Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch
Abstract
:1. Introduction
2. Materials and Methods
2.1. Analysis of MWCNTs
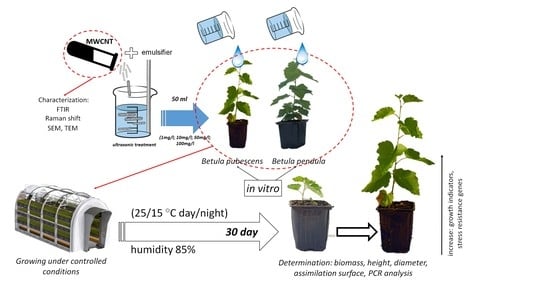
2.2. Obtaining and Studying Colloidal Solutions of MWCNTs
2.3. Objects of Study and Methodology for Conducting the Experiment
2.4. Conducting Gene Expression Analysis for the Effects of MWCNTs
2.5. Statistical Analysis
3. Results
3.1. Characterization of MWCNTs
3.2. Results of Determining the Stability of Colloidal Solutions
3.3. Results of Determining the Morphometric Parameters of Silver Birch (B. pendula Roth.) and Downy Birch (B. pubescens Ehrh.)
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mishra, S.; Keswani, C.; Abhilash, P.C.; Fraceto, L.F.; Singh, H.B. Integrated Approach of Agri-nanotechnology: Challenges and Future Trends. Front. Plant Sci. 2017, 8, 471. [Google Scholar] [CrossRef] [Green Version]
- Abideen, Z.; Hanif, M.; Munir, N.; Nielsen, B.L. Impact of Nanomaterials on the Regulation of Gene Expression and Metabolomics of Plants under Salt Stress. Plants 2022, 11, 691. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Z. Recent advances in nano-enabled agriculture for improving plant performance. Crop J. 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Khan, F.S.A.; Mubarak, N.M.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Ahmad, A.; Karri, R.R.; Pakalapati, H. Functionalized multi-walled carbon nanotubes and hydroxyapatite nanorods reinforced with polypropylene for biomedical application. Sci. Rep. 2021, 11, 843. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar] [CrossRef] [Green Version]
- Díez-Pascual, A.M. Chemical Functionalization of Carbon Nanotubes with Polymers: A Brief Overview. Macromol 2021, 1, 64–83. [Google Scholar] [CrossRef]
- Subagio, A.; Prihastanti, E.; Ngadiwiyana, N. Application of Functionalized Multi-Walled Carbon Nanotubes for Growth Enhancement of Mustard Seed Germination. Indones. J. Chem. 2019, 20, 120. [Google Scholar] [CrossRef]
- Sharifi, P.; Bidabadi, S.S.; Zaid, A.; Abdel Latef, A.A.H. Efficacy of multi-walled carbon nanotubes in regulating growth performance, total glutathione and redox state of Calendula officinalis L. cultivated on Pb and Cd polluted soil. Ecotoxicol. Environ. Saf. 2021, 213, 112051. [Google Scholar] [CrossRef]
- Rahmani, N.; Radjabian, T.; Soltani, B.M. Impacts of foliar exposure to multi-walled carbon nanotubes on physiological and molecular traits of Salvia verticillata L., as a medicinal plant. Plant Physiol. Biochem. 2020, 150, 27–38. [Google Scholar] [CrossRef]
- Zaytseva, O. Analysis of Phytotoxicity and Plant Growth Stimulation by Multi-Walled Carbon Nanotubes. Ph.D. Thesis, University of Hohenheim, Stuttgart, Germany, 2017. [Google Scholar]
- Joshi, A.; Sharma, L.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Plant Nanobionic Effect of Multi-walled Carbon Nanotubes on Growth, Anatomy, Yield and Grain Composition of Rice. BioNanoScience 2020, 10, 430–445. [Google Scholar] [CrossRef]
- Ghasempour, M.; Iranbakhsh, A.; Ebadi, M.; Oraghi Ardebili, Z. Multi-walled carbon nanotubes improved growth, anatomy, physiology, secondary metabolism, and callus performance in Catharanthus roseus: An in vitro study. 3 Biotech 2019, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Yan, S.; Zhao, L.; Li, H.; Zhang, Q.; Tan, J.; Huang, M.; He, S.; Li, L. Single-walled carbon nanotubes selectively influence maize root tissue development accompanied by the change in the related gene expression. J. Hazard. Mater. 2013, 246–247, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yue, M.; Zheng, X.; Xie, C.; Zhou, H.; Li, L. Physiological Effects of Single- and Multi-Walled Carbon Nanotubes on Rice Seedlings. IEEE Trans. Nanobiosci. 2017, 16, 563–570. [Google Scholar] [CrossRef]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef]
