Glutamic Acid Increased Methotrexate Polyglutamation and Cytotoxicity in a CCRF-SB Acute Lymphoblastic Leukemia Cell Line
Abstract
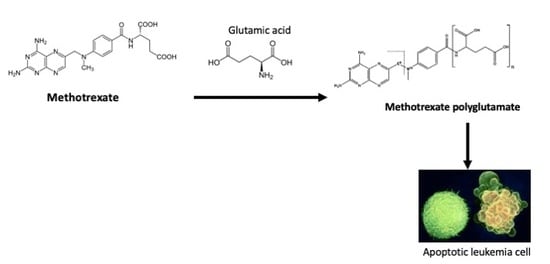
:1. Introduction
2. Materials and Methods
2.1. Maintenance of Cell Culture
2.2. Concentration–Response Curve for Methotrexate Citotoxicity
2.3. Drug Combination Treatment
2.4. HPLC Analysis of MTX Polyglutamate Formation
2.5. Immunocytochemical Analysis of FPGS Enzyme and GLAST Transporter
2.6. Assessment of Apoptosis with Annexin-V Staining
2.7. Glutamate Uptake
2.8. Statistical Analysis
3. Results
3.1. IC50 of MTX
3.2. Glutamic Acid Increases the Intracellular Accumulation of Long-Chain MTX-PG in Acute Lymphoblastic Leukemia Cells
3.3. Treatment with MTX or MTX/Glu Induces the Expression of the FPGS Enzyme and the GLAST Transporter
3.4. The Combination Treatment of Glutamic Acid–MTX Increases Apoptosis in CCRF-SB Cells
3.5. Effect of Glutamic Acid–MTX Treatment on Glutamate Uptake
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inaba, H.; Greaves, M.; Mullighan, C.G. Acute lymphoblastic leukaemia. Lancet. 2013, 381, 1943–1955. [Google Scholar] [CrossRef]
- Lee-Sherick, A.; Linger, R.M.; Gore, L.; Keating, A.K.; Graham, D.K. Targeting paediatric acute lymphoblastic leukaemia: Novel therapies currently in development. Br. J. Haematol. 2010, 151, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Goldman, D. Resistance to antifolates. Oncogene. 2003, 22, 7431–7457. [Google Scholar] [CrossRef] [PubMed]
- Xin, N.; Fen, Z.; Li, C.; Yan, X.; Runming, J. Intracranial Hemorrhage following Oral Low-Dose Methotrexaate after multiple toxicities caused by high-dose Metothrexate in Childhood Acute Lymphoblastic Leukemia. Front. Pharmacol. 2019, 10, 1072. [Google Scholar] [CrossRef]
- Visentin, M.; Zhao, R.; Goldman, I.D. The antifolates. Hematol. Oncol. Clin. N. Am. 2012, 26, 629–648. [Google Scholar] [CrossRef]
- De Beaumais, T.A.; Jacqz-Aigrain, E. Intracellular disposition of methotrexate in acute lymphoblastic leukemia in children. Curr. Drug Metab. 2012, 13, 822–834. [Google Scholar] [CrossRef]
- Barredo, J.C.; Synold, T.W.; Laver, J.; Relling, M.V.; Pui, C.H.; Priest, D.G.; Evans, W.E. Differences in constitutive and post-methotrexate folylpolyglutamate synthetase activity in B-lineage and T-lineage leukemia. Blood 1994, 84, 564–569. [Google Scholar] [CrossRef]
- Masson, E.; Relling, M.V.; Synold, T.W.; Liu, Q.; Schuetz, J.D.; Sandlund, J.T.; Pui, C.H. Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. J. Clin. Invest. 1996, 97, 73–80. [Google Scholar] [CrossRef]
- Leclerc, G.J.; Mou, C.; Leclerc, G.M.; Mian, A.M.; Barredo, J.C. Histone deacetylase inhibitors induce FPGS mRNA expression and intracellular accumulation of long-chain methotrexate polyglutamates in childhood acute lymphoblastic leukemia: Implications for combination therapy. Leukemia. 2010, 24, 552–562. [Google Scholar] [CrossRef]
- Koizumi, S.; Curt, G.A.; Fine, R.L.; Griffin, J.D.; Chabner, B.A. Formation of Methotrexate Polyglutamates in Purified Myeloid Precursor Cells from Normal Human Bone Marrow. J. Clin. Invest. 1985, 75, 1008–1014. [Google Scholar] [CrossRef]
- Barnes, M.J.; Estlin, E.J.; Taylor, G.A.; Aherne, G.W.; Hardcastle, A.; McGuire, J.J.; Calvete, J.A.; Lunec, J.; Pearson, A.D.; Newell, D.R. Impact of polyglutamation on sensitivity to raltitrexed and methotrexate in relation to drug-induced inhibition of de novo thymidylate and purine biosynthesis in CCRF-CEM cell lines. Clin. Cancer Res. 1999, 5, 2548–2558. [Google Scholar]
- Haandel, L.; Becker, M.; Leeder, S.; Williams, T.; Stobaugh, J. A novel high-performance liquid chromatography/mass spectrometry method for improved selective and sensitive measurement of methotrexate polyglutamation status in human red blood cells. Rapid Commun. Mass Spectrom. 2009, 23, 3693–3702. [Google Scholar] [CrossRef]
- Mikkelsen, T.S.; Sparreboom, A.; Cheng, C.; Zhou, Y.; Boyett, J.M.; Raimondi, S.C.; Panetta, J.C.; Bowman, W.P.; Sanlund, J.T.; Pui, C.H.; et al. Shortening infusion time for high-dose methotrexate alters antileukemic effects: A randomized prospective clinical trial. J. Clin. Oncol. 2011, 29, 1771–1778. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, Y.; Wang, L.; Liu, H.; Cui, G. Enhancer anticancer activity of glutamate prodrugs of all-trans retinoic acid. J. Pharm. Pharmacol. 2009, 61, 1353–1358. [Google Scholar] [CrossRef]
