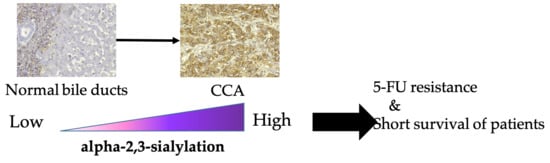
Increase of MAL-II Binding Alpha2,3-Sialylated Glycan Is Associated with 5-FU Resistance and Short Survival of Cholangiocarcinoma Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. CCA Tissues from Patients
2.2. Cholangiocyte and CCA Cell Lines
2.3. Lectin-Histochemistry Staining
2.4. Lectin-Cyto-Fluorescence Staining
2.5. Cell Proliferation and Chemosensitivity Assay
2.6. Statistical Analysis
3. Results
3.1. MAL-SG and SNA-SG Were Elevated in CCA Compared with Normal Bile Ducts and HP/DP
3.2. High Level of MAL-SG in CCA Was Associated with Shorter Survival of CCA Patients
3.3. Suppression of Sialylation by a Sialyltransferase Inhibitor Altered the Expression of MAL-SG
3.4. Suppression of Sialylation Enhances the 5-FU Susceptibility of CCA Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blechacz, B.; Gores, G.J. Cholangiocarcinoma: Advances in pathogenesis, diagnosis, and treatment. Hepatology 2008, 48, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Juntavee, A.; Sripa, B.; Pugkhem, A.; Khuntikeo, N.; Wongkham, S. Expression of sialyl Lewis(a) relates to poor prognosis in cholangiocarcinoma. World J. Gastroenterol. 2005, 11, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Indramanee, S.; Silsirivanit, A.; Pairojkul, C.; Wongkham, C.; Wongkham, S. Aberrant glycosylation in cholangiocarcinoma demonstrated by lectin-histochemistry. Asian Pac. J. Cancer Prev. 2012, 13, 119–124. [Google Scholar] [PubMed]
- Phoomak, C.; Silsirivanit, A.; Wongkham, C.; Sripa, B.; Puapairoj, A.; Wongkham, S. Overexpression of O-GlcNAc-transferase associates with aggressiveness of mass-forming cholangiocarcinoma. Asian Pac. J. Cancer Prev. 2012, 13, 101–105. [Google Scholar]
- Phoomak, C.; Silsirivanit, A.; Park, D.; Sawanyawisuth, K.; Vaeteewoottacharn, K.; Wongkham, C.; Lam, E.W.; Pairojkul, C.; Lebrilla, C.B.; Wongkham, S. O-GlcNAcylation mediates metastasis of cholangiocarcinoma through FOXO3 and MAN1A1. Oncogene 2018, 37, 5648–5665. [Google Scholar] [CrossRef]
- Wu, L.H.; Shao, X.T.; Guo, J.X.; Sun, H.; Chen, Q.; Pan, J.; Cai, Q.Q.; Dong, Y.W.; Chen, Z.Y.; Yan, X.M.; et al. Vimentin is important in the neural differentiation of PC12 cells promoted by sialylation. Glycoconj. J. 2017, 34, 51–59. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Tanabe, M.; Date, K.; Sakuda, K.; Sano, K.; Ogawa, H. Sialylation of vitronectin regulates stress fiber formation and cell spreading of dermal fibroblasts via a heparin-binding site. Glycoconj. J. 2016, 33, 227–236. [Google Scholar] [CrossRef]
- Sasaki, N.; Itakura, Y.; Toyoda, M. Sialylation regulates myofibroblast differentiation of human skin fibroblasts. Stem Cell Res. Ther. 2017, 8, 81. [Google Scholar] [CrossRef]
- Us, D. Cytokine storm in avian influenza. Mikrobiyoloji Bul. 2008, 42, 365–380. [Google Scholar]
- Qin, Y.; Zhong, Y.; Zhu, M.; Dang, L.; Yu, H.; Chen, Z.; Chen, W.; Wang, X.; Zhang, H.; Li, Z. Age- and sex-associated differences in the glycopatterns of human salivary glycoproteins and their roles against influenza A virus. J. Proteome Res. 2013, 12, 2742–2754. [Google Scholar] [CrossRef]
- Shi, Y.Q.; He, Q.; Zhao, Y.J.; Wang, E.H.; Wu, G.P. Lectin microarrays differentiate carcinoma cells from reactive mesothelial cells in pleural effusions. Cytotechnology 2013, 65, 355–362. [Google Scholar] [CrossRef] [PubMed]