- Pourkhaloee, A.; Haghighi, M.; Saharkhiz, M.J.; Jouzi, H.; Doroodmand, M.M. Carbon Nanotubes Can Promote Seed Germination via Seed Coat Penetration. Seed Technol. 2011, 33, 155–169. [Google Scholar]
- Khodakovskaya, M.V.; Kim, B.-S.; Kim, J.N.; Alimohammadi, M.; Dervishi, E.; Mustafa, T.; Cernigla, C.E. Carbon Nanotubes as Plant Growth Regulators: Effects on Tomato Growth, Reproductive System, and Soil Microbial Community. Small 2013, 9, 115–123. [Google Scholar] [CrossRef]
- Flores, D.; Schmidt-Duran, A.; Alvarado, L. Effect of Using Two Different Types of Carbon Nanotubes for Blackberry (Rubus adenotrichos) in Vitro Plant Rooting, Growth and Histology. Am. J. Plant Sci. 2014, 5, 3510–3518. [Google Scholar] [CrossRef] [Green Version]
- Khodakovskaya, M.V.; de Silva, K.; Biris, A.S.; Dervishi, E.; Villagarcia, H. Carbon Nanotubes Induce Growth Enhancement of Tobacco Cells. ACS Nano 2012, 6, 2128–2135. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Nano-Enabled Agriculture: How Do Nanoparticles Cross Barriers in Plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef]
- Hatami, M.; Hadian, J.; Ghorbanpour, M. Mechanisms underlying toxicity and stimulatory role of single-walled carbon nanotubes in Hyoscyamus niger during drought stress simulated by polyethylene glycol. J. Hazard. Mater. 2017, 324, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Shahrajabian, M.H.; Huang, Q. Soybean seeds treated with single walled carbon nanotubes (SwCNTs) showed enhanced drought tolerance during germination. Int. J. Adv. Biol. Biomed. Res. 2020, 8, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.C.; Zapata, L.; Chalbi, N.; Carvajal, M. Multiwalled carbon nanotubes enter broccoli cells enhancing growth and water uptake of plants exposed to salinity. J. Nanobiotechnol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Ballesta, M.C.; Chelbi, N.; Lopez-Zaplana, A.; Carvajal, M. Discerning the mechanism of the multiwalled carbon nanotubes effect on root cell water and nutrient transport. Plant Physiol. Biochem. 2020, 146, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Sonkar, S.K.; Sarkar, S. Growth stimulation of gram (Cicer arietinum) plant by water soluble carbon nanotubes. Nanoscale 2011, 3, 1176–1181. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Dervishi, E.; Chen, J.; Nima, Z.; Gaume, A.; Biris, A.S.; Khodakovskaya, M.V. Impact of Carbon Nanotube Exposure to Seeds of Valuable Crops. ACS Appl. Mater. Interfaces 2013, 5, 7965–7973. [Google Scholar] [CrossRef]
- Yousefi, S.; Kartoolinejad, D.; Naghdi, R. Effects of priming with multi-walled carbon nanotubes on seed physiological characteristics of Hopbush (Dodonaeaviscosa L.) under drought stress. Int. J. Environ. Stud. 2017, 74, 528–539. [Google Scholar] [CrossRef]
- Khodakovskaya, M.; Dervishi, E.; Mahmood, M.; Xu, Y.; Li, Z.; Watanabe, F.; Biris, A.S. Carbon Nanotubes Are Able to Penetrate Plant Seed Coat and Dramatically Affect Seed Germination and Plant Growth. ACS Nano 2009, 3, 3221–3227. [Google Scholar] [CrossRef]
- Mathew, S.; Tiwari, D.K.; Tripathi, D. Interaction of carbon nanotubes with plant system: A review. Carbon Lett. 2021, 31, 167–176. [Google Scholar] [CrossRef]
