Interdisciplinary Therapy Improves the Mediators of Inflammation and Cardiovascular Risk in Adolescents with Obesity
Abstract
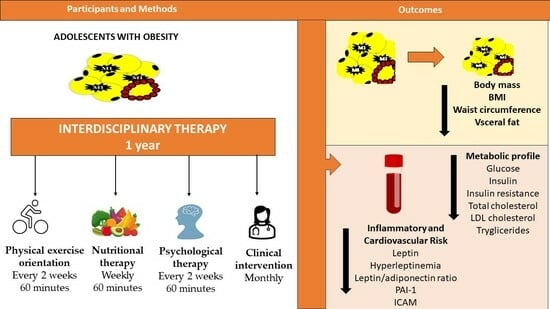
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometric Measurements and Body Composition
2.3. Visceral and Subcutaneous Adiposity
2.4. Blood Pressure Evaluation
2.5. Biochemical Analysis
2.6. Interdisciplinary Therapy
2.6.1. Nutritional Therapy
2.6.2. Psychological Therapy
2.6.3. Physical Exercise Counselling
2.6.4. Clinical Intervention
2.7. Statistical Analysis
3. Results
3.1. Effects of the Interdisciplinary Therapy
3.2. Correlation and Association between Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCD Risk Factor Collaboration. 2016. Available online: http://ncdrisc.org/obesity-prevalence-ranking.html (accessed on 23 March 2023).
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde. Atlas da Obesidade Infantile; Ministério da Saúde: Brasília, Brazil, 2019.
- Pesquisa Nacional de Saúde: 2019: Atenção Primária à Saúde e Informações Antropométricas; Brasil/IBGE, Coordenação de Trabalho e Rendimento: Rio de Janeiro, Brazil, 2020.
- Nogueira-de-Almeida, C.A.; Del Ciampo, L.A.; Ferraz, I.S.; Del Ciampo, I.R.L. COVID-19 and obesity in childhood and adolescence: A clinical review. J. Pediatr. 2020, 96, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Coleman, S.; Nixon, J.; Sharples, L.; Hamilton-Shield, J.; Rutter, H.; Bryant, M. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes. Rev. 2019, 20, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef] [PubMed]
- de Assis, G.G.; Murawska-Ciałowicz, E. Leptin-A Potential Bridge between Fat Metabolism and the Brain’s Vulnerability to Neuropsychiatric Disorders: A Systematic Review. J. Clin. Med. 2021, 10, 5714. [Google Scholar] [CrossRef] [PubMed]
- Khoramipour, K.; Chamari, K.; Hekmatikar, A.A.; Ziyaiyan, A.; Taherkhani, S.; Elguindy, N.M.; Bragazzi, N.L. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, Z.A.; Norris, S.A.; Roberts, M.; Singhal, A. The global challenge of childhood obesity and its consequences: What can be done? Lancet Glob. Health 2023, 11, e1172–e1173. [Google Scholar] [CrossRef]
- WHO Global Strategy on Diet, Physical Activity and Health. Childhood Overweight and Obesity. Available online: http://www.who.int/dietphysicalactivity/childhood/en/ (accessed on 22 March 2023).
- WHO. WHO Report of the Commission on Ending Childhood Obesity; WHO: Geneva, Switzerland, 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/204176/9789241510066_eng.pdf (accessed on 22 March 2022).
