Continuous Production of Highly Tuned Silk/Calcium-Based Composites: Exploring New Pathways for Skin Regeneration
Abstract
:1. Introduction
2. Results
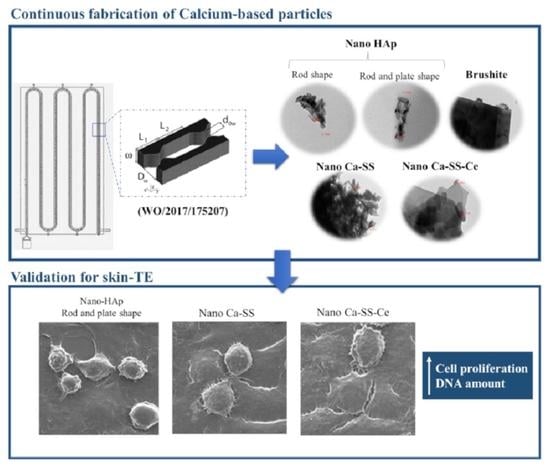
2.1. Physicochemical Properties of Produced Calcium-Based Particles
2.1.1. Phase Identification
2.1.2. Size, Morphology, and Crystallinity
2.2. In Vitro Cell Viability and Cell Behavior
2.2.1. MTT Assay and DNA Quantification
2.2.2. Cell Morphology
3. Discussion
3.1. Physicochemical Characterization (FTIR, XRD, EDX, SEM, TEM, Laser Diffraction)
3.2. In Vitro Evaluation (SEM, Confocal, MTT, DNA)
3.3. Antibacterial Activity
3.4. Potential Application in Skin-TE
4. Materials and Methods
4.1. Description of the Modular Oscillatory Flow Plate Reactor
4.2. Particles Synthesis
4.3. Particles Characterization
4.3.1. Phase Identification
4.3.2. Size, Morphology, and Crystallinity
4.4. Cell Culture and Sample Preparation
4.4.1. HDFs Seeding
4.4.2. Particle Preparation and Sterilization
4.4.3. Exposure of HDFs to Ca-Based Particles
4.5. Cell Interaction Evaluation
4.5.1. MTT Staining
4.5.2. DNA Quantification
4.5.3. SEM and Confocal
4.6. Antibacterial Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ribeiro, N.; Sousa, A.; Cunha-Reis, C.; Oliveira, A.L.; Granja, P.L.; Monteiro, F.J.; Sousa, S.R. New Prospects in Skin Regeneration and Repair Using Nanophased Hydroxyapatite Embedded in Collagen Nanofibers. Nanomed. Nanotechnol. Biol. Med. 2021, 33, 102353. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B.G. Calcium: A Potential Central Regulator in Wound Healing in the Skin. Wound Repair Regen. 2002, 10, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Castro, F.; Reis, C.C.; Sousa, A.; Oliveira, A.L.; Rocha, F. Hydroxyapatite/Sericin Composites: A Simple Synthesis Route under near-Physiological Conditions of Temperature and PH and Preliminary Study of the Effect of Sericin on the Biomineralization Process. Mater. Sci. Eng. C 2020, 108, 110400. [Google Scholar] [CrossRef] [PubMed]
- Veiga, A.; Castro, F.; Rocha, F.; Oliveira, A.L. Recent Advances in Silk Sericin/Calcium Phosphate Biomaterials. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Kamei, K.; Srichana, T. The Effect of Sericin with Variable Amino-Acid Content from Different Silk Strains on the Production of Collagen and Nitric Oxide. J. Biomater. Sci. Polym. Ed. 2009, 20, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a Protein Derived from Silkworms, Accelerates the Proliferation of Several Mammalian Cell Lines Including a Hybridoma. Cytotechnology 2002, 40, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Shuai, Y.; Zhang, C.; Chen, Y.; Zhu, L.; Mao, C.; OuYang, H. Biomimetic Nucleation of Hydroxyapatite Crystals Mediated by Antheraea Pernyi Silk Sericin Promotes Osteogenic Differentiation of Human Bone Marrow Derived Mesenchymal Stem Cells. Biomacromolecules 2014, 15, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Sunarintyas, S.; Siswomihardjo, W. The Effect of Sericin Application over Hydroxyapatite Surface on Osteoblast Cells Proliferation. In Proceedings of the International Conference on Instrumentation, Communication, Information Technology and Biomedical Engineering, Bandung, Indonesia, 8–9 November 2011; pp. 145–149. [Google Scholar]