- Larue, C.; Pinault, M.; Czarny, B.; Georgin, D.; Jaillard, D.; Bendiab, N.; Mayne-L’Hermite, M.; Taran, F.; Dive, V.; Carrière, M. Quantitative evaluation of multi-walled carbon nanotube uptake in wheat and rapeseed. J. Hazard. Mater. 2012, 227–228, 155–163. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Chen, J.; Tian, Y.; Shi, J.; Li, D.; Guo, C.; Ma, Q. Carboxylated multi-walled carbon nanotubes aggravated biochemical and subcellular damages in leaves of broad bean (Vicia faba L.) seedlings under combined stress of lead and cadmium. J. Hazard. Mater. 2014, 274, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhao, F.; Judy, J.D.; He, Z. Copper Stress Alleviation in Corn (Zea mays L.): Comparative Efficiency of Carbon Nanotubes and Carbon Nanoparticles. NanoImpact 2022, 25, 100381. [Google Scholar] [CrossRef] [PubMed]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-Dependent Phytotoxicity of Nanoparticles to Plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xing, B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007, 150, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Chakraborty, A.; Bandyopadhyay, M.; Mukherjee, A. Multi-walled carbon nanotubes (MWCNT): Induction of DNA damage in plant and mammalian cells. J. Hazard. Mater. 2011, 197, 327–336. [Google Scholar] [CrossRef]
- Zhai, G.; Gutowski, S.M.; Walters, K.S.; Yan, B.; Schnoor, J.L. Charge, Size, and Cellular Selectivity for Multiwall Carbon Nanotubes by Maize and Soybean. Environ. Sci. Technol. 2015, 49, 7380–7390. [Google Scholar] [CrossRef]
- Khodakovskaya, M.V.; de Silva, K.; Nedosekin, D.A.; Dervishi, E.; Biris, A.S.; Shashkov, E.V.; Galanzha, E.I.; Zharov, V.P. Complex genetic, photothermal, and photoacoustic analysis of nanoparticle-plant interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 1028–1033. [Google Scholar] [CrossRef] [Green Version]
- Landa, P.; Vankova, R.; Andrlova, J.; Hodek, J.; Marsik, P.; Storchova, H.; White, J.C.; Vanek, T. Nanoparticle-specific changes in Arabidopsis thaliana gene expression after exposure to ZnO, TiO2, and fullerene soot. J. Hazard. Mater. 2012, 241–242, 55–62. [Google Scholar] [CrossRef]
- Jordan, J.T.; Singh, K.P.; Cañas-Carrell, J.E. Carbon-based nanomaterials elicit changes in physiology, gene expression, and epigenetics in exposed plants: A review. Curr. Opin. Environ. Sci. Health 2018, 6, 29–35. [Google Scholar] [CrossRef]
- Hao, Y.; Ma, C.; Zhang, Z.; Song, Y.; Cao, W.; Guo, J.; Zhou, G.; Rui, Y.; Liu, L.; Xing, B. Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem. Environ. Pollut. 2018, 232, 123–136. [Google Scholar] [CrossRef]
- Green, M.J. Analysis and measurement of carbon nanotube dispersions: Nanodispersion versus macrodispersion. Polym. Int. 2010, 59, 1319–1322. [Google Scholar] [CrossRef]
- Irin, F.; Shrestha, B.; Cañas, J.E.; Saed, M.A.; Green, M.J. Detection of carbon nanotubes in biological samples through microwave-induced heating. Carbon 2012, 50, 4441–4449. [Google Scholar] [CrossRef]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liné, C.; Larue, C.; Flahaut, E. Carbon nanotubes: Impacts and behaviour in the terrestrial ecosystem—A review. Carbon 2017, 123, 767–785. [Google Scholar] [CrossRef] [Green Version]
- Husen, A.; Siddiqi, K.S. Carbon and fullerene nanomaterials in plant system. J. Nanobiotechnol. 2014, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Grodetskaya, T.; Fedorova, O.; Evlakov, P. Optimized method for RNA extraction from leaves of forest tree species. IOP Conf. Ser. Earth Environ. Sci. 2021, 875, 012008. [Google Scholar] [CrossRef]
- Grodetskaia, T.A.; Fedorova, O.A.; Evlakov, P.M.; Baranov, O.Y.; Zakharova, O.V.; Gusev, A.A. Effect of Copper Oxide and Silver Nanoparticles on the Development of Tolerance to Alternaria alternata in Poplar in Vitro Clones. Nanobiotechnol. Rep. 2021, 16, 231–238. [Google Scholar] [CrossRef]
- Ritonga, F.; Ngatia, J.; Song, R.; Farooq, U.; Somadona, S.; Lestari, A.T.; Chen, S. Abiotic stresses induced physiological, biochemical, and molecular changes in Betula platyphylla: A review. Silva Fenn. 2021, 55, 10516. [Google Scholar] [CrossRef]