- Cominato, L.; Franco, R.; Damiani, D. Adolescent obesity treatments: News, views, and evidence. Arch. Endocrinol. Metab. 2021, 65, 527–536. [Google Scholar] [CrossRef]
- Ho, M.; Garnett, S.P.; Baur, L.; Burrows, T.; Stewart, L.; Neve, M.; Collins, C. Effectiveness of lifestyle interventions in child obesity: Systematic review with meta-analysis. Pediatrics 2012, 130, e1647–e1671. [Google Scholar] [CrossRef]
- Wickham, E.P.; Stern, M.; Evans, R.K.; Bryan, D.L.; Moskowitz, W.B.; Clore, J.N.; Laver, J.H. Prevalence of the metabolic syndrome among obese adolescents enrolled in a multidisciplinary weight management program: Clinical correlates and response to treatment. Metab. Syndr. Relat. Disord. 2009, 7, 179–186. [Google Scholar] [CrossRef]
- Ryder, J.R.; Vega-López, S.; Gaesser, G.A.; Buman, M.P.; Shaibi, G.Q. Heterogeneous vascular responses to lifestyle intervention in obese Latino adolescents. Metab. Syndr. Relat. Disord. 2014, 12, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Dâmaso, A.R.; de Piano, A.; Campos, R.M.; Corgosinho, F.C.; Siegfried, W.; Caranti, D.A.; Masquio, D.C.; Carnier, J.; Sanches Pde, L.; Leão da Silva, P.; et al. Multidisciplinary approach to the treatment of obese adolescents: Effects on cardiovascular risk factors, inflammatory profile, and neuroendocrine regulation of energy balance. Int. J. Endocrinol. 2013, 2013, 541032. [Google Scholar] [CrossRef] [PubMed]
- Tanner, J.M.; Whitehouse, R.H. Clinical longitudinal standards for height, weight velocity and stages of puberty. Arch. Dis. Child 1976, 51, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Prevalence of Overweight among Children and Adolescents: United States 1999–2002; National Center for Health Statistics: Hyattsville, MD, USA, 2002; (Updates on 11 January 2007). Available online: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overwght99.htm (accessed on 15 August 2011).
- Lohman, T.G.; Roche, A.F.; Martorrel, R. Anthropometric Standardization Reference Manual; Human Kinetic Books: Champaign, IL, USA, 1991. [Google Scholar]
- Fields, D.A.; Higgins, P.B.; Radley, D. Air-displacement plethysmography: Here to stay. Curr. Opin. Clin. Nutr. Metab. Care 2005, 8, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Filho, F.F.; Faria, A.N.; Azjen, S.; Zanella, M.T.; Ferreira, S.R. Methods of estimation of visceral fat: Advantages of ultrasonography. Obes. Res. 2003, 11, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B.; Ramsey, L.; Barbeau, P.; Cannady, W.; Ferguson, M.; Litaker, M.; Owens, S. Plasma leptin concentrations in obese children: Changes during 4-mo periods with and without physical training. Am. J. Clin. Nutr. 1999, 69, 388–394. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Keskin, M.; Kurtoglu, S.; Kendirci, M.; Atabek, M.E.; Yazici, C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics 2005, 115, e500–e503. [Google Scholar] [CrossRef]
