Mathematical Modelling of Intravenous Thrombolysis in Acute Ischaemic stroke: Effects of Dose Regimens on Levels of Fibrinolytic Proteins and Clot Lysis Time
Abstract
:1. Introduction
2. Methods
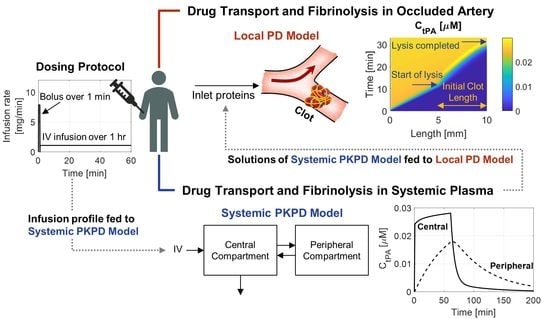
2.1. Overview of Modelling Strategy
2.2. Mathematical Models
2.2.1. Systemic Pharmacokinetics and Pharmacodynamics (PKPD) Model
2.2.2. Coupled Flow, Transport and Clot Lysis Model (“Local Pharmacodynamics Model”)
2.3. Model Integration and Numerical Procedure
2.4. Simulation Details
2.5. Remarks on Kinetics Parameters and Model Validation
3. Results and Discussion
3.1. Effects of tPA Dose on Systemic Concentrations of Thrombolytic Proteins
3.2. Effects of Delays between Bolus and Continuous IV Infusion on Systemic Concentrations
3.3. Effects of Bolus to Continuous Infusion Ratio on Systemic Concentrations
3.4. Systemic Concentrations of Thrombolytic Proteins for New Dosage Regimens
3.5. Effects of Different Dosage Regimens on Recanalisation Time
3.6. Temporal and Spatial Variations in Protein Concentration
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Peters, N.A.; Paciullo, C.A. Alteplase for the treatment of pulmonary embolism a review. Adv. Emerg. Nurs. J. 2015, 37, 258–272. [Google Scholar] [CrossRef] [PubMed]
- The National Institute of Neurological Disorders; Stroke rt-PA Stroke Study Group. Tissue Plasminogen Activator for Acute Ischemic Stroke. N. Engl. J. Med. 1995, 333, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Tiefenbrunn, A.J.; Graor, R.A.; Robison, A.K.; Lucas, F.V.; Hotchkiss, A.; Sobel, B.E. Pharmacodynamics of tissue-type plasminogen activator characterized by computer-assisted simulation. Circulation 1986, 73, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Tanswell, P.; Seifried, E.; Su, P.C.A.F.; Feuerer, W.; Rijken, D.C. Pharmacokinetics and systemic effects of tissue-type plasminogen activator in normal subjects. Clin. Pharmacol. Ther. 1989, 46, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, U.; Tanswell, P.; Seifried, E.; Feuerer, W.; Scholz, K.H.; Herrmann, K.S. Single-bolus injection of recombinant tissue-type plasminogen activator in acute myocardial infarction. Am. J. Cardiol. 1989, 64, 448–453. [Google Scholar] [CrossRef]
- Collen, D.; Topol, E.J.; Tiefenbrunn, A.J.; Gold, H.; Weisfeldt, M.; Sobel, B.; Leinbach, R.; Brinker, J.; Ludbrook, P.; Yasuda, I. Coronary thrombolysis with recombinant human tissue-type plasminogen activator: A prospective, randomized, placebo-controlled trial. Circulation 1984, 70, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, M.; Bounameaux, H.; de Cock, F.; Van de Werf, F.; Collen, D. Pharmacokinetics and systemic fibrinogenolytic effects of recombinant human tissue-type plasminogen activator (rt-PA) in humans. J. Pharmacol. Exp. Ther. 1985, 235, 506–512. [Google Scholar] [PubMed]
- Collen, D.; Bounameaux, H.; De Cock, F.; Lijnen, H.R.; Verstraete, M. Analysis of coagulation and fibrinolysis during intravenous infusion of recombinant human tissue-type plasminogen activator in patients with acute myocardial infarction. Circulation 1986, 73, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Chandler, W.L.; Alessi, M.C.; Aillaud, M.F.; Vague, P.; Juhan-Vague, I. Formation, inhibition and clearance of plasmin in vivo. Haemostasis 2001, 30, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Noe, D.A.; Bell, W.R. A kinetic analysis of fibrinogenolysis during plasminogen activator therapy. Clin. Pharmacol. Ther. 1987, 41, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Lindley, R.I.; Wardlaw, J.M.; Whiteley, W.N.; Cohen, G.; Blackwell, L.; Murray, G.D.; Sandercock, P.A.G. Alteplase for acute ischemic stroke: Outcomes by clinically important subgroups in the Third International Stroke Trial. Stroke 2015, 46, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Parsons, M.; Spratt, N.; Bivard, A.; Campbell, B.; Chung, K.; Miteff, F.; O’Brien, B.; Bladin, C.; McElduff, P.; Allen, C.; et al. A Randomized Trial of Tenecteplase versus Alteplase for Acute Ischemic Stroke. N. Engl. J. Med. 2012, 366, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Mori, E.; Minematsu, K.; Nakagawara, J.; Hashi, K.; Saito, I.; Shinohara, Y. Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 h of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke 2006, 37, 1810–1815. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Dowling, R.J.; Yan, B.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N. Engl. J. Med. 2018, 378, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Smadja, D.; Chausson, N.; Joux, J.; Saint-Vil, M.; Signat, A.; Edimonana, M.; Jeannin, S.; Bartoli, B.; Aveillan, M.; Cabre, P.; et al. A new therapeutic strategy for acute ischemic stroke: Sequential combined intravenous tpa-tenecteplase for proximal middle cerebral artery occlusion based on first results in 13 consecutive patients. Stroke 2011, 42, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Logallo, N.; Kvistad, C.E.; Nacu, A.; Naess, H.; Waje-Andreassen, U.; Asmuss, J.; Aamodt, A.H.; Lund, C.; Kurz, M.W.; Rønning, O.M.; et al. The Norwegian tenecteplase stroke trial (NOR-TEST): Randomised controlled trial of tenecteplase vs. alteplase in acute ischaemic stroke. BMC Neurol. 2014, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, P.; Ford, G.A. Pharmacokinetics of alteplase in the treatment of ischaemic stroke. Expert Opin. Drug Metab. Toxicol. 2012, 8, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Tanaka, E.; Murai, Y.; Tancharoen, S. Clinical trials in acute ischemic stroke. CNS Drugs 2014, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Diamond, S.L. Computer simulation of systemic circulation and clot lysis dynamics during thrombolytic therapy that accounts for inner clot transport and reaction. Circulation 1996, 94, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Piebalgs, A.; Gu, B.; Roi, D.; Lobotesis, K.; Thom, S.; Xu, X.Y. Computational Simulations of Thrombolytic Therapy in Acute Ischaemic Stroke. Sci. Rep. 2018, 8, 15810. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Rohan, V.; Baxa, J.; Tupy, R.; Cerna, L.; Sevcik, P.; Friesl, M.; Polivka, J.; Polivka, J.; Ferda, J. Length of occlusion predicts recanalization and outcome after intravenous thrombolysis in middle cerebral artery stroke. Stroke 2014, 45, 2010–2017. [Google Scholar] [CrossRef] [PubMed]
- Kamalian, S.; Morais, L.T.; Pomerantz, S.R.; Aceves, M.; Sit, S.P.; Bose, A.; Hirsch, J.A.; Lev, M.H.; Yoo, A.J. Clot length distribution and predictors in anterior circulation stroke: Implications for intra-arterial therapy. Stroke 2013, 44, 3553–3556. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.L. Engineering Design of Optimal Strategies for Blood Clot Dissolution. Annu. Rev. Biomed. Eng. 1999, 1, 427–461. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.L.; Anand, S. Inner clot diffusion and permeation during fibrinolysis. Biophys. J. 1993, 65, 2622–2643. [Google Scholar] [CrossRef] [Green Version]
- Friedrich, B.; Gawlitza, M.; Schob, S.; Hobohm, C.; Raviolo, M.; Hoffmann, K.T.; Lobsien, D. Distance to thrombus in acute middle cerebral artery occlusion: A predictor of outcome after intravenous thrombolysis for acute ischemic stroke. Stroke 2015, 46, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, D.E. PAI-1 and atherothrombosis. J. Thromb. Haemost. 2005, 3, 1879–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibo, H.; Carver, C.C.; Rhonton, A.L., Jr.; Carla, L.; Mitchell, R.J. Microsurgical anatomy of the middle cerebral artery. J. Neurosurg. 1981, 54, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Skeik, N.; Gits, C.C.; Ehrenwald, E.; Cragg, A.H. Fibrinogen level as a surrogate for the outcome of thrombolytic therapy using tissue plasminogen activator for acute lower extremity intravascular thrombosis. Vasc. Endovasc. Surg. 2013, 47, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Sobel, B.E.; Gross, R.W.; Robinson, A.K. Thrombolysis, clot selectivity, and kinetics. Circulation 1984, 70, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Al-Nuaimi, Y.; Wainwright, J.; Sherrington, C.; Singh, A.; Kallingal, J.; Douglass, C.; Parry-Jones, A.; Smith, C.; Dixit, A.; et al. The influence of bolus to infusion delays on plasma tissue plasminogen activator levels. Int. J. Stroke 2014, 9, 939–942. