Effects of Verapamil and Diltiazem on the Pharmacokinetics and Pharmacodynamics of Rivaroxaban
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animal Study
2.3. Rivaroxaban Plasma Concentrations Measured by LC-MS/MS
2.4. Measurement of Prothrombin Time
2.5. Model Independent Data Analysis
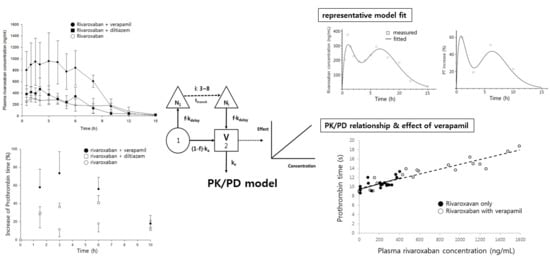
2.6. Model Dependent Data Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Pharmacokinetics of Rivaroxaban in the Presence of Verapamil or Diltiazem
3.2. Prothrombin Time Following Oral Administration of Rivaroxaban in the Presence of Verapamil or Diltiazem
3.3. PK/PD Modeling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perzborn, E.; Strassburger, J.; Wilmen, A.; Pohlmann, J.; Roehrig, S.; Schlemmer, K.H.; Straub, A. In vitro and in vivo studies of the novel antithrombotic agent BAY 59-7939-an oral, direct Factor Xa inhibitor. J. Thromb. Haemost. 2005, 3, 514–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayer Pharma AG. Xarelto_ (rivaroxaban) Summary of Product Characteristics. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR__Product_Information/human/000944/WC500057108.pdf (accessed on 23 July 2013).
- Janssen Pharmaceuticals Inc. Xarelto_ (rivaroxaban) Prescribing Information. 2013. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022406s004lbl.pdf (accessed on 23 July 2013).
- Wessler, J.D.; Grip, L.T.; Mendell, J.; Giugliano, R.P. The P-glycoprotein transport system and cardiovascular drugs. J. Am. Coll. Cardiol. 2013, 61, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Washam, J.B.; Hellkamp, A.S.; Lokhnygina, Y.; Piccini, J.P.; Berkowitz, S.D.; Nessel, C.C.; Becker, R.C.; Breithardt, G.; Fox, K.A.A.; Halperin, J.L.; et al. Efficacy and safety of rivaroxaban versus warfarin in patients taking nondihydropyridine calcium channel blockers for atrial fibrillation (from the ROCKET AF Trial). Am. J. Cardiol. 2017, 120, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.F. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr. Drug Metab. 2008, 9, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Frost, C.E.; Byon, W.; Song, Y.; Wang, J.; Schuster, A.E.; Boyd, R.A.; Zhang, D.; Yu, Z.; Dias, C.; Shenker, A.; LaCreta, F. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br. J. Clin. Pharmacol. 2015, 79, 838–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.H.; Chou, I.J.; Yeh, Y.H.; Chiou, M.J.; Wen, M.S.; Kuo, C.T.; See, L.C.; Kuo, C.F. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of major bleeding in nonvalvular atrial fibrillation. JAMA 2017, 318, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Greenblatt, D.J.; Patel, M.; Harmatz, J.S.; Nicholson, W.T.; Rubino, C.M.; Chow, C.R. Impaired rivaroxaban clearance in mild renal insufficiency with verapamil coadministration: Potential implications for bleeding risk and dose selection. J. Clin. Pharmacol. 2018, 58, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Kang, W.K.; Kwon, K.I. Pharmacokinetic and pharmacodynamic modeling of the antiplatelet and cardiovascular effects of cilostazol in healthy humans. Clin. Pharmacol. Ther. 2002, 71, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Kang, W. P-glycoprotein inhibitors enhance saturable uptake of idarubicin in rat heart: Pharmacokinetic/pharmacodynamic modeling. J. Pharmacol. Exp. Ther. 2002, 300, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kubitza, D.; Willmann, S.; Becka, M.; Thelen, K.; Young, G.; Brandão, L.R.; Monagle, P.; Male, C.; Chan, A.; Kennet, G.; et al. Exploratory evaluation of pharmacodynamics, pharmacokinetics and safety of rivaroxaban in children and adolescents: An EINSTEIN-Jr phase I study. Thromb. J. 2018, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Girgis, I.G.; Patel, M.R.; Peters, G.R.; Moore, K.T.; Mahaffey, K.W.; Nessel, C.C.; Halperin, J.L.; Califf, R.M.; Fox, K.A.; Becker, R.C. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: Results from ROCKET AF. J. Clin. Pharmacol. 2014, 54, 917–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanigawa, T.; Kaneko, M.; Hashizume, K.; Kajikawa, M.; Ueda, H.; Tajiri, M.; Paolini, J.F.; Mueck, W. Model-based dose selection for phase III rivaroxaban study in Japanese patients with non-valvular atrial fibrillation. Drug Metab. Pharmacokinet. 2013, 28, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.S.; Moore, K.; Burton, P.; Stuyckens, K.; Mueck, W.; Rossenu, S.; Plotnikov, A.; Gibson, M.; Vermeulen, A. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br. J. Clin. Pharmacol. 2012, 74, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueck, W.; Lensing, A.W.; Agnelli, G.; Decousus, H.; Prandoni, P.; Misselwitz, F. Rivaroxaban: Population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin. Pharmacokinet. 2011, 50, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Mueck, W.; Eriksson, B.I.; Bauer, K.A.; Borris, L.; Dahl, O.E.; Fisher, W.D.; Gent, M.; Haas, S.; Huisman, M.V.; Kakkar, A.K.; et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban-an oral, direct factor Xa inhibitor-in patients undergoing major orthopaedic surgery. Clin. Pharmacokinet. 2008, 47, 203–216. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Rivaroxaban | Rivaroxaban + Verapamil | Rivaroxaban + Diltiazem |
|---|---|---|---|
| Cmax(ng/mL) | 373 (3) | 1079 (39) ** | 532 (31) * |
| Tmax (h) | 2.8 (111) | 4.0 (63) | 1.6 (44) |
| AUCinf (ng/mL) | 2699 (25) | 7606 (30) *** | 2915 (27) |
| CL/F (mL/h/kg) | 774 (22) | 286 (35) *** | 723 (24) |
| Parameter | Rivaroxaban | Rivaroxaban + Verapamil | Rivaroxaban + Diltiazem |
|---|---|---|---|
| Emax(%) | 30 (11) | 75 (14) * | 40 (7) |
| AUEC(%∙h) | 188 (9) | 491 (10) * | 247 (14) |
| Parameter | Rivaroxaban | Rivaroxaban + Verapamil |
|---|---|---|
| ka (h−1) | 0.63 (22) | 1.02 (19) ** |
| V/F (mL/kg) | 960 (37) | 369 (37) ** |
| ke (h−1) | 0.85 (27) | 0.80 (19) |
| f (fraction) | 0.61 (30) | 0.61 (14) |
| ttransit (h) | 6.37 (21) | 4.55 (15) * |
| Slope (%·mL/ng) | 0.08 (44) | 0.08 (24) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Son, H.; Noh, K.; Kim, E.; Shin, B.S.; Kang, W. Effects of Verapamil and Diltiazem on the Pharmacokinetics and Pharmacodynamics of Rivaroxaban. Pharmaceutics 2019, 11, 133. https://doi.org/10.3390/pharmaceutics11030133
Kim M, Son H, Noh K, Kim E, Shin BS, Kang W. Effects of Verapamil and Diltiazem on the Pharmacokinetics and Pharmacodynamics of Rivaroxaban. Pharmaceutics. 2019; 11(3):133. https://doi.org/10.3390/pharmaceutics11030133
Chicago/Turabian StyleKim, Minsoo, Heebin Son, Keumhan Noh, Eunyoung Kim, Beom Soo Shin, and Wonku Kang. 2019. "Effects of Verapamil and Diltiazem on the Pharmacokinetics and Pharmacodynamics of Rivaroxaban" Pharmaceutics 11, no. 3: 133. https://doi.org/10.3390/pharmaceutics11030133