- Schwimmer, J.B.; Deutsch, R.; Rauch, J.B.; Behling, C.; Newbury, R.; Lavine, J.E. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J. Pediatr. 2003, 143, 500–505. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescentsd—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academic Press: Washington, DC, USA, 2000. [Google Scholar]
- ACSM. ACMS position stand on the appropriate intervention strategies for weight loss and prevention of weight regain for adults. Med. Sci. Sports Exerc. 2001, 33, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Horesh, A.; Tsur, A.M.; Bardugo, A.; Twig, G. Adolescent and Childhood Obesity and Excess Morbidity and Mortality in Young Adulthood—A Systematic Review. Curr. Obes. Rep. 2021, 10, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hintze, L.J.; Kravchychyn, A.C.P.; Ferreira, Y.A.M.; Campos, R.M.D.S.; Dantas, A.D.A.; Masquio, D.C.L.; Caranti, D.A.; Thivel, D.; Dâmaso, A.R. Semi-intensive and Intensive Interdisciplinary Treatments Have Similar Effects on Metabolic Syndrome and Selected Inflammatory Markers in Adolescents with Obesity. J. Obes. Metab. Syndr. 2021, 30, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585887. [Google Scholar] [CrossRef] [PubMed]
- Agostinis-Sobrinho, C.A.; Lacerda Mendes, E.; Moreira, C.; Abreu, S.; Lopes, L.; Oliveira-Santos, J.; Skurvydas, A.; Mota, J.; Santos, R. Association between Leptin, Adiponectin, and Leptin/Adiponectin Ratio with Clustered Metabolic Risk Factors in Portuguese Adolescents: The LabMed Physical Activity Study. Ann. Nutr. Metab. 2017, 70, 321–328. [Google Scholar] [CrossRef]
- López-Jaramillo, P.; Gómez-Arbeláez, D.; López-López, J. Relationship among the leptin-to-adiponectin ratio, systemic inflammation, and anisocytosis: A plausible pathophysiological mechanism of a novel cardiovascular risk marker. Kardiol. Pol. 2020, 78, 381–382. [Google Scholar] [CrossRef]
- Stakos, D.A.; Papaioannou, H.I.; Angelidou, I.; Mantadakis, E.; Paraskakis, E.; Tsigalou, C.; Chatzimichael, A. Plasma leptin and adiponectin concentrations correlate with cardiometabolic risk and systemic inflammation in healthy, non-obese children. J. Pediatr. Endocrinol. Metab. 2014, 27, 221–228. [Google Scholar] [CrossRef]
- Shah, N.; Khadilkar, A.; Oza, C.; Bhor, S.; Ladkat, D.; Gondhalekar, K.; More, C.; Khadilkar, V. Adiponectin-leptin ratio as a marker of cardio-metabolic risk in Indian children and youth with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2023, 36, 561–567. [Google Scholar] [CrossRef]
- Frühbeck, G.; Catalán, V.; Rodríguez, A.; Gómez-Ambrosi, J. Adiponectin-leptin ratio: A promising index to estimate adipose tissue dysfunction. Relation with obesity-associated cardiometabolic risk. Adipocyte 2018, 7, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Morell-Azanza, L.; Rendo-Urteaga, T.; García-Calzón, S.; Ojeda-Rodríguez, A.; Martín-Calvo, N.; Moreno-Aliaga, M.J.; Martínez, J.A.; Azcona-San Julián, M.C. Serum and gene expression levels of CT-1, IL-6, and TNF-α after a lifestyle intervention in obese children. Pediatr. Diabetes 2018, 19, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.; Declerck, P.J. A Narrative Review on Plasminogen Activator Inhibitor-1 and Its (Patho)Physiological Role: To Target or Not to Target? Int. J. Mol. Sci. 2021, 22, 2721. [Google Scholar] [CrossRef]