- Cai, Y.; Jin, J.; Mei, D.; Xia, N.; Yao, J. Effect of Silk Sericin on Assembly of Hydroxyapatite Nanocrystals into Enamel Prism-like Structure. J. Mater. Chem. 2009, 19, 5751. [Google Scholar] [CrossRef]
- Vieira, E.; Silva, M.; Maia-Filho, A.; Ferreira, D.; Figuerêdo-Silva, J.; Rovaris, K.; Fialho, A.C.; Leite-Oliveira, A.; de Oliveira, A.L.M.; da Fonseca, M.G.; et al. Effect of Cerium-Containing Hydroxyapatite in Bone Repair in Female Rats with Osteoporosis Induced by Ovariectomy. Minerals 2021, 11, 377. [Google Scholar] [CrossRef]
- Chigurupati, S.; Mughal, M.R.; Okun, E.; Das, S.; Kumar, A.; McCaffery, M.; Seal, S.; Mattson, M.P. Effects of Cerium Oxide Nanoparticles on the Growth of Keratinocytes, Fibroblasts and Vascular Endothelial Cells in Cutaneous Wound Healing. Biomaterials 2013, 34, 2194–2201. [Google Scholar] [CrossRef] [Green Version]
- Castro, F.; Ribeiro, V.P.; Ferreira, A.; Oliveira, A.L.; Reis, R.L.; Teixeira, J.A.; Rocha, F. Continuous-Flow Precipitation as a Route to Prepare Highly Controlled Nanohydroxyapatite: In Vitro Mineralization and Biological Evaluation. Mater. Res. Express 2016, 3, 075404. [Google Scholar] [CrossRef] [Green Version]
- Veiga, A.; Castro, F.; Oliveira, A.; Rocha, F. High Efficient Strategy for the Production of Hydroxyapatite/Silk Sericin Nanocomposites. J. Chem. Technol. Biotechnol. 2021, 96, 241–248. [Google Scholar] [CrossRef]
- Cruz, P.C.; Silva, C.R.; Rocha, F.A.; Ferreira, A.M. Mixing Performance of Planar Oscillatory Flow Reactors with Liquid Solutions and Solid Suspensions. Ind. Eng. Chem. Res. 2021, 60, 2663–2676. [Google Scholar] [CrossRef]
- Castro, F.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Continuous-Flow Precipitation of Hydroxyapatite at 37 °C in a Meso Oscillatory Flow Reactor. AIChE J. 2013, 59, 4483–4493. [Google Scholar] [CrossRef] [Green Version]
- Veiga, A.; Castro, F.; Magalhães, R.; Rocha, F.; Oliveira, A.L. Fabrication of Highly Tuned Calcium Phosphate/Silk Sericin Composites: Exploring New Pathways on Skin Regeneration. In Proceedings of the 31st Conference of the European Society For Biomaterials, Porto, Portugal, 5–9 September 2021; pp. 1–5. [Google Scholar]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef]
- Cama, G.; Gharibi, B.; Sait, M.S.; Knowles, J.C.; Lagazzo, A.; Romeed, S.; Di Silvio, L.; Deb, S. A Novel Method of Forming Micro- and Macroporous Monetite Cements. J. Mater. Chem. B 2013, 1, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Idowu, B.; Cama, G.; Deb, S.; Di Silvio, L. In Vitro Osteoinductive Potential of Porous Monetite for Bone Tissue Engineering. J. Tissue Eng. 2014, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Azuri, I.; Addadi, L.; Weiner, S.; Yang, K.; Curtarolo, S.; Kronik, L. Infrared Absorption Spectrum of Brushite from First Principles. Chem. Mater. 2014, 26, 2934–2942. [Google Scholar] [CrossRef]
- Landi, E.; Celotti, G.; Logroscino, G.; Tampieri, A. Carbonated Hydroxyapatite as Bone Substitute. J. Eur. Ceram. Soc. 2003, 23, 2931–2937. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, X.; Ji, Z.; Zhu, L.; Ma, N.; Chen, D.; Jia, X.; Tang, J.; Cao, Y. DFT-Calculated IR Spectrum Amide I, II, and III Band Contributions of N-Methylacetamide Fine Components. ACS Omega 2020, 5, 8572–8578. [Google Scholar] [CrossRef] [Green Version]
- RRUFF. Brushite R070554. Available online: https://rruff.info/Brushite (accessed on 25 May 2021).