- Wen, X.; Wang, J.; Zhang, D.; Wang, Y. A Gene Regulatory Network Controlled by BpERF2 and BpMYB102 in Birch under Drought Conditions. Int. J. Mol. Sci. 2019, 20, 3071. [Google Scholar] [CrossRef] [Green Version]
- Zan, T.; Li, L.; Li, J.; Zhang, L.; Li, X. Genome-wide identification and characterization of late embryogenesis abundant protein-encoding gene family in wheat: Evolution and expression profiles during development and stress. Gene 2020, 736, 144422. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Fe2O3 nanoparticles induced biochemical responses and expression of genes involved in rosmarinic acid biosynthesis pathway in Moldavian balm under salinity stress. Physiol. Plant. 2020, 169, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Luo, Y.; Zhang, Z.; Li, J.; Li, C.; Li, C.; Guo, Z.; Wang, L.; Zhang, W.; Zhao, H.; et al. The Development of Real-Time Digital PCR Technology Using an Improved Data Classification Method. Biosens. Bioelectron. 2022, 199, 113873. [Google Scholar] [CrossRef] [PubMed]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Goh, B.; Kim, K.J.; Park, C.-L.; Kim, E.S.; Kim, S.H.; Choi, J. In-Plane Thermal Conductivity of Multi-Walled Carbon Nanotube Yarns under Mechanical Loading. Carbon 2021, 184, 452–462. [Google Scholar] [CrossRef]
- Dobrzańska-Danikiewicz, A.; Wolany, W.; Lukowiec, D.; Jurkiewicz, K.; Niedziałkowski, P. Characteristics of multiwalled carbon nanotubes-rhenium nanocomposites with varied rhenium mass fractions. Nanomater. Nanotechnol. 2017, 7, 184798041770717. [Google Scholar] [CrossRef] [Green Version]
- Shuaib, M.; Mohammed, N.; Burhanudin, Z. Optimization of the Production of Aligned CNTs Array as the Gas Sensing Element. Mater. Sci. Forum 2013, 756, 156–163. [Google Scholar] [CrossRef]
- Kumar, A.; Ghosh, P.K.; Yadav, K.L.; Kumar, K. Thermo-mechanical and anti-corrosive properties of MWCNT/epoxy nanocomposite fabricated by innovative dispersion technique. Compos. Part B Eng. 2017, 113, 291–299. [Google Scholar] [CrossRef]
- Chen, J.; Han, J. Effect of Hydroxylated Carbon Nanotubes on the Thermal and Electrical Properties of Derived Epoxy Composite Materials. Results Phys. 2020, 18, 103246. [Google Scholar] [CrossRef]
- Nitesh; Kumar, A.; Saini, S.; Yadav, K.L.; Ghosh, P.K.; Rathi, A. Morphology and tensile performance of MWCNT/TiO2-epoxy nanocomposite. Mater. Chem. Phys. 2022, 277, 125336. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Dharamvir, K.; Nayyar, H.; Verma, G. Multi-walled carbon nanotubes applied through seed-priming influence early germination, root hair, growth and yield of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 3148–3160. [Google Scholar] [CrossRef]
- Seddighinia, F.S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Nejad Satari, T.; Soleimanpour, S. Seed Priming with Cold Plasma and Multi-walled Carbon Nanotubes Modified Growth, Tissue Differentiation, Anatomy, and Yield in Bitter Melon (Momordica charantia). J. Plant Growth Regul. 2020, 39, 87–98. [Google Scholar] [CrossRef]
- Joshi, A.; Kaur, S.; Singh, P.; Dharamvir, K.; Nayyar, H.; Verma, G. Tracking multi-walled carbon nanotubes inside oat (Avena sativa L.) plants and assessing their effect on growth, yield, and mammalian (human) cell viability. Appl. Nanosci. 2018, 8, 1399–1414. [Google Scholar] [CrossRef]
- Agrawal, S.; Kumar, V.; Kumar, S.; Shahi, S.K. Plant development and crop protection using phytonanotechnology: A new window for sustainable agriculture. Chemosphere 2022, 299, 134465. [Google Scholar] [CrossRef] [PubMed]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2018, 66, 6654–6662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, H.; Wang, H.; Zhang, R.; Jia, H.; Huang, J.; Zhao, J.; Yao, J. Effects of graphene on morphology, microstructure and transcriptomic profiling of Pinus tabuliformis Carr. roots. PLoS ONE 2021, 16, e0253812. [Google Scholar] [CrossRef]
