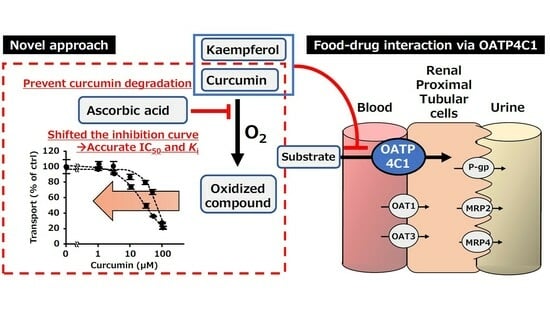
The Use of an Antioxidant Enables Accurate Evaluation of the Interaction of Curcumin on Organic Anion-Transporting Polypeptides 4C1 by Preventing Auto-Oxidation
Abstract
:1. Introduction
2. Results
2.1. Screening of the Effect of Food Ingredients on OATP4C1-Mediated Transport
2.2. Using an Antioxidant to Evaluate the Inhibitory Effects of Food Ingredients
2.3. Concentration-Dependent Inhibition by Kaempferol and Curcumin
2.4. Kinetic Analysis of OATP4C1-Mediated T3 Uptake in The Presence or Absence of Kaempferol and Curcumin
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Transport Study
4.4. Using an Antioxidant to Evaluate the Inhibitory Effects of Food Ingredients
- (1)
- The effect of 10, 100, and 1000 µM AA on OATP4C1-mediated transport was evaluated.
- (2)
- The inhibitory effect of curcumin was evaluated in the absence or presence of 10, 100, and 1000 µM AA.
4.5. Concentration-Dependent Inhibition by Kaempferol and Curcumin
4.6. Kinetic Analysis of OATP4C1-Mediated T3 Uptake in the Presence or Absence of Kaempferol and Curcumin
4.7. Sample Preparation
4.8. Liquid Chromatography/Tandem Mass Spectrometry Conditions
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Makiabadi, E.; Jalali, S.; Heidari, Z.; Assadi, M.; RashidKhani, B. Dietary intake of polyphenols and the risk of breast cancer: A case-control study. Clin. Nutr. Res. 2021, 10, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Cheon, M.; Chung, M.; Park, Y. Association between dietary intake of flavonoids and cancer recurrence among breast cancer survivors. Nutrients 2021, 13, 3049. [Google Scholar] [CrossRef] [PubMed]
- Hatono, M.; Ikeda, H.; Suzuki, Y.; Kajiwara, Y.; Kawada, K.; Tsukioki, T.; Kochi, M.; Suzawa, K.; Iwamoto, T.; Yamamoto, H.; et al. Effect of isoflavones on breast cancer cell development and their impact on breast cancer treatments. Breast Cancer Res. Treat. 2021, 185, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar] [PubMed]
- Koch, W. Dietary polyphenols—Important non-nutrients in the prevention of chronic noncommunicable diseases. A systematic review. Nutrients 2019, 11, 1039. [Google Scholar] [CrossRef]
- Petrescu, A.M.; Paunescu, V.; Ilia, G. The antiviral activity and cytotoxicity of 15 natural phenolic compounds with previously demonstrated antifungal activity. J. Environ. Sci. Health B 2019, 54, 498–504. [Google Scholar] [CrossRef]
- Mueller, S.C.; Uehleke, B.; Woehling, H.; Petzsch, M.; Majcher-Peszynska, J.; Hehl, E.-M.; Sievers, H.; Frank, B.; Riethling, A.-K.; Drewelow, B. Effect of St John’s wort dose and preparations on the pharmacokinetics of digoxin. Clin. Pharmacol. Ther. 2004, 75, 546–557. [Google Scholar] [CrossRef]
- Qiao, J.; Gu, C.; Shang, W.; Du, J.; Yin, W.; Zhu, M.; Wang, W.; Han, M.; Lu, W. Effect of green tea on pharmacokinetics of 5-fluorouracil in rats and pharmacodynamics in human cell lines in vitro. Food Chem. Toxicol. 2011, 49, 1410–1415. [Google Scholar] [CrossRef]