- RRUFF. Hydroxylapatite R050512. Available online: https://rruff.info/hydrox/display=default/R050512 (accessed on 25 May 2021).
- Castro, F.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J. Precipitation of Hydroxyapatite at 37o C in a Meso Oscillatory Flow Reactor Operated in Batch at Constant Power Density. AIChE J. 2014, 7, 405–410. [Google Scholar] [CrossRef]
- Rivera-Muñoz, E.M. Hydroxyapatite-Based Materials: Synthesis and Characterization. In Biomedical Engineering—Frontiers and Challenges; IntechOpen: London, UK, 2011; Volume 1, p. 13. [Google Scholar] [CrossRef]
- Phatai, P.; Futalan, C.M.; Utara, S.; Khemthong, P.; Kamonwannasit, S. Structural Characterization of Cerium-Doped Hydroxyapatite Nanoparticles Synthesized by an Ultrasonic-Assisted Sol-Gel Technique. Results Phys. 2018, 10, 956–963. [Google Scholar] [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium Phosphates in Biomedical Applications: Materials for the Future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Feng, Z.; Liao, Y.; Ye, M. Synthesis and Structure of Cerium-Substituted Hydroxyapatite. J. Mater. Sci. Med. 2005, 6, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Quadros, P.; Laranjeira, P.; Dias, M.; Lopes, J. A Novel Continuous Industrial Process for Producing Hydroxyapatite Nanoparticles. J. Dispers. Sci. Technol. 2008, 29, 542–547. [Google Scholar] [CrossRef]
- Bastan, F.E.; Erdogan, G.; Moskalewicz, T.; Ustel, F. Spray Drying of Hydroxyapatite Powders: The Effect of Spray Drying Parameters and Heat Treatment on the Particle Size and Morphology. J. Alloys Compd. 2017, 724, 586–596. [Google Scholar] [CrossRef]
- Elliot, J.C. Struture and Chemistry of the Apatites and Other Calcium Orthophosphates, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Mullin, J.W. Crystallisation. Org. Process Res. Dev. 2002, 6, 201–202. [Google Scholar] [CrossRef]
- Sivkumar, G.R.; Girija, E.K.; Narayana Kalkura, S.; Subramanian, C. Crystallization and Characterization of Calcium Phosphates: Brushite and Monetite. Cryst. Res. Technol. 1998, 33, 197–205. [Google Scholar] [CrossRef]
- Matsumoto, T.; Okazaki, M.; Inoue, M.; Hamada, Y.; Taira, M.; Takahashi, J. Crystallinity and Solubility Characteristics of Hydroxyapatite Adsorbed Amino Acid. Biomaterials 2002, 23, 2241–2247. [Google Scholar] [CrossRef]
- Castro, M.D.; Priego-Capote, F. Ultrasound-Assisted Crystallization (Sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef]
- Castro, F.; Kuhn, S.; Jensen, K.; Ferreira, A.; Rocha, F.; Vicente, A.; Teixeira, J.A. Process Intensification and Optimization for Hydroxyapatite Nanoparticles Production. Chem. Eng. Sci. 2013, 100, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Sinko, P.J. The Role of Crystallinity on Differential Attachment/Proliferation of Osteoblasts and Fibroblasts on Poly (Caprolactone-Co-Glycolide) Polymeric Surfaces. Front. Mater. Sci. 2012, 6, 47–59. [Google Scholar] [CrossRef]