- dos-Santos, P.B.; Zanetti, J.S.; Vieira-de-Mello, G.S.; Rego, M.B.; Ribeiro-Silva, A.A.; Beltrao, E.I. Lectin histochemistry reveals SNA as a prognostic carbohydrate-dependent probe for invasive ductal carcinoma of the breast: A clinicopathological and immunohistochemical auxiliary tool. Int. J. Clin. Exp. Pathol. 2014, 7, 2337–2349. [Google Scholar]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kaptan, E.; Sancar-Bas, S.; Sancakli, A.; Bektas, S.; Bolkent, S. The effect of plant lectins on the survival and malignant behaviors of thyroid cancer cells. J. Cell. Biochem. 2018, 119, 6274–6287. [Google Scholar] [CrossRef] [PubMed]
- Maignien, C.; Santulli, P.; Chouzenoux, S.; Gonzalez-Foruria, I.; Marcellin, L.; Doridot, L.; Jeljeli, M.; Grange, P.; Reis, F.M.; Chapron, C.; et al. Reduced alpha-2,6 sialylation regulates cell migration in endometriosis. Hum. Reprod. 2019, 34, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Shiozaki, K.; Yamaguchi, K.; Miyazaki, S.; Satomi, S.; Kato, K.; Sakuraba, H.; Miyagi, T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene 2009, 28, 1218–1229. [Google Scholar] [CrossRef]
- Kolasinska, E.; Przybylo, M.; Janik, M.; Litynska, A. Towards understanding the role of sialylation in melanoma progression. Acta Biochim. Pol. 2016, 63, 533–541. [Google Scholar] [CrossRef]
- Schultz, M.J.; Swindall, A.F.; Wright, J.W.; Sztul, E.S.; Landen, C.N.; Bellis, S.L. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 2013, 6, 25. [Google Scholar] [CrossRef]
- Britain, C.M.; Holdbrooks, A.T.; Anderson, J.C.; Willey, C.D.; Bellis, S.L. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 2018, 11, 12. [Google Scholar] [CrossRef]
- Santos, S.N.; Junqueira, M.S.; Francisco, G.; Vilanova, M.; Magalhaes, A.; Dias Baruffi, M.; Chammas, R.; Harris, A.L.; Reis, C.A.; Bernardes, E.S. O-glycan sialylation alters galectin-3 subcellular localization and decreases chemotherapy sensitivity in gastric cancer. Oncotarget 2016, 7, 83570–83587. [Google Scholar] [CrossRef]
- Park, J.J.; Yi, J.Y.; Jin, Y.B.; Lee, Y.J.; Lee, J.S.; Lee, Y.S.; Ko, Y.G.; Lee, M. Sialylation of epidermal growth factor receptor regulates receptor activity and chemosensitivity to gefitinib in colon cancer cells. Biochem. Pharmacol. 2012, 83, 849–857. [Google Scholar] [CrossRef]
- Tatsuzuki, A.; Ezaki, T.; Makino, Y.; Matsuda, Y.; Ohta, H. Characterization of the sugar chain expression of normal term human placental villi using lectin histochemistry combined with immunohistochemistry. Arch. Histol. Cytol. 2009, 72, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Akasov, R.; Haq, S.; Haxho, F.; Samuel, V.; Burov, S.V.; Markvicheva, E.; Neufeld, R.J.; Szewczuk, M.R. Sialylation transmogrifies human breast and pancreatic cancer cells into 3D multicellular tumor spheroids using cyclic RGD-peptide induced self-assembly. Oncotarget 2016, 7, 66119–66134. [Google Scholar] [CrossRef]
- Arrighi, S.; Ventriglia, G.; Aralla, M.; Zizza, S.; Di Summa, A.; Desantis, S. Absorptive activities of the efferent ducts evaluated by the immunolocalization of aquaporin water channels and lectin histochemistry in adult cats. Histol. Histopathol. 2010, 25, 433–444. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, H.; Yang, Y.; Wang, B. Expression of Neu5Acalpha2,3Gal and Neu5Acalpha2,6Gal on the nasal mucosa of patients with chronic rhinosinusitis and its possible effect on bacterial biofilm formation. Microb. Pathog. 2018, 123, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Kobayashi, N.; Westerman, K.A.; Sakaguchi, M.; Allain, J.E.; Totsugawa, T.; Okitsu, T.; Fukazawa, T.; Weber, A.; Stolz, D.B.; et al. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation 2004, 77, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Uthaisar, K.; Vaeteewoottacharn, K.; Seubwai, W.; Talabnin, C.; Sawanyawisuth, K.; Obchoei, S.; Kraiklang, R.; Okada, S.; Wongkham, S. Establishment and characterization of a novel human cholangiocarcinoma cell line with high metastatic activity. Oncol. Rep. 2016, 36, 1435–1446. [Google Scholar] [CrossRef]