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, A.V.; Burgin, W.S.; Demchuk, A.M.; El-mitwalli, A.; Grotta, C. Speed of Intracranial Clot Lysis With Intravenous Tissue. Circulation 2001, 103, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.S.; Robinson, T.; Lindley, R.I.; Arima, H.; Lavados, P.M.; Lee, T.-H.; Broderick, J.P.; Chen, X.; Chen, G.; Sharma, V.K.; et al. Low-Dose versus Standard-Dose Intravenous Alteplase in Acute Ischemic Stroke. N. Engl. J. Med. 2016, 374, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Han, M.K.; Park, T.H.; Park, S.S.; Lee, K.B.; Lee, B.C.; Yu, K.H.; Oh, M.S.; Cha, J.K.; Kim, D.H.; et al. Low-Versus Standard-Dose Alteplase for Ischemic Strokes Within 4.5 Hours: A Comparative Effectiveness and Safety Study. Stroke 2015, 46, 2541–2548. [Google Scholar] [CrossRef] [PubMed]









| No. | Description | |
|---|---|---|
| Plasma | 1 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| Clot | 1 | |
| 2 | ||
| 3 | ||
| 4 | ||
| 5 | ||
| 6 |
| No. | Total Dose | Regimen Description |
|---|---|---|
| 1 | 0.9 mg/kg | 10% as bolus + 90% as continuous over 1 h (reference case) |
| 2 | 0.6 mg/kg | 10% as bolus + 90% as continuous over 1 h |
| 3 | 1.2 mg/kg | 10% as bolus + 90% as continuous over 1 h |
| 4 | 0.9 mg/kg | 10% as bolus + 5-min delay + 90% as continuous over 1 h |
| 5 | 0.9 mg/kg | 10% as bolus + 10-min delay + 90% as continuous over 1 h |
| 6 | 0.9 mg/kg | 10% as bolus + 30-min delay + 90% as continuous over 1 h |
| 7 | 0.9 mg/kg | the total dose as a continuous infusion over 1 h |
| 8 | 0.9 mg/kg | 20% as bolus + 80% as continuous over 1h |
| 9 | 0.9 mg/kg | 50% as bolus + 50% as continuous over 1h |
| 10 | 0.9 mg/kg | 15% as bolus + 50% as continuous over 30 min + 35% as continuous over 1 h |
| 11 | 0.9 mg/kg | 15% as bolus + 35% as continuous over 30 min + 50% as continuous over 1 h |
| 12 | 0.9 mg/kg | 10% as bolus + 60% as continuous over 30 min + 30% as continuous over 1 h |
| 13 | 0.9 mg/kg | 10% as bolus + 30% as continuous over 30 min + 60% as continuous over 1 h |
| Symbol | Definition | Values [unit] | Source |
|---|---|---|---|
| CtPA,0 | Initial tPA concentration | 0.05 [nM] | [24] |
| CPLG,0 | Initial PLG concentration | 2.2 [μM] | [24] |
| CPLS,0 | Initial PLS concentration | 0 [μM] | [24] |
| CFBG,0 | Initial FBG concentration | 8 [μM] | [24] |
| CAP,0 | Initial AP concentration | 1 [μM] | [24] |
| CMG,0 | Initial MG concentration | 3 [μM] | [24] |
| CPAI,0 | Initial PAI concentration | 5.23 × 10−4 [μM] | [27] |
| εclot,0 | Initial porosity of the clot | 0.95 [-] | [24] |
| Rf | Radius of fibrin fibre in the clot | 100 [nm] | [24] |
| μ | Blood viscosity | 0.0037 [Pa∙s] | [20] |
| ntot,0 | Initial total concentration of binding sites in the clot | 1.74 [μM] | [20] |
| ∆Px | Pressure drop per unit length across the clot | 60 [mmHg/cm] | [24] |
| dclot | Distance from the bifurcation to the clot front | 5 [mm] | [26] |
| Lclot | Length of clot | 5 [mm] | [22] |
| Da | Diameter of occluded artery | 3 [mm] | [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, B.; Piebalgs, A.; Huang, Y.; Longstaff, C.; Hughes, A.D.; Chen, R.; Thom, S.A.; Xu, X.Y. Mathematical Modelling of Intravenous Thrombolysis in Acute Ischaemic stroke: Effects of Dose Regimens on Levels of Fibrinolytic Proteins and Clot Lysis Time. Pharmaceutics 2019, 11, 111. https://doi.org/10.3390/pharmaceutics11030111
Gu B, Piebalgs A, Huang Y, Longstaff C, Hughes AD, Chen R, Thom SA, Xu XY. Mathematical Modelling of Intravenous Thrombolysis in Acute Ischaemic stroke: Effects of Dose Regimens on Levels of Fibrinolytic Proteins and Clot Lysis Time. Pharmaceutics. 2019; 11(3):111. https://doi.org/10.3390/pharmaceutics11030111
Chicago/Turabian StyleGu, Boram, Andris Piebalgs, Yu Huang, Colin Longstaff, Alun D. Hughes, Rongjun Chen, Simon A. Thom, and Xiao Yun Xu. 2019. "Mathematical Modelling of Intravenous Thrombolysis in Acute Ischaemic stroke: Effects of Dose Regimens on Levels of Fibrinolytic Proteins and Clot Lysis Time" Pharmaceutics 11, no. 3: 111. https://doi.org/10.3390/pharmaceutics11030111