- Szőllősi, R.; Molnár, Á.; Kondak, S.; Kolbert, Z. Dual Effect of Nanomaterials on Germination and Seedling Growth: Stimulation vs. Phytotoxicity. Plants 2020, 9, 1745. [Google Scholar] [CrossRef]
- Samadi, S.; Saharkhiz, M.J.; Azizi, M.; Samiei, L.; Ghorbanpour, M. Multi-walled carbon nanotubes stimulate growth, redox reactions and biosynthesis of antioxidant metabolites in Thymus daenensis Celak. In vitro. Chemosphere 2020, 249, 126069. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef]
- Samadi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rodríguez-Couto, S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ. Technol. Innov. 2021, 21, 101323. [Google Scholar] [CrossRef]
- Giorgetti, L. Chapter 4—Effects of Nanoparticles in Plants: Phytotoxicity and Genotoxicity Assessment. In Nanomaterials in Plants, Algae and Microorganisms; Tripathi, D.K., Ahmad, P., Sharma, S., Chauhan, D.K., Dubey, N.K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 65–87. [Google Scholar]
- Pacheco, I.; Buzea, C. Nanoparticle Uptake by Plants: Beneficial or Detrimental? In Phytotoxicity of Nanoparticles; Faisal, M., Saquib, Q., Alatar, A.A., Al-Khedhairy, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–61. [Google Scholar]
- Raeisi Sadati, S.Y.; Jahanbakhsh Godehkahriz, S.; Ebadi, A.; Sedghi, M. Zinc Oxide Nanoparticles Enhance Drought Tolerance in Wheat via Physio-Biochemical Changes and Stress Genes Expression. Iran. J. Biotechnol. 2022, 20, 12–24. [Google Scholar] [CrossRef]
- Linh, T.; Mai, N.; Hoe, P.; Lien, L.; Ban, N.; Hien, L.; Hoai Chau, N.; Van, N. Metal-Based Nanoparticles Enhance Drought Tolerance in Soybean. J. Nanomater. 2020, 2020, 4056563. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Karimian, Z.; Mostafaei Dehnavi, M.; Samiei, L. Chitosan nanoparticles improve physiological and biochemical responses of Salvia abrotanoides (Kar.) under drought stress. BMC Plant Biol. 2022, 22, 364. [Google Scholar] [CrossRef]
- Saha, G.; Mostofa, M.G.; Rahman, M.M.; Tran, L.-S.P. Silicon-mediated heat tolerance in higher plants: A mechanistic outlook. Plant Physiol. Biochem. 2021, 166, 341–347. [Google Scholar] [CrossRef]
- Ali, F.; Bano, A.; Fazal, A. Recent methods of drought stress tolerance in plants. Plant Growth Regul. 2017, 82, 363–375. [Google Scholar] [CrossRef]
- Moradi, P.; Vafaee, Y.; Mozafari, A.A.; Tahir, N.A.-r. Silicon Nanoparticles and Methyl Jasmonate Improve Physiological Response and Increase Expression of Stress-related Genes in Strawberry cv. Paros Under Salinity Stress. Silicon 2022, 14, 10559–10569. [Google Scholar] [CrossRef]
- Mertens, J.; Aliyu, H.; Cowan, D.A. LEA Proteins and the Evolution of the WHy Domain. Appl. Environ. Microbiol. 2018, 84, e00539-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yumurtaci, A. Diverse Aspects of ABA Signaling under Drought Stress in Wheat Glob. J. Bot. Sci. 2020, 8, 30–39. [Google Scholar] [CrossRef]
- Erpen, L.; Devi, H.S.; Grosser, J.W.; Dutt, M. Potential use of the DREB/ERF, MYB, NAC and WRKY transcription factors to improve abiotic and biotic stress in transgenic plants. Plant Cell Tissue Organ Cult. 2018, 132, 1–25. [Google Scholar] [CrossRef]
- Abedi, S.; Iranbakhsh, A.; Oraghi Ardebili, Z.; Ebadi, M. Nitric oxide and selenium nanoparticles confer changes in growth, metabolism, antioxidant machinery, gene expression, and flowering in chicory (Cichorium intybus L.): Potential benefits and risk assessment. Environ. Sci. Pollut. Res. 2021, 28, 3136–3148. [Google Scholar] [CrossRef]