- Saentaweesuk, W.; Araki, N.; Vaeteewoottacharn, K.; Silsirivanit, A.; Seubwai, W.; Talabnin, C.; Muisuk, K.; Sripa, B.; Wongkham, S.; Okada, S.; et al. Activation of Vimentin Is Critical to Promote a Metastatic Potential of Cholangiocarcinoma Cells. Oncol. Res. 2018, 26, 605–616. [Google Scholar] [CrossRef]
- Fitzgibbons, P.L.; Dillon, D.A.; Alsabeh, R.; Berman, M.A.; Hayes, D.F.; Hicks, D.G.; Hughes, K.S.; Nofech-Mozes, S. Template for reporting results of biomarker testing of specimens from patients with carcinoma of the breast. Arch. Pathol. Labor. Med. 2014, 138, 595–601. [Google Scholar] [CrossRef]
- Shah, M.; Telang, S.; Raval, G.; Shah, P.; Patel, P.S. Serum fucosylation changes in oral cancer and oral precancerous conditions: Alpha-L-fucosidase as a marker. Cancer 2008, 113, 336–346. [Google Scholar] [CrossRef]
- Sung, P.L.; Wen, K.C.; Horng, H.C.; Chang, C.M.; Chen, Y.J.; Lee, W.L.; Wang, P.H. The role of alpha2,3-linked sialylation on clear cell type epithelial ovarian cancer. Taiwan J. Obstet. Gynecol. 2018, 57, 255–263. [Google Scholar] [CrossRef]
- Pihikova, D.; Kasak, P.; Kubanikova, P.; Sokol, R.; Tkac, J. Aberrant sialylation of a prostate-specific antigen: Electrochemical label-free glycoprofiling in prostate cancer serum samples. Anal. Chim. Acta 2016, 934, 72–79. [Google Scholar] [CrossRef]
- Shen, L.; Luo, Z.; Wu, J.; Qiu, L.; Luo, M.; Ke, Q.; Dong, X. Enhanced expression of alpha2,3-linked sialic acids promotes gastric cancer cell metastasis and correlates with poor prognosis. Int. J. Oncol. 2017, 50, 1201–1210. [Google Scholar] [CrossRef]
- Wongkham, S.; Boonla, C.; Kongkham, S.; Wongkham, C.; Bhudhisawasdi, V.; Sripa, B. Serum total sialic acid in cholangiocarcinoma patients: An ROC curve analysis. Clin. Biochem. 2001, 34, 537–541. [Google Scholar] [CrossRef]
- Kongtawelert, P.; Tangkijvanich, P.; Ong-Chai, S.; Poovorawan, Y. Role of serum total sialic acid in differentiating cholangiocarcinoma from hepatocellular carcinoma. World J. Gastroenterol. 2003, 9, 2178–2181. [Google Scholar] [CrossRef]
- Miyagi, T.; Takahashi, K.; Moriya, S.; Hata, K.; Yamamoto, K.; Wada, T.; Yamaguchi, K.; Shiozaki, K. Altered expression of sialidases in human cancer. Adv. Exp. Med. Biol. 2012, 749, 257–267. [Google Scholar] [CrossRef]
- Wen, K.C.; Sung, P.L.; Hsieh, S.L.; Chou, Y.T.; Lee, O.K.; Wu, C.W.; Wang, P.H. alpha2,3-sialyltransferase type I regulates migration and peritoneal dissemination of ovarian cancer cells. Oncotarget 2017, 8, 29013–29027. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Wei, A.; Yu, X.; Niang, B.; Zhang, J. Alpha2,6-linked sialic acids on N-glycans modulate the adhesion of hepatocarcinoma cells to lymph nodes. Tumour Biol. 2015, 36, 885–892. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Ma, H.; Dong, W.; Zhou, H.; Song, X.; Zhang, J.; Jia, L. Withdrawal: Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol. Cell. Proteom. 2019, 18, 1269. [Google Scholar] [CrossRef] [Green Version]
- Wei, A.; Fan, B.; Zhao, Y.; Zhang, H.; Wang, L.; Yu, X.; Yuan, Q.; Yang, D.; Wang, S. ST6Gal-I overexpression facilitates prostate cancer progression via the PI3K/Akt/GSK-3beta/beta-catenin signaling pathway. Oncotarget 2016, 7, 65374–65388. [Google Scholar] [CrossRef] [Green Version]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef] [Green Version]