- Jeon, H.; Jang, I.J.; Lee, S.; Ohashi, K.; Kotegawa, T.; Ieiri, I.; Cho, J.Y.; Yoon, S.H.; Shin, S.G.; Yu, K.S.; et al. Apple juice greatly reduces systemic exposure to atenolol. Br. J. Clin. Pharmacol. 2013, 75, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Albassam, A.A.; Markowitz, J.S. An appraisal of drug-drug interactions with green tea (Camellia sinensis). Planta Med. 2017, 83, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.E.; Shin, K.H.; Park, J.E.; Kim, M.G.; Yun, Y.M.; Choi, D.H.; Kwon, K.J.; Lee, J. Effect of green tea catechins on the pharmacokinetics of digoxin in humans. Drug Des. Devel. Ther. 2018, 12, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Prely, H.; Herledan, C.; Caffin, A.G.; Baudouin, A.; Larbre, V.; Maire, M.; Schwiertz, V.; Vantard, N.; Ranchon, F.; Rioufol, C. Real-life drug–drug and herb–drug interactions in outpatients taking oral anticancer drugs: Comparison with databases. J. Cancer Res. Clin. Oncol. 2022, 148, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Palleria, C.; Roberti, R.; Iannone, L.F.; Tallarico, M.; Barbieri, M.A.; Vero, A.; Manti, A.; De Sarro, G.; Spina, E.; Russo, E. Clinically relevant drug interactions between statins and antidepressants. J. Clin. Pharm. Ther. 2020, 45, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Kumondai, M.; Kikuchi, M.; Mizuguchi, A.; Hayashi, N.; Ui, M.; Hirama, T.; Okada, Y.; Sato, Y.; Sato, T.; Maekawa, M.; et al. Therapeutic drug monitoring of blood sirolimus and tacrolimus concentrations for polypharmacy management in a lymphangioleiomyomatosis patient taking two cytochrome P450 3A inhibitors. Tohoku J. Exp. Med. 2023, 260, 29–34. [Google Scholar] [CrossRef]
- Sato, T.; Yamaguchi, H.; Kogawa, T.; Abe, T.; Mano, N. Organic anion transporting polypeptides 1B1 and 1B3 play an important role in uremic toxin handling and drug-uremic toxin interactions in the liver. J. Pharm. Pharm. Sci. 2014, 17, 475–484. [Google Scholar] [CrossRef]
- Sato, T.; Mishima, E.; Mano, N.; Abe, T.; Yamaguchi, H. Potential drug interactions mediated by renal organic anion transporter OATP4C1. J. Pharmacol. Exp. Ther. 2017, 362, 271–277. [Google Scholar] [CrossRef]
- Sato, T.; Maekawa, M.; Mano, N.; Abe, T.; Yamaguchi, H. Role of OATP4C1 in renal handling of remdesivir and its nucleoside analog GS-441524: The first approved drug for patients with COVID-19. J. Pharm. Pharm. Sci. 2021, 24, 227–236. [Google Scholar] [CrossRef]
- Petric, Z.; Žuntar, I.; Putnik, P.; Bursać Kovačević, D. Food–drug interactions with fruit juices. Foods 2020, 10, 33. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sato, T.; Otake, A.; Kumondai, M.; Sato, Y.; Kikuchi, M.; Maekawa, M.; Yamaguchi, H.; Abe, T.; Mano, N. Bile acid–drug interaction via organic anion-transporting Polypeptide 4C1 is a potential mechanism of altered pharmacokinetics of renally excreted drugs. Int. J. Mol. Sci. 2022, 23, 8508. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-X.; Guo, C.-X.; Chen, W.-Q.; Yu, J.; Qu, Q.; Chen, Y.; Tan, Z.-R.; Wang, G.; Fan, L.; Li, Q.; et al. Inhibition of the organic anion-transporting polypeptide 1B1 by quercetin: An in vitro and in vivo assessment. Br. J. Clin. Pharmacol. 2012, 73, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Whitley, A.C.; Sweet, D.H.; Walle, T. The dietary polyphenol ellagic acid is a potent inhibitor of hOAT1. Drug Metab. Dispos. 