- Tamiello, C.; Buskermolen, A.B.C.; Baaijens, F.P.T.; Broers, J.L.V.; Bouten, C.V.C. Heading in the Right Direction: Understanding Cellular Orientation Responses to Complex Biophysical Environments. Cell. Mol. Bioeng. 2016, 9, 12–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gliga, A.R.; Edoff, K.; Caputo, F.; Källman, T.; Blom, H.; Karlsson, H.L.; Ghibelli, L.; Traversa, E.; Ceccatelli, S.; Fadeel, B. Cerium Oxide Nanoparticles Inhibit Differentiation of Neural Stem Cells. Sci. Rep. 2017, 7, 9284. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.; Fisher, G.J.; Voorhees, J.J.; Quan, T. Actin Cytoskeleton Assembly Regulates Collagen Production via TGF-β Type II Receptor in Human Skin Fibroblasts. J. Cell. Mol. Med. 2018, 22, 4085–4096. [Google Scholar] [CrossRef] [Green Version]
- Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [Green Version]
- Tamimi, F.; Kumarasami, B.; Doillon, C.; Gbureck, U.; Le, D.; Lopez, E.; Barralet, J.E. Brushite—Collagen Composites for Bone Regeneration. Acta Biomater. 2008, 4, 1315–1321. [Google Scholar] [CrossRef]
- Mansour, S.F.; El-dek, S.I.; Ahmed, M.A.; Abd-Elwahab, S.M.; Ahmed, M.K. Effect of Preparation Conditions on the Nanostructure of Hydroxyapatite and Brushite Phases. Appl. Nanosci. 2016, 6, 991–1000. [Google Scholar] [CrossRef] [Green Version]
- Seydlová, M.; Teuberová, Z.; Dostálová, T.; Kríz, P.; Dvoránková, B.; Smetana, K.; Jelínek, M.; Kocourek, T. Influence of Hydroxyapatite Crystallinity on the Growth of Keratinocytes. Prague Med. Rep. 2008, 109, 23. [Google Scholar]
- Liu, L.; Wang, J.; Duan, S.; Chen, L.; Xiang, H.; Dong, Y.; Wang, W. Systematic Evaluation of Sericin Protein as a Substitute for Fetal Bovine Serum in Cell Culture. Sci. Rep. 2016, 6, 31516. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.; Wei, J.; Sun, J. Effects of Four Types of Hydroxyapatite Nanoparticles with Different Nanocrystal Morphologies and Sizes on Apoptosis in Rat Osteoblasts. J. Appl. Toxicol. 2012, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, A.C.; Stan, G.E.; Maidaniuc, A.; Miculescu, M.; Antoniac, I.V.; Ciocoiu, R.C.; Voicu, Ş.I.; Mitran, V.; Cîmpean, A.; Miculescu, F. Naturally-Derived Biphasic Calcium Phosphates through Increased Phosphorus-Based Reagent Amounts for Biomedical Applications. Materials 2019, 12, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arros, C.; Rouillon, T.; Veziers, J.; Malard, O.; Borget, P.; Daculsi, G. Bioactivity of Biphasic Calcium Phosphate Granules, the Control of a Needle-Like Apatite Layer Formation for Further Medical Device Developments. Front. Bioeng. Biotechnol. 2020, 7, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janny, S.; Bert, F.; Dondero, F.; Nicolas Chanoine, M.-H.; Belghiti, J.; Mantz, J.; Paugam-Burtz, C. Fatal Escherichia Coli Skin and Soft Tissue Infections in Liver Transplant Recipients: Report of Three Cases. Transpl. Infect. Dis. 2013, 15, E49–E53. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.V.B.; Oliveira, A.L.; Osajima, J.A.; Silva-Filho, E.C. Development of Composites Scaffolds with Calcium and Cerium-Hydroxyapatite and Gellan Gum. Ceram. Int. 2020, 46, 3811–3817. [Google Scholar] [CrossRef]