- Marino, D.; Leone, F.; Cavalloni, G.; Cagnazzo, C.; Aglietta, M. Biliary tract carcinomas: From chemotherapy to targeted therapy. Crit. Rev. Oncol./Hematol. 2013, 85, 136–148. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Ruan, Y.; Sun, L.; Xu, C.; Jiang, H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-beta1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018, 9, 1102. [Google Scholar] [CrossRef] [Green Version]
- Perego, P.; Gatti, L.; Beretta, G.L. The ABC of glycosylation. Nature reviews. Cancer 2010, 10, 523. [Google Scholar] [CrossRef]
- Fletcher, J.I.; Haber, M.; Henderson, M.J.; Norris, M.D. ABC transporters in cancer: More than just drug efflux pumps. Nature reviews. Cancer 2010, 10, 147–156. [Google Scholar] [CrossRef]
- da Fonseca, L.M.; da Silva, V.A.; Freire-de-Lima, L.; Previato, J.O.; Mendonca-Previato, L.; Capella, M.A. Glycosylation in Cancer: Interplay between Multidrug Resistance and Epithelial-to-Mesenchymal Transition? Front. Oncol. 2016, 6, 158. [Google Scholar] [CrossRef]



| Variables | n | MAL-SG | SNA-SG | ||||
|---|---|---|---|---|---|---|---|
| Low | High | p | Low | High | p | ||
| (<50) | (≥50) | (<20) | (≥20) | ||||
| Histological type (n = 74) | 0.242 | 0.474 | |||||
| Papillary | 22 | 13 | 9 | 13 | 9 | ||
| Non-papillary | 52 | 23 | 29 | 26 | 26 | ||
| Age (years) (n = 74) | 0.496 | 0.668 | |||||
| ≤56 | 34 | 18 | 16 | 17 | 17 | ||
| >56 | 40 | 18 | 22 | 22 | 18 | ||
| Gender (n = 74) | 0.864 | 0.022 | |||||
| Female | 26 | 13 | 13 | 9 | 17 | ||
| Male | 48 | 23 | 25 | 30 | 18 | ||
| Tumor size (n = 73) | 0.887 | 0.233 | |||||
| <5 cm | 13 | 6 | 7 | 5 | 8 | ||
| ≥5 cm | 60 | 29 | 31 | 34 | 26 | ||
| Tumor stage (n = 74) | 0.814 | 0.963 | |||||
| I-III | 29 | 15 | 14 | 15 | 14 | ||
| IVA | 35 | 17 | 18 | 19 | 16 | ||
| IVB | 10 | 4 | 6 | 5 | 5 | ||
| Variables | n | Hazard Ratio (HR) | 95% (CI) | p |
|---|---|---|---|---|
| Histological type (n=74) | 1.117–3.572 | 0.020 | ||
| Papillary | 22 | 1 | ||
| Non-papillary | 52 | 1.997 | ||
| Age (years) (n = 74) | 0.844–2.288 | 0.195 | ||
| ≤56 | 34 | 1 | ||
| >56 | 40 | 1.390 | ||
| Gender (n = 74) | 0.691–1.873 | 0.613 | ||
| Female | 26 | 1 | ||
| Male | 48 | 1.137 | ||
| Tumor stage (n = 74) | ||||
| I-III | 29 | 1 | 0.092 | |
| IVA | 35 | 0.840 | 0.499–1.415 | 0.513 |
| IVB | 10 | 2.005 | 0.930–4.322 | 0.076 |
| MAL-II expression (n = 74) | 1.139–3.246 | 0.014 | ||
| Low | 36 | 1 | ||
| High | 38 | 1.923 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wattanavises, S.; Silsirivanit, A.; Sawanyawisuth, K.; Cha’on, U.; Waraasawapati, S.; Saentaweesuk, W.; Luang, S.; Chalermwat, C.; Wongkham, C.; Wongkham, S. Increase of MAL-II Binding Alpha2,3-Sialylated Glycan Is Associated with 5-FU Resistance and Short Survival of Cholangiocarcinoma Patients. Medicina 2019, 55, 761. https://doi.org/10.3390/medicina55120761
Wattanavises S, Silsirivanit A, Sawanyawisuth K, Cha’on U, Waraasawapati S, Saentaweesuk W, Luang S, Chalermwat C, Wongkham C, Wongkham S. Increase of MAL-II Binding Alpha2,3-Sialylated Glycan Is Associated with 5-FU Resistance and Short Survival of Cholangiocarcinoma Patients. Medicina. 2019; 55(12):761. https://doi.org/10.3390/medicina55120761
Chicago/Turabian StyleWattanavises, Sasiprapa, Atit Silsirivanit, Kanlayanee Sawanyawisuth, Ubon Cha’on, Sakda Waraasawapati, Waraporn Saentaweesuk, Sukanya Luang, Chalongchai Chalermwat, Chaisiri Wongkham, and Sopit Wongkham. 2019. "Increase of MAL-II Binding Alpha2,3-Sialylated Glycan Is Associated with 5-FU Resistance and Short Survival of Cholangiocarcinoma Patients" Medicina 55, no. 12: 761. https://doi.org/10.3390/medicina55120761