- Adly, A.A.; Elbarbary, N.S.; Ismail, E.A.; Hassan, S.R. Plasminogen activator inhibitor-1 (PAI-1) in children and adolescents with type 1 diabetes mellitus: Relation to diabetic micro-vascular complications and carotid intima media thickness. J. Diabetes Complicat. 2014, 28, 340–347. [Google Scholar] [CrossRef]
- Varona, J.F.; Ortiz-Regalón, R.; Sánchez-Vera, I.; López-Melgar, B.; García-Durango, C.; Castellano Vázquez, J.M.; Solís, J.; Fernández-Friera, L.; Vidal-Vanaclocha, F. Soluble ICAM 1 and VCAM 1 Blood Levels Alert on Subclinical Atherosclerosis in Non Smokers with Asymptomatic Metabolic Syndrome. Arch. Med. Res. 2019, 50, 20–28. [Google Scholar] [CrossRef]
- Moschonis, G.; Karatzi, K.; Polychronopoulou, M.C.; Manios, Y. Waist circumference, trunk and visceral fat cutoff values for detecting hyperinsulinemia and insulin resistance in children: The Healthy Growth Study. Eur. J. Nutr. 2016, 55, 2331–2334. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Toledo-Corral, C.; Lane, C.J.; Weigensberg, M.J.; Spruijt-Metz, D.; Davies, J.N.; Goran, M.I. Insulin sensitivity as an independent predictor of fat mass gain in Hispanic adolescents. Diabetes Care 2009, 32, 2114–2115. [Google Scholar] [CrossRef]
- Fragoso, A.; Mendes, F.; Silva, A.P.; Neves, P.L. Insulin resistance as a predictor of cardiovascular morbidity and end-stage renal disease. J. Diabetes Complicat. 2015, 29, 1098–1104. [Google Scholar] [CrossRef]
- Caprio, S.; Perry, R.; Kursawe, R. Adolescent Obesity and Insulin Resistance: Roles of Ectopic Fat Accumulation and Adipose Inflammation. Gastroenterology 2017, 152, 1638–1646. [Google Scholar] [CrossRef]
- Savoye, M.; Caprio, S.; Dziura, J.; Camp, A.; Germain, G.; Summers, C.; Li, F.; Shaw, M.; Nowicka, P.; Kursawe, R.; et al. Reversal of early abnormalities in glucose metabolism in obese youth: Results of an intensive lifestyle randomized controlled trial. Diabetes Care 2014, 37, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; Lass, N.; Toschke, C.; Rothermel, J.; Lanzinger, S.; Holl, R.W. Which Amount of BMI-SDS Reduction Is Necessary to Improve Cardiovascular Risk Factors in Overweight Children? J. Clin. Endocrinol. Metab. 2016, 101, 3171–3179. [Google Scholar] [CrossRef] [PubMed]