2005, 33, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Meier, P.J. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta 2003, 1609, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hagenbuch, B.; Meier, P.J. Organic anion transporting polypeptides of the OATP/SLC21 family: Phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflug. Arch. 2004, 447, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Kullak-Ublick, G.A.; Stieger, B.; Meier, P.J. Enterohepatic bile salt transporters in normal physiology and liver disease. Gastroenterology 2004, 126, 322–342. [Google Scholar] [CrossRef]
- Suga, T.; Yamaguchi, H.; Sato, T.; Maekawa, M.; Goto, J.; Mano, N. Preference of conjugated bile acids over unconjugated bile acids as substrates for OATP1B1 and OATP1B3. PLoS ONE 2017, 12, e0169719. [Google Scholar] [CrossRef]
- Mikkaichi, T.; Suzuki, T.; Onogawa, T.; Tanemoto, M.; Mizutamari, H.; Okada, M.; Chaki, T.; Masuda, S.; Tokui, T.; Eto, N.; et al. Isolation and characterization of a digoxin transporter and its rat homologue expressed in the kidney. Proc. Natl Acad. Sci. USA 2004, 101, 3569–3574. [Google Scholar] [CrossRef]
- Toyohara, T.; Suzuki, T.; Morimoto, R.; Akiyama, Y.; Souma, T.; Shiwaku, H.O.; Takeuchi, Y.; Mishima, E.; Abe, M.; Tanemoto, M.; et al. SLCO4C1 transporter eliminates uremic toxins and attenuates hypertension and renal inflammation. J. Am. Soc. Nephrol. 2009, 20, 2546–2555. [Google Scholar] [CrossRef]
- Mandery, K.; Balk, B.; Bujok, K.; Schmidt, I.; Fromm, M.F.; Glaeser, H. Inhibition of hepatic uptake transporters by flavonoids. Eur. J. Pharm. Sci. 2012, 46, 79–85. [Google Scholar] [CrossRef]
- Kondo, A.; Narumi, K.; Ogura, J.; Sasaki, A.; Yabe, K.; Kobayashi, T.; Furugen, A.; Kobayashi, M.; Iseki, K. Organic anion-transporting polypeptide (OATP) 2B1 contributes to the cellular uptake of theaflavin. Drug Metab. Pharmacokinet. 2017, 32, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Nimiya, Y.; Wang, W.; Du, Z.; Sukamtoh, E.; Zhu, J.; Decker, E.; Zhang, G. Redox modulation of curcumin stability: Redox active antioxidants increase chemical stability of curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Leung, M.H.M.; Kee, T.W. Effective stabilization of curcumin by association to plasma proteins: Human serum albumin and fibrinogen. Langmuir 2009, 25, 5773–5777. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, J.; Guo, C.; Xing, H.; Xu, J.; Wen, Y.; Qiu, Z.; Zhang, Q.; Zheng, Y.; Chen, X.; et al. Pharmacokinetic effects of curcumin on docetaxel mediated by OATP1B1, OATP1B3 and CYP450s. Drug Metab. Pharmacokinet. 2016, 31, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Kimira, M.; Arai, Y.; Shimoi, K.; Watanabe, S. Japanese intake of flavonoids and isoflavonoids from foods. J. Epidemiol. 1998, 8, 168–175. [Google Scholar] [CrossRef]
- Jun, S.; Shin, S.; Joung, H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br. J. Nutr. 2016, 115, 480–489. [Google Scholar] [CrossRef]
- Jacques, P.F.; Cassidy, A.; Rogers, G.; Peterson, J.J.; Dwyer, J.T. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br. J. Nutr. 2015, 114, 1496–1503. [Google Scholar] [CrossRef]