- Baskaran, P.; Udduttula, A.; Uthirapathy, V. Development and Characterisation of Novel Ce-Doped Hydroxyapatite–Fe3O4 Nanocomposites and Their in Vitro Biological Evaluations for Biomedical Applications. IET Nanobiotechnology 2018, 12, 138–146. [Google Scholar] [CrossRef]
- Ciobanu, G.; Maria Bargan, A.; Luca, C. New Cerium(IV)-Substituted Hydroxyapatite Nanoparticles: Preparation and Characterization. Ceram. Int. 2015, 41, 12192–12201. [Google Scholar] [CrossRef]
- Kawai, K.; Larson, B.J.; Ishise, H.; Carre, A.L.; Nishimoto, S.; Longaker, M.; Lorenz, H.P. Calcium-Based Nanoparticles Accelerate Skin Wound Healing. PLoS ONE 2011, 6, e27106. [Google Scholar] [CrossRef] [Green Version]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- WOUNDS. The Use of Novel New Multi-tissue Biomaterial Powder Focused on Cell Signaling in Wound Healing Applications: A Case Series. Available online: https://www.hmpgloballearningnetwork.com/site/wounds/poster/use-novel-new-multi-tissue-biomaterial-powder-focused-cell-signaling-wound-healing (accessed on 2 August 2021).
- Young, J.D.; Martel, J.; Young, D.; Young, A.; Hung, C.-M.; Young, L.; Chao, Y.-J.; Young, J.; Wu, C.-Y. Characterization of Granulations of Calcium and Apatite in Serum as Pleomorphic Mineralo-Protein Complexes and as Precursors of Putative Nanobacteria. PLoS ONE 2009, 4, e5421. [Google Scholar] [CrossRef] [Green Version]
- Takeda, S. Sericulture. In Encyclopedia of Insects; Elsevier: Amsterdam, The Netherlands, 2009; Volume 1, pp. 912–914. [Google Scholar] [CrossRef]
- Takechi, T.; Wada, R.; Fukuda, T.; Harada, K.; Takamura, H. Antioxidant Activities of Two Sericin Proteins Extracted from Cocoon of Silkworm (Bombyx Mori) Measured by DPPH, Chemiluminescence, ORAC and ESR Methods. Biomed. Rep. 2014, 2, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Q. Applications of Natural Silk Protein Sericin in Biomaterials. Biotechnol. Adv. 2002, 20, 91–100. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Expanded Applications, Shifting Paradigms and an Improved Understanding of Host–Biomaterial Interactions. Acta Biomater. 2013, 9, 4948–4955. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.; Silva, C.; Rocha, F.; Ferreira, A. The Axial Dispersion of Liquid Solutions and Solid Suspensions in Planar Oscillatory Flow Crystallizers. AIChE J. 2019, 65, e16683. [Google Scholar] [CrossRef]
- Baptista-Silva, S.; Borges, S.; Costa-Pinto, A.R.; Costa, R.; Amorim, M.; Dias, J.R.; Ramos, Ó.; Alves, P.; Granja, P.L.; Soares, R.; et al. In Situ Forming Silk Sericin-Based Hydrogel: A Novel Wound Healing Biomaterial. ACS Biomater. Sci. Eng. 2021, 7, 1573–1586. [Google Scholar] [CrossRef]
- Angel, R.; Petrova, E. Quant-iTTMPicoGreen® dsDNA Assay Kit (ThermoFisher). Available online: https://www.protocols.io/view/qant-it-picogreen-dsdna-quantification-bftfjnjn?step=12 (accessed on 26 July 2021).