- Qorbani, M.; Kelishadi, R.; Farrokhi-Khajeh-Pasha, Y.; Motlagh, M.; Aminaee, T.; Ardalan, G.; Asayesh, H.; Shafiee, G.; Taslimi, M.; Poursafa, P.; et al. Association of anthropometric measures with cardiovascular risk factors and metabolic syndrome in normal-weight children and adolescents: The CASPIAN III study. Obes. Facts. 2013, 6, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Bacha, F.; Edmundowicz, D.; Sutton-Tyrell, K.; Lee, S.; Tfayli, H.; Arslanian, S.A. Coronary artery calcification in obese youth: What are the phenotypic and metabolic determinants? Diabetes Care 2014, 37, 2632–2639. [Google Scholar] [CrossRef]
- Kelishadi, R.; Hashemipour, M.; Sarrafzadegan, N.; Mohammadifard, N.; Alikhasy, H.; Beizaei, M.; Sajjadi, F.; Poursafa, P.; Amin, Z.; Ghatreh-Samani, S.; et al. Effects of a lifestyle modification trial among phenotypically obese metabolically normal and phenotypically obese metabolically abnormal adolescents in comparison with phenotypically normal metabolically obese adolescents. Matern Child Nutr. 2010, 6, 275–286. [Google Scholar] [CrossRef]
- Pacifico, L.; Arca, M.; Anania, C.; Cantisani, V.; Di Martino, M.; Chiesa, C. Arterial function and structure after a 1-year lifestyle intervention in children with nonalcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1010–1016. [Google Scholar] [CrossRef]



| Baseline | After Therapy | p | Δ | |
|---|---|---|---|---|
| Age (years) | 15.7 ± 1.2 | 16.6 ± 1.2 | ||
| Body Mass (kg) | 92.6 ± 15.9 | 88.9 ± 16.1 | <0.001 | −3.7 ± 3.9 |
| Height (m) | 1.6 ± 0.1 | 1.6 ± 0.1 | 0.01 | −0.0 ± 0.0 |
| BMI (kg/m2) | 34.5 ± 4.8 | 33.4 ± 4.9 | 0.01 | −1.1 ± 1.4 |
| Body Fat (%) | 43.1 ± 5.1 | 42.7 ± 6.5 | 0.62 | −0.4 ± 3.4 |
| Fat-Free Mass (%) | 56.9 ± 5.2 | 57.3 ± 6.5 | 0.62 | 0.4 ± 3.4 |
| Body Fat (kg) | 40.3 ± 10.3 | 38.4 ± 11.4 | 0.06 | −1.8 ± 4.4 |
| Fat-Free Mass (kg) | 52.3 ± 7.7 | 50.3 ± 7.7 | <0.001 | −1.9 ±2.5 |
| Waist Circumference (cm) | 94.2 ± 11.6 | 92.2 ± 12.3 | 0.03 | −2.0 ± 3.9 |
| Visceral Fat (cm) | 4.4 ± 1.1 | 3.9 ± 1.3 | 0.01 | −0.5 ± 0.8 |
| Subcutaneous Fat (cm) | 3.8 ± 0.8 | 3.8 ± 0.9 | 0.89 | 0.0 ± 0.5 |
| Glucose (mg/dL) | 95.7 ± 5.1 | 91.4 ± 6.6 | <0.001 | −4.3 ± 5.1 |
| Insulin (uU/mL) | 15.5 ± 7.2 | 10.9 ± 5.8 | <0.001 | −4.6 ± 5.6 |
| HOMA-IR | 3.6 ± 1.7 | 2.5 ± 1.4 | <0.001 | −1.2 ± 1.3 |
| QUICKI | 0,32 ± 0,02 | 0,34 ± 0,03 | <0.001 | 0.02 ± 0.02 |
| Total Cholesterol (mg/dL) | 167.8 ± 26.7 | 143.8 ± 26.7 | <0.001 | −24.0 ± 14.6 |
| HDL-Cholesterol (mg/dL) | 46.7 ± 10.2 | 46.6 ± 11.0 | 0.95 | −0.09 ± 6.3 |
| LDL-Cholesterol (mg/dL) | 101.7 ± 20.3 | 82.8 ± 18.8 | <0.001 | −18.9 ± 12.4 |
| VLDL-Cholesterol (mg/dL) | 27.1 ± 36.9 | 14.4 ± 6.4 | 0.12 | −12.7 ± 36.7 |
| Triglycerides (mg/dL) | 96.6 ± 48.2 | 72.3 ± 32.1 | <0.001 | −23.4 ± 35.8 |
| Free Fatty Acids | 0.7 ± 0.2 | 0.8 ± 0.2 | 0.30 | 0.06 ± 0.4 |
| Systolic Blood Pressure (mmHg) | 120.9 ± 8.3 | 118.0 ± 7.9 | 0.17 | −3.1 ± 9.3 |
| Diastolic Blood Pressure (mmHg) | 78.1 ± 5.1 | 75.9 ± 4.1 | 0.09 | −2.4 ± 5.6 |
| Baseline | After Therapy | p | Δ | |
|---|---|---|---|---|
| Adiponectin (ng/mL) | 25.8 (9.5–97.2) | 28.2 (9.5–143.3) | 0.49 | 4.3 (−38.9–108.6) |