- Ferreira, A.; Santos, A.O.; Falcão, A.; Alves, G. In vitro screening of dual flavonoid combinations for reversing P-glycoprotein-mediated multidrug resistance: Focus on antiepileptic drugs. Food Chem. Toxicol. 2018, 111, 84–93. [Google Scholar] [CrossRef]
- Ullmann, U.; Haller, J.; Decourt, J.P.; Girault, N.; Girault, J.; Richard-Caudron, A.S.; Pineau, B.; Weber, P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003, 31, 88–101. [Google Scholar] [CrossRef] [PubMed]
- Antony, B.; Merina, B.; Iyer, V.S.; Judy, N.; Lennertz, K.; Joyal, S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95® CG (BiocurcumaxTM), a novel bioenhanced preparation of curcumin. Indian J. Pharm. Sci. 2008, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, K.L.R.; Keppler, D.; Hoffmaster, K.A.; Bow, D.A.J.; Cheng, Y.; Lai, Y.; Palm, J.E.; Stieger, B.; Evers, R.; International Transporter Consortium. In vitro methods to support transporter evaluation in drug discovery and development. Clin. Pharmacol. Ther. 2013, 94, 95–112. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wolkoff, A.W.; Morris, M.E. Flavonoids as a novel class of human organic anion-transporting polypeptide OATP1B1 (OATP-C) modulators. Drug Metab. Dispos. 2005, 33, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Mandery, K.; Bujok, K.; Schmidt, I.; Keiser, M.; Siegmund, W.; Balk, B.; König, J.; Fromm, M.F.; Glaeser, H. Influence of the flavonoids apigenin, kaempferol, and quercetin on the function of organic anion transporting polypeptides 1A2 and 2B1. Biochem. Pharmacol. 2010, 80, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Meng, Q.; Wang, C.; Wu, J.; Zhu, Y.; Sun, P.; Ma, X.; Sun, H.; Liu, K. Targeting renal OATs to develop renal protective agent from traditional Chinese medicines: Protective effect of Apigenin against Imipenem-induced nephrotoxicity. Phytotherapy Res. 2020, 34, 2998–3010. [Google Scholar] [CrossRef] [PubMed]
- Navrátilová, L.; Applová, L.; Horký, P.; Mladěnka, P.; Pávek, P.; Trejtnar, F. Interaction of soy isoflavones and their main metabolites with hOATP2B1 transporter. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 1063–1071. [Google Scholar] [CrossRef]
- An, G.; Wang, X.; Morris, M.E. Flavonoids Are Inhibitors of Human Organic Anion Transporter 1 (OAT1)–Mediated Transport. Drug Metab. Dispos. 2014, 42, 1357–1366. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, Y.; Wang, C.; Meng, Q.; Wu, J.; Sun, P.; Ma, X.; Sun, H.; Huo, X.; Liu, K.; et al. Molecular pharmacokinetic mechanism of the drug-drug interaction between genistein and repaglinide mediated by P-gp. Biomed. Pharmacother. 2020, 125, 110032. [Google Scholar] [CrossRef]
- Mohos, V.; Fliszár-Nyúl, E.; Ungvári, O.; Kuffa, K.; Needs, P.W.; Kroon, P.A.; Telbisz, Á.; Özvegy-Laczka, C.; Poór, M. Inhibitory Effects of Quercetin and Its Main Methyl, Sulfate, and Glucuronic Acid Conjugates on Cytochrome P450 Enzymes, and on OATP, BCRP and MRP2 Transporters. Nutrients 2020, 12, 2306. [Google Scholar] [CrossRef]
- Li, Z.; Tian, S.; Wu, Z.; Xu, X.; Lei, L.; Li, Y.; Wang, B.; Huang, Y. Pharmacokinetic herb-disease-drug interactions: Effect of ginkgo biloba extract on the pharmacokinetics of pitavastatin, a substrate of Oatp1b2, in rats with non-alcoholic fatty liver disease. J. Ethnopharmacol. 2021, 280, 114469. [Google Scholar] [CrossRef]
