Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
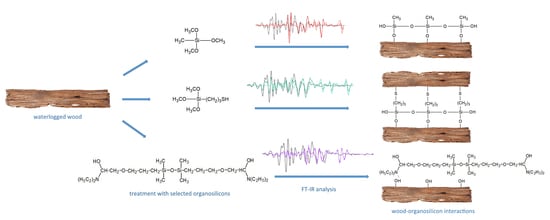
2.1. Materials
2.2. Methods
2.2.1. Scanning Electron Microscopy
2.2.2. Fourier Transform Infrared Spectroscopy
3. Results and Discussion
3.1. Structural Evaluation of Archaeological Elm Wood
3.2. Interactions between Organosilicons and Archaeological Elm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Y.; Hou, P.; Duan, C.; Zhang, R.; Zhou, Z.; Cheng, X.; Shah, S. The use of tetraethyl orthosilicate silane (TEOS) for surface-treatment of hardened cement-based materials: A comparison study with normal treatment agents. Constr. Build. Mater. 2016, 117, 144–151. [Google Scholar] [CrossRef]
- Kregiel, D. Advances in biofilm control for food and beverage industry using organo-silane technology: A review. Food Control 2014, 40, 32–40. [Google Scholar] [CrossRef]
- Mojsiewicz-Pieńkowska, K.; Jamrógiewicz, M.; Szymkowska, K.; Krenczkowska, D. Direct Human Contact with Siloxanes (Silicones)–Safety or Risk Part 1. Characteristics of Siloxanes (Silicones). Front. Pharmacol. 2016, 7, 132. [Google Scholar] [CrossRef]
- Onar, N.; Mete, G.; Aksit, A.; Kutlu, B.; Celik, E. Water-and oil-repellency properties of cotton fabric treated with Silane, Zr, Ti based nanosols. Int. J. Text. Sci. 2015, 4, 84–96. [Google Scholar]
- Szymanowski, H.; Olesko, K.; Kowalski, J.; Fijalkowski, M.; Gazicki-Lipman, M.; Sobczyk-Guzenda, A. Thin SiNC/SiOC Coatings with a Gradient of Refractive Index Deposited from Organosilicon Precursor. Coatings 2020, 10, 794. [Google Scholar] [CrossRef]
- Wang, M.; Hao, X.; Wang, W. Reinforcing Behaviors of Sulfur-Containing Silane Coupling Agent in Natural Rubber-Based Magnetorheological Elastomers with Various Vulcanization Systems. Materials 2020, 13, 5163. [Google Scholar] [CrossRef]
- Donath, S.; Militz, H.; Mai, C. Wood modification with alkoxysilanes. Wood Sci. Technol. 2004, 38, 555–566. [Google Scholar] [CrossRef]
- Mai, C.; Militz, H. Modification of wood with silicon compounds. Inorganic silicon compounds and sol-gel systems: A review. Wood Sci. Technol. 2004, 37, 339–348. [Google Scholar] [CrossRef]
- Levy, D.; Zayat, M. (Eds.) The Sol-Gel Handbook: Synthesis, Characterization and Applications; 3-Volume Set; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Panov, D.; Terziev, N. Study on some alkoxysilanes used for hydrophobation and protection of wood against decay. Int. Biodeter. Biodegr. 2009, 63, 456–461. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane coupling agents used for natural fiber/polymer composites: A review. Compos. Part A Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Farahani, M.M.; Hale, M.D. The use of organo alkoxysilane coupling agents for wood preservation. Holzforschung 2004, 58, 316–325. [Google Scholar] [CrossRef]
- De Vetter, L.; Van den Bulcke, J.; Van Acker, J. Impact of organosilicon treatments on the wood-water relationship of solid wood. Holzforschung 2010, 64, 463–468. [Google Scholar] [CrossRef]
- Kartal, S.N.; Yoshimura, T.; Imamura, Y. Modification of wood with Si compounds to limit boron leaching from treated wood and to increase termite and decay resistance. Int. Biodeter. Biodegr. 2009, 63, 187–190. [Google Scholar] [CrossRef]
- Giudice, C.A.; Alfieri, P.V.; Canosa, G. Decay resistance and dimensional stability of Araucaria angustifolia using siloxanes synthesized by sol–gel process. Int. Biodeter. Biodegr. 2013, 83, 166–170. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L.; Wang, W.; Sun, Y.; Wang, H. Modifying wood veneer with silane coupling agent for decorating wood fiber/high-density polyethylene composite. Constr. Build. Mater. 2019, 224, 691–699. [Google Scholar] [CrossRef]
- Dodangeh, F.; Dorraji, M.S.; Rasoulifard, M.H.; Ashjari, H.R. Synthesis and characterization of alkoxy silane modified polyurethane wood adhesive based on epoxidized soybean oil polyester polyol. Compos. B Eng. 2020, 187, 107857. [Google Scholar] [CrossRef]
- Canosa, G.; Alfieri, P.V.; Giudice, C.A. Low Density Wood Impregnation with Water-Repellent Organosilicic Compounds. MSCE 2018, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.W.; Hamilton, D.L. Treatment of Waterlogged Wood Using Hydrolyzable, Multi-Functional Alkoxysilane Polymers. In Proceedings of the 8th ICOM Group on Wet Organic Archaeological Materials Conference, Stockholm, Sweden, 11–15 June 2001; pp. 614–615. [Google Scholar]