| Wavenumber (cm−1) Functional Groups | HAp Reference | Commercial HAp | Sericin | CaP1-Nano | CaP2-Nano/Micro | CaP3-Micro | CaP-SS | CaP-SS-Ce |
|---|---|---|---|---|---|---|---|---|
| PO43− | 1087, ν3 | 1090 | - | - | - | - | - | - |
| 1032, ν3 | 1024 | 1020 | 1020 | 1020 | 1023 | |||
| 962, ν1 | 962 | 961 | 960 | 961 | - | |||
| 602, ν4 | 600 | 600 | 597 | 600 | 600 | |||
| 561, ν4 | 561 | 558 | 561 | 559 | 530 | |||
| 472, ν2 | - | - | - | - | - | |||
| HPO43− | - | - | - | - | - | 983 | - | - |
| 1053 | ||||||||
| P-O | - | - | - | - | - | 574 | - | - |
| 541 | ||||||||
| CO32− | 875 | 874 | - | 866 | 875 | 869 | 865 | - |
| 1410 | 1418 | - | - | - | ||||
| OH− | 631 | 630 | - | - | - | - | - | - |
| 3572 | 3571 | - | - | - | - | - | ||
| ⱱO-H | - | - | - | - | - | 3533 | ||
| 3477 | ||||||||
| 3151 | ||||||||
| H-O-H | - | - | - | - | - | 1647 | - | - |
| P=O | - | - | - | - | - | 1205 | ||
| 1099 | ||||||||
| P-O-P | 777 | |||||||
| 653 | ||||||||
| Amide I | - | - | 1657 | - | - | - | 1644 | 1644 |
| Amide II | - | - | 1551 | - | - | - | - | - |
| Amide III | - | - | 1251 | - | - | - | - | - |
| Sample | °2 Theta | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Miller Index | HAp Reference (JCPDS 00-009-0432) | Brushite (JCPDS 72-0713 | Commercial HAp | [17] | CaP1-Nano | CaP2-Nano/Micro | CaP3-Micro | CaP-SS | CaP-SS-Ce |
| 020 | - | 11.71 | - | 11.71 | 11.70 | - | - | ||
| 121 | 20.62 | 21.00 | 21.04 | ||||||
| 104 | 23.47 | - | 23.53 | ||||||
| 002 | 25.79 | - | 26.09 | 25.90 | 25.95 | 26.07 | - | 25.96 | |
| 141 | - | 29.15 | - | 29.33 | 29.38 | ||||
| 211 | 31.08 | - | 32.08 | 31.86 | 31.78 | 31.64 | - | 31.77 | |
| 112 | 32.20 | 32.41 | 32.20 | 32.26 | 32.29 | 32.27 | |||
| 300 | 32.92 | 33.17 | 32.90 | 33.73 | - | 33.79 | |||
| 121 | - | 34.19 | - | 34.18 | 34.21 | ||||
| 222 | 36.49 | - | 36.57 | ||||||
| 310 | 39.81 | - | 40.12 | 39.86 | 39.88 | 39.78 | - | 40.59 | |
| 152 | - | 41.01 | - | 41.67 | 41.69 | ||||
| 222 | 46.69 | - | 46.95 | 46.69 | 46.67 | 46.61 | - | 46.81 | |
| 213 | 49.43 | 49.74 | 48.16 | 49.82 | 50.31 | 49.60 | |||
| 143 | - | 50.75 | - | 50.25 | - | ||||
| 004 | 53.21 | - | 53.43 | 53.27 | 53.37 | 53.56 | - | 53.51 | |
| Calcium-Based Material | Oxygen (O) | Phosphate (P) | Calcium (Ca) | Cerium (Ce) | Ca/P Molar Ratio | ||||
|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | Weight (%) | Atomic (%) | ||
| Commercial HAp | 40.26 | 60.68 | 19.09 | 14.86 | 40.64 | 24.45 | - | ||
| CaP1-nano | 40.76 | 60.97 | 20.87 | 16.10 | 38.38 | 22.92 | 1.68 | ||