- Fan, X.; Bai, J.; Hu, M.; Xu, Y.; Zhao, S.; Sun, Y.; Wang, B.; Hu, J.; Li, Y. Drug interaction study of flavonoids toward OATP1B1 and their 3D structure activity relationship analysis for predicting hepatoprotective effects. Toxicology 2020, 437, 152445. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Nabekura, T.; Takahashi, T.; Nakamura, Y.; Sakamoto, H.; Tano, H.; Hirai, M.; Tsukahara, G. Structure-Activity Relationships of the Inhibitory Effects of Flavonoids on P-Glycoprotein-Mediated Transport in KB-C2 Cells. Biol. Pharm. Bull. 2005, 28, 2274–2278. [Google Scholar] [CrossRef] [PubMed]
- Kaci, H.; Bodnárová, S.; Fliszár-Nyúl, E.; Lemli, B.; Pelantová, H.; Valentová, K.; Bakos, É.; Özvegy-Laczka, C.; Poór, M. Interaction of luteolin, naringenin, and their sulfate and glucuronide conjugates with human serum albumin, cytochrome P450 (CYP2C9, CYP2C19, and CYP3A4) enzymes and organic anion transporting polypeptide (OATP1B1 and OATP2B1) transporters. Biomed. Pharmacother. 2023, 157, 114078. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, X.; Bi, Y.; Yu, H.; Wei, J.; Zhang, Y.; Han, L.; Zhang, Y. Potent Inhibitors of Organic Anion Transporters 1 and 3 From Natural Compounds and Their Protective Effect on Aristolochic Acid Nephropathy. Toxicol. Sci. 2020, 175, 279–291. [Google Scholar] [CrossRef]
- Hong, S.S.; Seo, K.; Lim, S.-C.; Han, H.-K. Interaction characteristics of flavonoids with human organic anion transporter 1 (hOAT1) and 3 (hOAT3). Pharmacol. Res. 2007, 56, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Morris, M.E. Effects of the Flavonoids Biochanin A, Morin, Phloretin, and Silymarin on P-Glycoprotein-Mediated Transport. J. Pharmacol. Exp. Ther. 2002, 304, 1258–1267. [Google Scholar] [CrossRef]
- Wang, M.; Qi, H.; Li, J.; Xu, Y.; Zhang, H. Transmembrane transport of steviol glucuronide and its potential interaction with selected drugs and natural compounds. Food Chem. Toxicol. 2015, 86, 217–224. [Google Scholar] [CrossRef]
- Wen, F.; Shi, M.; Bian, J.; Zhang, H.; Gui, C. Identification of natural products as modulators of OATP2B1 using LC-MS/MS to quantify OATP-mediated uptake. Pharm. Biol. 2015, 54, 293–302. [Google Scholar] [CrossRef]
- Mohana, S.; Ganesan, M.; Agilan, B.; Karthikeyan, R.; Srithar, G.; Mary, R.B.; Ananthakrishnan, D.; Velmurugan, D.; Prasad, N.R.; Ambudkar, S.V. Screening dietary flavonoids for the reversal of P-glycoprotein-mediated multidrug resistance in cancer. Mol. Biosyst. 2016, 12, 2458–2470. [Google Scholar] [CrossRef]
- Johnson, E.J.; Won, C.S.; Köck, K.; Paine, M.F. Prioritizing pharmacokinetic drug interaction precipitants in natural products: Application to OATP inhibitors in grapefruit juice. Biopharm. Drug Dispos. 2016, 38, 251–259. [Google Scholar] [CrossRef]