| Leptin (ng/mL) | 30.8 ± 12.0 | 20.9 ± 14.3 | <0.001 | −9.9 ± 10.7 |
| Leptin/Adiponectin ratio | 1.4 ± 1.3 | 1.0 ± 1.6 | 0.02 | −0.4 ± 0.8 |
| Adiponectin/Leptin ratio | 0.9 (0.2–4.6) | 1.7 (0.2–17.6) | 0.05 | 0.4 (−2.7–16.9) |
| TNF-α (ng/mL) | 8.4 (2.3–116.0) | 9.1 (1.9–48.4) | 0.29 | −0.7 (−67.6–5.7) |
| IL-1RA (pg/mL) | 12.6 (0.3–327.0) | 22.4 (0.3–60.1) | 0.25 | −0.6 (−210.7–53.4) |
| IL-6 (pg/mL) | 1.2 (0.1–59.8) | 0.9 (0.1–59.6) | 0.66 | −0.1 (−29.2–16.3) |
| IL-10 (pg/mL) | 0.4 (0.1–20.3) | 0.9 (0.1–30.6) | 1.00 | −0.1 (−3.6–12.2) |
| IL-15 (pg/mL) | 0.1 (0.1–106.0) | 0.5 (0.1–107.0) | 0.30 | 106.0 (0.44–153.0) |
| CRP (ng/mL) | 0.5 (0.1–1.8) | 0.3 (0.1–6.3) | 0.96 | −0.1 (−1.5–5.2) |
| MCP 1 (pg/mL) | 214.2 ± 106.1 | 168.2 ± 81.4 | 0.06 | −23.6 ± 124.6 |
| PAI-1 (ng/mL) | 181.5 ± 61.8 | 111.3 ± 33.8 | <0.001 | −70.2 ± 54.3 |
| ICAM (ng/mL) | 146.8 ± 52.0 | 113.9 ± 45.6 | 0.01 | −32.8 ± 52.9 |
| HOMA-IR | ||||
| −95.00% | +95.00% | |||
| Beta (ß) | p | Cnf.Lmt | Cnf.Lmt | |
| Adiponectin | −0.57 | 0.01 | −0.08 | −0.02 |
| Leptin/adiponectin ratio | 0.46 | 0.03 | 0.06 | 1.10 |
| Visceral fat | 0.47 | 0.03 | 0.09 | 1.32 |
| Leptin/Adiponectin Ratio | ||||
| −95.00% | +95.00% | |||
| Beta (ß) | p | Cnf.Lmt | Cnf.Lmt | |
| Body fat | 0.43 | 0.04 | 0.00 | 0.11 |
| Visceral fat | 0.46 | 0.03 | 0.05 | 1.04 |
| Insulin | 0.49 | 0.02 | 0.02 | 0.16 |
| Number of Metabolic Alterations | ||||
| −95.00% | +95.00% | |||
| Beta (ß) | p | Cnf.Lmt | Cnf.Lmt | |
| Waist circumference | 0.42 | 0.04 | 0.00 | 0.06 |
| Visceral fat | 0.45 | 0.04 | 0.02 | 0.68 |
| QUICKI | 0.43 | 0.04 | −29.76 | −0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masquio, D.C.L.; Campos, R.M.d.S.; Netto, B.D.M.; Carvalho-Ferreira, J.P.d.; Bueno, C.R., Jr.; Alouan, S.; Poletto, G.T.; Ganen, A.d.P.; Tufik, S.; de Mello, M.T.; et al. Interdisciplinary Therapy Improves the Mediators of Inflammation and Cardiovascular Risk in Adolescents with Obesity. Int. J. Environ. Res. Public Health 2023, 20, 7114. https://doi.org/10.3390/ijerph20237114
Masquio DCL, Campos RMdS, Netto BDM, Carvalho-Ferreira JPd, Bueno CR Jr., Alouan S, Poletto GT, Ganen AdP, Tufik S, de Mello MT, et al. Interdisciplinary Therapy Improves the Mediators of Inflammation and Cardiovascular Risk in Adolescents with Obesity. International Journal of Environmental Research and Public Health. 2023; 20(23):7114. https://doi.org/10.3390/ijerph20237114
Chicago/Turabian StyleMasquio, Deborah Cristina Landi, Raquel Munhoz da Silveira Campos, Bárbara Dal Molin Netto, Joana Pereira de Carvalho-Ferreira, Carlos Roberto Bueno, Jr., Stella Alouan, Gabriela Tronca Poletto, Aline de Piano Ganen, Sergio Tufik, Marco Túlio de Mello, and et al. 2023. "Interdisciplinary Therapy Improves the Mediators of Inflammation and Cardiovascular Risk in Adolescents with Obesity" International Journal of Environmental Research and Public Health 20, no. 23: 7114. https://doi.org/10.3390/ijerph20237114