- Broda, M.; Mazela, B. Application of methyltrimethoxysilane to increase dimensional stability of waterlogged wood. J. Cult. Herit. 2017, 25, 149–156. [Google Scholar] [CrossRef]
- Broda, M.; Dąbek, I.; Dutkiewicz, A.; Dutkiewicz, M.; Popescu, C.-M.; Mazela, B.; Maciejewski, H. Organosilicons of different molecular size and chemical structure as consolidants for waterlogged archaeological wood–a new reversible and retreatable method. Sci. Rep. 2020, 10, 2188. [Google Scholar] [CrossRef] [Green Version]
- Broda, M.; Mazela, B.; Dutkiewicz, A. Organosilicon compounds with various active groups as consolidants for the preservation of waterlogged archaeological wood. J. Cult. Herit. 2019, 35, 123–128. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Popescu, C.-M.; Lisa, G.; Sakata, Y. Evaluation of morphological and chemical aspects of different wood species by spectroscopy and thermal methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Popescu, C.-M.; Popescu, M.-C.; Vasile, C. Characterization of fungal degraded lime wood by FT-IR and 2D IR correlation spectroscopy. Microchem. J. 2010, 95, 377–387. [Google Scholar] [CrossRef]
- Gelbrich, J.; Mai, C.; Militz, H. Evaluation of bacterial wood degradation by Fourier Transform Infrared (FTIR) measurements. J. Cult. Herit. 2012, 13, S135–S138. [Google Scholar] [CrossRef]
- Tolvaj, L.; Popescu, C.-M.; Molnar, Z.; Preklet, E. Dependence of the Air Relative Humidity and Temperature on the Photodegradation Processes of Beech and Spruce Wood Species. BioResourses 2016, 11, 296–305. [Google Scholar]
- Łucejko, J.J.; Modugno, F.; Ribechini, E.; Tamburini, D.; Colombini, M.P. Analytical instrumental techniques to study archaeological wood degradation. Appl. Spectrosc. Rev. 2015, 50, 584–625. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef]
- Cesar, T.; Danevčič, T.; Kavkler, K.; Stopar, D. Melamine polymerization in organic solutions and waterlogged archaeological wood studied by FTIR spectroscopy. J. Cult. Herit. 2017, 23, 106–110. [Google Scholar] [CrossRef]
- McHale, E.; Steindal, C.C.; Kutzke, H.; Benneche, T.; Harding, S.E. In situ polymerisation of isoeugenol as a green consolidation method for waterlogged archaeological wood. Sci. Rep. 2017, 7, 46481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliç, N.; Kiliç, A.G. An attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopic study of waterlogged woods treated with melamine formaldehyde. Vib. Spectrosc. 2019, 105, 102985. [Google Scholar] [CrossRef]
- Popescu, M.-C.; Froidevaux, J.; Navi, P.; Popescu, C.-M. Structural modifications of Tilia cordata wood during heat treatment investigated by FT-IR and 2D IR correlation spectroscopy. J. Mol. Struct. 2013, 1033, 176–186. [Google Scholar] [CrossRef]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by FTIR spectroscopy of silica aerogels prepared using several Si (OR) 4 and R′′ Si (OR′) 3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Benmouhoub, C.; Gauthier-Manuel, B.; Zegadi, A.; Robert, L. A Quantitative Fourier Transform Infrared Study of the Grafting of Aminosilane Layers on Lithium Niobate Surface. Appl. Spectrosc. 2017, 71, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Pasteur, G.A.; Schonhorn, H. Interaction of Silanes with Antimony Oxide to Facilitate Particulate Dispersion in Organic Media and to Enhance Flame Retardance. Appl. Spectrosc. 1975, 29, 512–517. [Google Scholar] [CrossRef]
- Kavale, M.S.; Mahadik, D.B.; Parale, V.G.; Wagh, P.B.; Gupta, S.C.; Rao, A.V.; Barshilia, H.C. Optically transparent, superhydrophobic methyltrimethoxysilane based silica coatings without silylating reagent. Appl. Surf. Sci. 2011, 258, 158–162. [Google Scholar] [CrossRef]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Rao, A.V. Porous superhydrophobic silica films by sol–gel process. Microporous Mesoporous Mater. 2010, 130, 115–121. [Google Scholar] [CrossRef]
- Lin, J.; Chen, H.; Fei, T.; Zhang, J. Highly transparent superhydrophobic organic–inorganic nanocoating from the aggregation of silica nanoparticles. Colloids Surf. 2013, 421, 51–62. [Google Scholar] [CrossRef]
