Mol. Pharm. 2020, 17, 4616–4628. [Google Scholar] [CrossRef] [PubMed]
- Sarisuta, N.; Parrott, E.L. Effects of temperature, humidity, and aging on the disintegration and dissolution of acetaminophen tablets. Drug Dev. Ind. Pharm. 1988, 14, 1877–1881. [Google Scholar] [CrossRef]










| Starches | PVP (% w/w) | Yield (% w/w) | Moisture Content a (% w/w) | Bulk Density a (g cm−3) | Tapped Density a (g cm−3) | Carr’s Index a (%) | Angle of Repose a (°) |
|---|---|---|---|---|---|---|---|
| TS | 0 | 34.88 | 12.06 ± 0.16 | 0.46 ± 0.01 | 0.70 ± 0.01 | 34.41 ± 0.22 | 42.82 ± 0.21 |
| 2 | 46.21 | 9.92 ± 0.25 | 0.48 ± 0.01 | 0.58 ± 0.01 | 17.71 ± 0.17 | 35.65 ± 0.73 | |
| 4 | 56.86 | 9.43 ± 0.37 | 0.45 ± 0.01 | 0.52 ± 0.01 | 14.08 ± 0.14 | 35.86 ± 0.36 | |
| 6 | 55.37 | 9.42 ± 0.14 | 0.46 ± 0.01 | 0.54 ± 0.01 | 13.91 ± 0.06 | 35.33 ± 0.36 | |
| 0.1 N AMTS | 4 | 55.37 | 9.08 ± 0.23 | 0.49 ± 0.01 | 0.60 ± 0.01 | 18.80 ± 0.12 | 35.17 ± 0.23 |
| 0.5 N AMTS | 4 | 63.66 | 9.34 ± 0.48 | 0.47 ± 0.01 | 0.59 ± 0.01 | 19.97 ± 0.58 | 35.27 ± 0.28 |
| 1.0 N AMTS | 4 | 72.63 | 9.51 ± 0.27 | 0.45 ± 0.02 | 0.56 ± 0.03 | 19.88 ± 0.42 | 35.42 ± 0.84 |
| Starches | Thickness a (mm) | Hardness a (N) | 0.1 N HCl b | pH 6.8 Phosphate Buffer b | ||
|---|---|---|---|---|---|---|
| DT (min) | T50% (min) | DT (min) | T50% (min) | |||
| TS, 0% PVP | 2.62 ± 0.01 | 5.69 ± 0.96 | 0.47 ± 0.01 | 3.27 ± 0.02 | 0.50 ± 0.01 | 3.15 ± 0.03 |
| TS, 2% PVP | 2.60 ± 0.01 | 37.85 ± 7.51 | 2.17 ± 0.01 | 7.84 ± 0.27 | 2.68 ± 0.07 | 9.04 ± 0.48 |
| TS, 4% PVP | 2.65 ± 0.02 | 44.72 ± 8.45 | 2.47 ± 0.01 | 9.31 ± 0.36 | 2.98 ± 0.03 | 9.86 ± 0.51 |
| TS, 6% PVP | 2.63 ± 0.02 | 52.96 ± 1.64 | 4.04 ± 0.01 | 12.10 ± 1.21 | 4.61 ± 0.18 | 14.73 ± 0.83 |
| 0.1 N AMTS, 4% PVP | 2.68 ± 0.02 | 43.35 ± 3.48 | 3.28 ± 0.11 | 8.97 ± 0.29 | 3.77 ± 0.01 | 13.02 ± 0.32 |
| 0.5 N AMTS, 4% PVP | 2.66 ± 0.01 | 42.17 ± 2.97 | 4.22 ± 0.03 | 9.93 ± 0.62 | 4.75 ± 0.16 | 13.37 ± 0.55 |
| 1.0 N AMTS, 4% PVP | 2.66 ± 0.01 | 54.59 ± 9.45 | 4.19 ± 0.04 | 13.04 ± 0.68 | 4.68 ± 0.07 | 14.73 ± 0.39 |
| ACT Added in Mixture (% w/w) | Thickness a (mm) | Tablet Weight b (mg) | Hardness a (N) | Friability (%) | ACT Content in Tablets c (%) | DT c (min) |
|---|---|---|---|---|---|---|
| 0 | 3.44 ± 0.01 | 353.26 ± 1.34 (%RSD = 0.38) | 69.14 ± 2.76 | 0.48 | - | 5.86 ± 0.01 |
| 10 | 3.45 ± 0.01 | 349.59 ± 2.29 (%RSD = 0.66) | 69.46 ± 2.22 | 0.77 | 100.78 ± 0.38 | 7.34 ± 0.12 |
| 20 | 3.46 ± 0.02 | 351.42 ± 3.44 (%RSD = 0.98) | 71.20 ± 2.02 | 0.83 | 98.11 ± 0.42 | 6.49 ± 0.12 |
| 30 | 3.47 ± 0.03 | 355.64 ± 5.75 (%RSD = 1.62) | 46.58 ± 3.61 | 1.19 | 100.83 ± 0.79 | 4.76 ± 0.23 |
| 40 | 3.42 ± 0.06 | 349.59 ± 16.53 (%RSD = 4.73) | 41.51 ± 7.33 | 1.92 | 101.45 ± 1.30 | 3.63 ± 0.52 |
| Condition | Thickness a (mm) | Hardness a (N) | ACT Content b (%) | DT b (min) | T50% b (min) | Similarity Factor (f2) |
|---|---|---|---|---|---|---|
| Initial | 3.46 ± 0.02 | 71.20 ± 2.02 | 98.11 ± 0.42 | 6.49 ± 0.12 | 15.55 ± 2.23 | - |
| 3 months storage | ||||||
| Ambient condition 26 ± 2 °C, 52 ± 3% RH | 3.55 ± 0.03 | 80.02 ± 2.31 | 98.33 ± 3.99 | 6.62 ± 0.13 | 20.93 ± 3.00 | 56.60 |
| Accelerated condition 45 ± 1 °C, 75% RH | 3.60 ± 0.05 | 136.15 ± 9.44 | 99.35 ± 4.08 | 7.65 ± 0.20 | 12.95 ± 1.37 | 68.75 |
| Refrigerator 2–8 °C, 20 ± 2% RH | 3.52 ± 0.03 | 79.43 ± 2.06 | 99.23 ± 3.29 | 7.19 ± 0.40 | 17.15 ± 2.45 | 70.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siriwachirachai, C.; Pongjanyakul, T. Particle Agglomeration of Acid-Modified Tapioca Starches: Characterization and Use as Direct Compression Fillers in Tablets. Pharmaceutics 2022, 14, 1245. https://doi.org/10.3390/pharmaceutics14061245
Siriwachirachai C, Pongjanyakul T. Particle Agglomeration of Acid-Modified Tapioca Starches: Characterization and Use as Direct Compression Fillers in Tablets. Pharmaceutics. 2022; 14(6):1245. https://doi.org/10.3390/pharmaceutics14061245
Chicago/Turabian StyleSiriwachirachai, Chaipat, and Thaned Pongjanyakul. 2022. "Particle Agglomeration of Acid-Modified Tapioca Starches: Characterization and Use as Direct Compression Fillers in Tablets" Pharmaceutics 14, no. 6: 1245. https://doi.org/10.3390/pharmaceutics14061245