- Bajraktari-Sylejmani, G.; Weiss, J. Potential Risk of Food-Drug Interactions: Citrus Polymethoxyflavones and Flavanones as Inhibitors of the Organic Anion Transporting Polypeptides (OATP) 1B1, 1B3, and 2B1. Eur. J. Drug Metab. Pharmacokinet. 2020, 45, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; Akiyoshi, T.; Sato, R.; Uekusa, Y.; Katayama, K.; Yajima, K.; Imaoka, A.; Sugimoto, Y.; Kiuchi, F.; Ohtani, H. Citrus Fruit-Derived Flavanone Glycoside Narirutin is a Novel Potent Inhibitor of Organic Anion-Transporting Polypeptides. J. Agric. Food Chem. 2020, 68, 14182–14191. [Google Scholar] [CrossRef]
- Shirasaka, Y.; Li, Y.; Shibue, Y.; Kuraoka, E.; Spahn-Langguth, H.; Kato, Y.; Langguth, P.; Tamai, I. Concentration-Dependent Effect of Naringin on Intestinal Absorption of β1-Adrenoceptor Antagonist Talinolol Mediated by P-Glycoprotein and Organic Anion Transporting Polypeptide (Oatp). Pharm. Res. 2008, 26, 560–567. [Google Scholar] [CrossRef]
- Roth, M.; Timmermann, B.N.; Hagenbuch, B. Interactions of Green Tea Catechins with Organic Anion-Transporting Polypeptides. Drug Metab. Dispos. 2011, 39, 920–926. [Google Scholar] [CrossRef]
- Fuchikami, H.; Satoh, H.; Tsujimoto, M.; Ohdo, S.; Ohtani, H.; Sawada, Y. Effects of herbal extracts on the function of human organic anion-transporting polypeptide OATP-B. Drug Metab. Dispos. 2006, 34, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kondo, M.; Hiramatsu, R.; Nabekura, T. (−)-Epigallocatechin-3-gallate Inhibits Human and Rat Renal Organic Anion Transporters. ACS Omega 2021, 6, 4347–4354. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Barron, D.; Orfila, C.; Dionisi, F.; Krajcsi, P.; Williamson, G. Interaction of hydroxycinnamic acids and their conjugates with organic anion transporters and ATP-binding cassette transporters. Mol. Nutr. Food Res. 2011, 55, 979–988. [Google Scholar] [CrossRef]
- Uwai, Y.; Ozeki, Y.; Isaka, T.; Honjo, H.; Iwamoto, K. Inhibitory Effect of Caffeic Acid on Human Organic Anion Transporters hOAT1 and hOAT3: A Novel Candidate for Food–Drug Interaction. Drug Metab. Pharmacokinet. 2011, 26, 486–493. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Wang, C.C.N.; Liao, W.-C.; Lan, Y.-H.; Hung, C.-C. Caffeic Acid Attenuates Multi-Drug Resistance in Cancer Cells by Inhibiting Efflux Function of Human P-Glycoprotein. Molecules 2020, 25, 247. [Google Scholar] [CrossRef]
- Wang, L.; Sweet, D.H. Potential for food-drug interactions by dietary phenolic acids on human organic anion transporters 1 (SLC22A6), 3 (SLC22A8), and 4 (SLC22A11). Biochem. Pharmacol. 2012, 84, 1088–1095. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, G.; Balupillai, A.; Ramasamy, K.; Shanmugam, M.; Gunaseelan, S.; Mary, B.; Prasad, N.R. Ferulic acid reverses ABCB1-mediated paclitaxel resistance in MDR cell lines. Eur. J. Pharmacol. 2016, 786, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.-L.; Takahashi, K.; Tanaka, K.; Tougou, K.; Qiu, F.; Komatsu, K.; Takahashi, K.; Azuma, J. Curcuma drugs and curcumin regulate the expression and function of P-gp in Caco-2 cells in completely opposite ways. Int. J. Pharm. 2008, 358, 224–229. [Google Scholar] [CrossRef]