- Wu, Z.; Liang, J.; Zhang, S.; Zhang, B.; Zhao, Q.; Li, G.; Yang, X.; Wang, C.; He, J.; Yi, M. A Canonical DREB2-Type Transcription Factor in Lily Is Post-translationally Regulated and Mediates Heat Stress Response. Front. Plant Sci. 2018, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Sheikh-Mohamadi, M.-H.; Etemadi, N.; Arab, M.M.; Aalifar, M.; Arab, M. Physiological and Ascorbate-Glutathione pathway-related genes responses under drought and heat stress in crested wheatgrass. Sci. Hortic. 2018, 242, 195–206. [Google Scholar] [CrossRef]
- Akbudak, M.A.; Filiz, E.; Kontbay, K. DREB2 (dehydration-responsive element-binding protein 2) type transcription factor in sorghum (Sorghum bicolor): Genome-wide identification, characterization and expression profiles under cadmium and salt stresses. 3 Biotech 2018, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Arabbeigi, M.; Arzani, A.; Majidi, M.M. Expression Profiles of P5CS and DREB2 Genes under Salt Stress in Aegilops cylindrica. Russ. J. Plant Physiol. 2019, 66, 583–590. [Google Scholar] [CrossRef]
- Vargas-Hernandez, M.; Macias-Bobadilla, I.; Guevara-Gonzalez, R.G.; Rico-Garcia, E.; Ocampo-Velazquez, R.V.; Avila-Juarez, L.; Torres-Pacheco, I. Nanoparticles as Potential Antivirals in Agriculture. Agriculture 2020, 10, 444. [Google Scholar] [CrossRef]
- Mitra, M.; Agarwal, P.; Roy, S. Plant Response to Heavy Metal Stress: An Insight into the Molecular Mechanism of Transcriptional Regulation. In Plant Transcription Factors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 337–367. [Google Scholar]
- Chalker-Scott, L.; Fuchigami, L.H. The role of phenolic compounds in plant stress responses. In Low Temperature Stress Physiology in Crops; CRC Press: Boca Raton, FL, USA, 1989; pp. 68–76. [Google Scholar]
- Rajaee Behbahani, S.; Iranbakhsh, A. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE 2020, 15, e0235556. [Google Scholar] [CrossRef] [PubMed]
- Asgari, F.; Majd, A.; Jonoubi, P.; Najafi, F. Effects of silicon nanoparticles on molecular, chemical, structural and ultrastructural characteristics of oat (Avena sativa L.). Plant Physiol. Biochem. 2018, 127, 152–160. [Google Scholar] [CrossRef]










| № | Gene | Sequence (5′→3′) |
|---|---|---|
| 1 | Pal | F: CTGTGGCTGCAACGGTTT |
| R: TCAATTTGAGGTCCGAGCCA | ||
| 2 | PR-10 | F: GGCCCGGAACCATTAAGAAG |
| R: CCACCCTCGATCAAGCTGTA | ||
| 3 | PR-1 | F: CCTCAAAGCCCACAATGACG |
| R: TCTCGTCCACCCATAGCTTC | ||
| 4 | lea8 | F: AATGACTTTGACATGGGCGT |
| R: TATCCCAAACTGCAGAGCCA | ||
| 5 | GAPDH | F: CAGCCGAAGATGTCAATGCA |
| R: GGCCACTTGTTTGCTACCAA | ||
| 6 | DREB2 | F: AGGCAGAGAACATGGGGAAA |
| R: GAAAGTTGAGGCGAGCGTAA |
| Element | C | Al | Cl | Co | S |
|---|---|---|---|---|---|
| Content, % | 97.34 | 0.25 | 0.98 | 1.16 | 0.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuzhukin, K.V.; Evlakov, P.M.; Grodetskaya, T.A.; Gusev, A.A.; Zakharova, O.V.; Shuklinov, A.V.; Tomina, E.V. Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch. Forests 2023, 14, 163. https://doi.org/10.3390/f14010163
Zhuzhukin KV, Evlakov PM, Grodetskaya TA, Gusev AA, Zakharova OV, Shuklinov AV, Tomina EV. Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch. Forests. 2023; 14(1):163. https://doi.org/10.3390/f14010163
Chicago/Turabian StyleZhuzhukin, Konstantin V., Peter M. Evlakov, Tatiana A. Grodetskaya, Alexander A. Gusev, Olga V. Zakharova, Aleksey V. Shuklinov, and Elena V. Tomina. 2023. "Effect of Multi-Walled Carbon Nanotubes on the Growth and Expression of Stress Resistance Genes in Birch" Forests 14, no. 1: 163. https://doi.org/10.3390/f14010163