- Robles, E.; Csóka, L.; Labidi, J. Effect of reaction conditions on the surface modification of cellulose nanofibrils with aminopropyl triethoxysilane. Coatings 2018, 8, 139. [Google Scholar] [CrossRef] [Green Version]
- Pacaphol, K.; Aht-Ong, D. The influences of silanes on interfacial adhesion and surface properties of nanocellulose film coating on glass and aluminum substrates. Surf. Coat. Technol. 2017, 320, 70–81. [Google Scholar] [CrossRef]
- Broda, M.; Majka, J.; Olek, W.; Mazela, B. Dimensional stability and hygroscopic properties of waterlogged archaeological wood treated with alkoxysilanes. Int. Biodeter. Biodegr. 2018, 133, 34–41. [Google Scholar] [CrossRef]
- Broda, M.; Curling, S.F.; Spear, M.J.; Hill, C.A.S. Effect of methyltrimethoxysilane impregnation on the cell wall porosity and water vapour sorption of archaeological waterlogged oak. Wood Sci. Technol. 2019, 53, 703–726. [Google Scholar] [CrossRef] [Green Version]









| Bands Assignment | Bands Position, cm−1 | |||
|---|---|---|---|---|
| A | A1 | A2 | A3 | |
| symmetric stretching vibration of C–H bonds in CH3 groups in wood and silanes/siloxanes | 2966 | 2974 | 2961 | 2968 |
| asymmetric stretching vibration of C–H bonds in CH3 groups in wood and silanes/siloxanes | 2935 | 2933 | 2929 | 2932 |
| symmetric stretching vibration of C–H bonds in CH2 groups in wood and silanes/siloxanes | 2877 | 2883 | 2885 | 2882 |
| asymmetric stretching vibration of C–H bonds in CH2 groups in wood and silanes/siloxanes | 2842 | 2841 | 2853 | 2843 |
| C=O stretching vibration of carbonyl, carboxyl and acetyl groups | 1738 | 1738 | 1736 | 1738 |
| conjugated C–O in quinines coupled with C=O stretching of various groups; N-H bending vibration | 1663 | 1661 | 1659 | 1660 |
| C=C stretching of aromatic skeletal (lignin) | 1595 | 1598 | 1598 | 1594 |
| conjugated C–O; C-N stretching vibration in secondary amines | 1552 | 1552 | 1551 | 1554 |
| C=C stretching of aromatic skeletal (lignin) | 1509 | 1510 | 1509 | 1511 |
| C–H deformation in lignin and carbohydrates C–H deformation in -CH2-CH3 | 1462 | 1462 | 1460 | 1462 |
| C–H deformation in lignin and carbohydrates; C–H deformation in -CH2-CH3 | 1417 | 1419 | 1418 | 1419 |
| C–H deformation in cellulose and hemicellulose; C–H asymmetric deformation in Si-R | 1371 | 1382 | ---- | 1380 |
| C–H vibration in cellulose and Cl–O vibration in syringyl derivatives—condensed structures in lignin; CH2 deformation vibration and C-N stretching vibration in primary amines | 1329 | 1333 | 1338 | 1333 |
| -CH2 groups twisting | ---- | ---- | 1307 | 1299 |
| C–O stretching in lignin; Si-C stretching vibration in Si-CH3 | 1268 | 1272 | 1260 | 1256 |
| C−O−C stretching mode of the pyranose ring; | 1222 | 1224 | 1229 | ---- |
| asymmetric stretching vibration of Si-O-C | 1187 | 1189 | ||
| C–O stretching; Si-O-C asymmetric stretching vibration | 1126 | 1131 | 1131 | 1123 |
| glucose ring stretching vibration; asymmetric stretching vibration of Si-O-Si | 1082 | 1075 | ---- | 1056 |
| C−O ester stretching vibrations in methoxyl and β–O–4 linkages in lignin; Si-O-C asymmetric stretching vibration | 1027 | 1027 | 1031 | ---- |
| Si-O in-plane stretching vibration of the silanol Si-OH groups | 955 | 954 | ---- | 974 946 |
| Si-O-C bending vibration | ---- | 906 | 913 | 904 |
| Si-C and Si-O-C symmetric stretching vibration | 840 | 848 | 865 | 842 |
| Si-O-Si bonds stretching vibration | ---- | 818 | 806 | |
| -Si-C rocking in -SiCH3 | ---- | 773 | 745 | 787 |
| Si-O-Si bonds symmetric stretching vibration C-S- stretching vibration | ---- | 715 | 700 645 | 700 |
| Si-O stretching vibration | ---- | 582 | 597 | ---- |
| O-Si-O deformation vibration | ---- | 497 444 | 470 | 467 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, C.-M.; Broda, M. Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy. Forests 2021, 12, 268. https://doi.org/10.3390/f12030268
Popescu C-M, Broda M. Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy. Forests. 2021; 12(3):268. https://doi.org/10.3390/f12030268
Chicago/Turabian StylePopescu, Carmen-Mihaela, and Magdalena Broda. 2021. "Interactions between Different Organosilicons and Archaeological Waterlogged Wood Evaluated by Infrared Spectroscopy" Forests 12, no. 3: 268. https://doi.org/10.3390/f12030268