- Li, Z.; Wang, K.; Zheng, J.; Cheung, F.S.G.; Chan, T.; Zhu, L.; Zhou, F. Interactions of the active components of Punica granatum(pomegranate) with the essential renal and hepatic human Solute Carrier transporters. Pharm. Biol. 2014, 52, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Du, X.; Li, Y.; Wang, R.; Liu, C.; Cao, Y.; Wu, W.; Sun, J.; Wang, B.; Huang, Y. Pharmacokinetics of gallic acid and protocatechuic acid in humans after dosing with Relinqing (RLQ) and the potential for RLQ-perpetrated drug–drug interactions on organic anion transporter (OAT) 1/3. Pharm. Biol. 2021, 59, 757–768. [Google Scholar] [CrossRef] [PubMed]
- Athukuri, B.L.; Neerati, P. Enhanced Oral Bioavailability of Diltiazem by the Influence of Gallic Acid and Ellagic Acid in Male Wistar Rats: Involvement of CYP3A and P-gp Inhibition. Phytotherapy Res. 2017, 31, 1441–1448. [Google Scholar] [CrossRef]
- Poór, M.; Kaci, H.; Bodnárová, S.; Mohos, V.; Fliszár-Nyúl, E.; Kunsági-Máté, S.; Özvegy-Laczka, C.; Lemli, B. Interactions of resveratrol and its metabolites (resveratrol-3-sulfate, resveratrol-3-glucuronide, and dihydroresveratrol) with serum albumin, cytochrome P450 enzymes, and OATP transporters. Biomed. Pharmacother. 2022, 151, 113136. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, Z.; Wang, C.; Meng, Q.; Huo, X.; Liu, Q.; Sun, H.; Sun, P.; Yang, X.; Ma, X.; et al. P-gp, MRP2 and OAT1/OAT3 mediate the drug-drug interaction between resveratrol and methotrexate. Toxicol. Appl. Pharmacol. 2016, 306, 27–35. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, S.; Abdelghany, A.A.; Al-Amoudi, H.S.; Efferth, T.; Wink, M. Resveratrol mediated cancer cell apoptosis, and modulation of multidrug resistance proteins and metabolic enzymes. Phytomedicine 2019, 55, 269–281. [Google Scholar] [CrossRef]







| IC50 (µM) | Ki (µM) | Average of Ki (µM) | SE (µM) | |
|---|---|---|---|---|
| AA (−) | 49.4 | 42.2 | 52.2 | 5.1 |
| 69.4 | 59.3 | |||
| 64.5 | 55.2 | |||
| AA (+) | 26.5 | 22.7 | 23.5 | 0.5 |
| 28.6 | 24.5 | |||
| 27.4 | 23.4 |
| Km (µM) | Vmax (pmol/mg Protein/10 min) | Vmax/Km (µL/mg Protein/10 min) | |
|---|---|---|---|
| Control | 6.06 ± 0.83 | 524 ± 18.2 | 88.8 ± 8.90 |
| Curcumin | 20.8 ± 4.89 | 757 ± 76.0 | 39.7 ± 6.79 |
| Kaempferol | 17.6 ± 5.01 | 517 ± 92.6 | 31.8 ± 4.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, T.; Yagi, A.; Yamauchi, M.; Kumondai, M.; Sato, Y.; Kikuchi, M.; Maekawa, M.; Yamaguchi, H.; Abe, T.; Mano, N. The Use of an Antioxidant Enables Accurate Evaluation of the Interaction of Curcumin on Organic Anion-Transporting Polypeptides 4C1 by Preventing Auto-Oxidation. Int. J. Mol. Sci. 2024, 25, 991. https://doi.org/10.3390/ijms25020991
Sato T, Yagi A, Yamauchi M, Kumondai M, Sato Y, Kikuchi M, Maekawa M, Yamaguchi H, Abe T, Mano N. The Use of an Antioxidant Enables Accurate Evaluation of the Interaction of Curcumin on Organic Anion-Transporting Polypeptides 4C1 by Preventing Auto-Oxidation. International Journal of Molecular Sciences. 2024; 25(2):991. https://doi.org/10.3390/ijms25020991
Chicago/Turabian StyleSato, Toshihiro, Ayaka Yagi, Minami Yamauchi, Masaki Kumondai, Yu Sato, Masafumi Kikuchi, Masamitsu Maekawa, Hiroaki Yamaguchi, Takaaki Abe, and Nariyasu Mano. 2024. "The Use of an Antioxidant Enables Accurate Evaluation of the Interaction of Curcumin on Organic Anion-Transporting Polypeptides 4C1 by Preventing Auto-Oxidation" International Journal of Molecular Sciences 25, no. 2: 991. https://doi.org/10.3390/ijms25020991