- Mersting, J.; Nielsen, S.N.; Grell, K.; Paerregaard, M.; Abrahamsson, J.; Lund, B.; Jonsson, O.G.; Pruunsild, K.; Vaitkevicience, G.; Kanerva, J.; et al. Methotrexate polyglutamate levels and co- distributions in childhood acute lymphoblastic leukemia maintenance therapy. Cancer Chemother. Pharmacol. 2019, 83, 53–60. [Google Scholar] [CrossRef]
- Reyes-Haro, D.; Garcí-Alcocer, G.; Miledi, R.; Garcí-Colunga, J. Uptake of serotonin by adult rat corpus callosum is partially reduced by common antidepressants. J. Neurosci. Res. 2003, 74, 97–102. [Google Scholar] [CrossRef]
- Schmiegelow, K. Advances in individual prediction of methotrexate toxicity: A review. Br. J. Haematol. 2009, 146, 489–503. [Google Scholar] [CrossRef]
- Egan, M.G.; Sirlin, S.; Rumberger, B.G.; Garrow, T.A.; Shane, B.; Sirotnak, F.M. Rapid decline in folylpolyglutamate synthetase activity and gene expression during maturation of HL-60 cells. J. Biol. Chem. 1995, 270, 5462–5468. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors and downsatream signalling. Int. J. Biol. Sci. 2013, 9, 548–559. [Google Scholar] [CrossRef]
- Dervieux, T.; Orentas, D.; Marcelletti, J.; Pischel, K.; Smith, K.; Walsh, M.; Richerson, R. HPLC determination of erythrocyte methotrexate polyglutamates after low-dose methotrexate therapy in patients with rheumatoid arthritis. Clin. Chem. 2003, 49, 1632–1641. [Google Scholar] [CrossRef]
- Kager, L.; Cheok, M.; Yang, W.; Zaza, G.; Cheng, Q.; Panetta, J.C.; Pui, C.H.; Downing, J.R.; Relling, M.V.; Evans, W.E. Folate pathway gene expression differs in subtypes of acute lymphoblastic leukemia and influences methotrexate pharmacodynamics. J. Clin. Invest. 2005, 115, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Fotoohi, A.K.; Yehuda, G.A.; Ali, M.; Jamileh, H.; Gerrit, J.; Godefridus, J.P.; Catharina, R.; Freidoun, A. Gene expression profiling of leukemia T-cells resistant to methotrexate and 7-hydroxymethotrexate reveals alterations that preserve intracellular levels of folate and nucleotide biosynthesis. Biochem. Pharmacol. 2009, 77, 1410. [Google Scholar] [CrossRef] [PubMed]
- Thornton, C.C.; Al-Rashed, F.; Calay, D.; Birdsey, G.M.; Bauer, A.; Mylroie, H.; Morley, B.J.; Randi, A.M.; Haskard, D.O.; Boyle, J.J.; et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: A novel mechanism for vascular protection in chronic systemic inflammation. Ann. Rheum. Dis. 2015, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. AMPK and transcriptional regulation. Front. Biosci. 2008, 13, 3022–3033. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.L.; Van, Y.T.; Shen, M.H. Biomedical applications of chemically and microbiologically synthesized poly(glutamic acid) and poly(lysine). Med. Chem. 2004, 4, 179–188. [Google Scholar] [CrossRef] [PubMed]







| Control | Group 1 | Group 2 | Group 3 | Group 4 | |
|---|---|---|---|---|---|
| MTX IC25 | ---- | + | ---- | + | + |
| MTX IC50 | ---- | ---- | + | ---- | ---- |
| Glu 5 mM | ---- | ---- | ---- | + | ---- |
| Glu 10 mM | ---- | ---- | ---- | ---- | + |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendoza-Santiago, A.; Becerra, E.; Garay, E.; Bah, M.; Berumen-Segura, L.; Escobar-Cabrera, J.; Hernández-Pérez, A.; García-Alcocer, G. Glutamic Acid Increased Methotrexate Polyglutamation and Cytotoxicity in a CCRF-SB Acute Lymphoblastic Leukemia Cell Line. Medicina 2019, 55, 758. https://doi.org/10.3390/medicina55120758
Mendoza-Santiago A, Becerra E, Garay E, Bah M, Berumen-Segura L, Escobar-Cabrera J, Hernández-Pérez A, García-Alcocer G. Glutamic Acid Increased Methotrexate Polyglutamation and Cytotoxicity in a CCRF-SB Acute Lymphoblastic Leukemia Cell Line. Medicina. 2019; 55(12):758. https://doi.org/10.3390/medicina55120758
Chicago/Turabian StyleMendoza-Santiago, Alma, Edgardo Becerra, Edith Garay, Moustapha Bah, Laura Berumen-Segura, Jesica Escobar-Cabrera, Abigail Hernández-Pérez, and Guadalupe García-Alcocer. 2019. "Glutamic Acid Increased Methotrexate Polyglutamation and Cytotoxicity in a CCRF-SB Acute Lymphoblastic Leukemia Cell Line" Medicina 55, no. 12: 758. https://doi.org/10.3390/medicina55120758