| CaP2-nano/micro | 35.19 | 55.24 | 22.45 | 18.20 | 42.37 | 26.44 | 1.69 | ||
| CaP3-micro | 45.75 | 65.32 | 22.37 | 16.50 | 31.88 | 18.17 | 1.23 | ||
| CaP-SS | 41.30 | 61.42 | 21.42 | 16.44 | 37.28 | 22.15 | 1.65 | ||
| CaP-SS-Ce | 30.38 | 58.78 | 18.67 | 18.65 | 20.00 | 15.44 | 30.51 | 6.74 | 1.01 |
| Experimental Conditions | Cell Viability (%) |
|---|---|
| Commercial HAp | 87.4 ± 27.5 |
| CaP1-nano | 90.9 ± 21.26 |
| CaP2-nano/micro | 79.4 ± 18.88 |
| CaP3-micro | 67.7 ± 2.26 |
| CaP-SS | 90.5 ± 28.74 |
| CaP-SS-Ce | 96.8 ± 24.56 |
| CaP1-Nano | CaP2-Nano/Micro | CaP3-Micro | CaP-SS | CaP-SS-Ce | Control | |
|---|---|---|---|---|---|---|
| Commercial HAp | ns | ** | ns | **** | **** | **** |
| CaP1-nano | *** | ns | **** | **** | **** | |
| CaP2-nano/micro | *** | ns | ns | ns | ||
| CaP3-micro | **** | **** | **** | |||
| CaP-SS | ns | ns | ||||
| CaP-SS-Ce | ns | |||||
| Experimental Conditions | Initial Reagents Concentration | Description | Physicochemical Characteristics | Frequency (Hz) | Amplitude (mm) | [Sericin] g/L | [Cerium] g/L |
|---|---|---|---|---|---|---|---|
| 1. | CaCl2.2H2O (0.02 M) | CaP1-nano | HAp nanoparticles | 1.9 | 4 | - | - |
| 2. | Na2HPO4 (0.012 M) | CaP2-nano/micro | HAp nano and microparticles | 4 | 4 | ||
| 3. | CaCl2.2H2O (0.2 M) | CaP3-micro | Brushite particles | 1.9 | 4 | ||
| Na2HPO4 (0.12 M) | |||||||
| 4. | CaCl2.2H2O (0.02 M) | CaP-SS | To evaluate | 0.1 | |||
| 5. | Na2HPO4 (0.012 M) | CaP-SS-Ce | 0.1 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veiga, A.; Magalhães, R.; Duarte, M.M.; Dias, J.R.; Alves, N.M.; Costa-Pinto, A.R.; Castro, F.; Rocha, F.; Oliveira, A.L. Continuous Production of Highly Tuned Silk/Calcium-Based Composites: Exploring New Pathways for Skin Regeneration. Molecules 2022, 27, 2249. https://doi.org/10.3390/molecules27072249
Veiga A, Magalhães R, Duarte MM, Dias JR, Alves NM, Costa-Pinto AR, Castro F, Rocha F, Oliveira AL. Continuous Production of Highly Tuned Silk/Calcium-Based Composites: Exploring New Pathways for Skin Regeneration. Molecules. 2022; 27(7):2249. https://doi.org/10.3390/molecules27072249
Chicago/Turabian StyleVeiga, Anabela, Rui Magalhães, Marta M. Duarte, Juliana R. Dias, Nuno M. Alves, Ana Rita Costa-Pinto, Filipa Castro, Fernando Rocha, and Ana L. Oliveira. 2022. "Continuous Production of Highly Tuned Silk/Calcium-Based Composites: Exploring New Pathways for Skin Regeneration" Molecules 27, no. 7: 2249. https://doi.org/10.3390/molecules27072249