Biological Activity of Extracts of Red and Yellow Fruits of Cornus mas L.—An In Vitro Evaluation of Antioxidant Activity, Inhibitory Activity against α-Glucosidase, Acetylcholinesterase, and Binding Capacity to Human Serum Albumin
Abstract
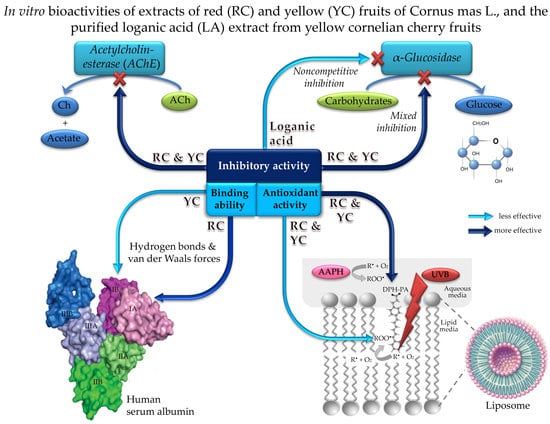
:1. Introduction
2. Results and Discussion
2.1. The Chemical Composition of Red and Yellow Fruits Extracts of Cornus mas L.
2.2. Antioxidant Activity
| Inducer Free Radical | Red Cornelian Cherries (μg/mL) | Yellow Cornelian Cherries (μg/mL) | L(+)-Ascorbic Acid (μg/mL) |
|---|---|---|---|
| AAPH | 12.60 ± 0.87 | 12.39 ± 0.96 | 22.80 ± 2.19 * |
| UVB | 26.48 ± 3.90 | 26.24 ± 2.11 | 115.3 ± 2.50 * |
2.3. Alpha-Glucosidase Inhibition
2.4. Acetylcholinesterase Inhibition
2.5. Binding to Human Serum Albumin
3. Materials and Methods
3.1. Materials
3.2. Plant Materials and Preparation of Cornelian Cherry Extracts
3.3. Identification of Compounds by Liquid Chromatography-Mass Spectrometry (LC-MS)
3.4. Determination of Compounds by HPLC
3.5. Liposome Preparation
3.6. Liposome Oxidation Assay–Spectrophotometric Method
3.7. Liposome Oxidation Assay–Fluorometric Method
3.8. α-Glucosidase Inhibitory Assay
3.9. Determination of α-Glucosidase Inhibition Type
3.10. Acetylcholinesterase Inhibition Assay
3.11. Binding to Human Serum Albumin
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Klymenko, S.; Kucharska, A.; Sokół-Łętowska, A.; Piórecki, N.; Przybylska, D.; Grygorieva, O. Iridoids, Flavonoids, and Antioxidant Capacity of Cornus mas, C. officinalis, and C. mas × C. officinalis Fruits. Biomolecules 2021, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Tural, S.; Koca, I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci. Hortic. 2008, 116, 362–366. [Google Scholar] [CrossRef]
- Han, Y.; Jung, H.W.; Park, Y.-K. Selective Therapeutic Effect of Cornus officinalis Fruits on the Damage of Different Organs in STZ-Induced Diabetic Rats. Am. J. Chin. Med. 2014, 42, 1169–1182. [Google Scholar] [CrossRef]
- Yamabe, N.; Kang, K.S.; Goto, E.; Tanaka, T.; Yokozawa, T. Beneficial Effect of Corni Fructus, a Constituent of Hachimi-jio-gan, on Advanced Glycation End-product-Mediated Renal Injury in Streptozotocin-Treated Diabetic Rats. Biol. Pharm. Bull. 2007, 30, 520–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwińska, M.E.; Melzig, M.F. Cornus mas and Cornus Officinalis—Analogies and Differences of Two Medicinal Plants Traditionally Used. Front. Pharmacol. 2018, 9, 894. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, Z.-L.; Chen, H.-B.; Wang, F.-S.; Lu, J.-H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czerwińska, M.E.; Bobińska, A.; Cichocka, K.; Buchholz, T.; Woliński, K.; Melzig, M.F. Cornus mas and Cornus officinalis—A Comparison of Antioxidant and Immunomodulatory Activities of Standardized Fruit Extracts in Human Neutrophils and Caco-2 Models. Plants 2021, 10, 2347. [Google Scholar] [CrossRef] [PubMed]
- Dinda, B.; Kyriakopoulos, A.M.; Dinda, S.; Zoumpourlis, V.; Thomaidis, N.S.; Velegraki, A.; Markopoulos, C.; Dinda, M. Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J. Ethnopharmacol. 2016, 193, 670–690. [Google Scholar] [CrossRef]
- Sugihara, H.; Nagao, M.; Harada, T.; Nakajima, Y.; Tanimura-Inagaki, K.; Okajima, F.; Tamura, H.; Inazawa, T.; Otonari, T.; Kawakami, M.; et al. Comparison of three α-glucosidase inhibitors for glycemic control and bodyweight reduction in Japanese patients with obese type 2 diabetes. J. Diabetes Investig. 2013, 5, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, J.; Tian, J.; Chen, S.; Ye, X.; Hu, Y.; Chen, J. Inhibitory mechanism of novel allosteric inhibitor, Chinese bayberry (Myrica rubra Sieb. et Zucc.) leaves proanthocyanidins against α-glucosidase. J. Funct. Foods 2019, 56, 286–294. [Google Scholar] [CrossRef]
- Kazeem, M.I.; Adamson, J.O.; Ogunwande, I.A. Modes of Inhibition ofα-Amylase andα-Glucosidase by Aqueous Extract of Morinda lucida Benth Leaf. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bila, I.; Dzydzan, O.; Brodyak, I.; Sybirna, N. Agmatine prevents oxidative-nitrative stress in blood leukocytes under streptozotocin-induced diabetes mellitus. Open Life Sci. 2019, 14, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Joubert, E.; Muller, C.J.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-d-glucoside. Nutr. Metab. 2017, 14, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Patel, R.; Rinker, L.; Peng, J.; Chilian, W.M. Reactive Oxygen Species: The Good and the Bad. In Reactive Oxygen Species (ROS) in Living Cells; Filip, C., Albu, E., Eds.; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Catalá, A.; Díaz, M. Editorial: Impact of Lipid Peroxidation on the Physiology and Pathophysiology of Cell Membranes. Front. Physiol. 2016, 7, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch. Biochem. Biophys. 1991, 288, 481–487. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Abirami, A.; Sinsinwar, S.; Rajalakshmi, P.; Brindha, P.; Rajesh, Y.B.R.D.; Vadivel, V. Antioxidant and cytoprotective properties of loganic acid isolated from seeds of Strychnos potatorum L. against heavy metal induced toxicity in PBMC model. Drug Chem. Toxicol. 2019, 45, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.K.; Krishnakumari, S.; Chandrakala, S. Evaluation of antioxidant properties of different parts of Amorphophallus commutatus, an endemic aroid of Western Ghats, South India. Int. J. Pharm. Bio. Sci. 2012, 3, 443–455. [Google Scholar]
- Ravi, K.; Ramachandran, B.; Subramanian, S. Protective Effect of Eugenia jambolana Seed Kernel on Tissue Antioxidants in Streptozotocin-Induced Diabetic Rats. Biol. Pharm. Bull. 2004, 27, 1212–1217. [Google Scholar] [CrossRef] [Green Version]
- Saha, S.; Panieri, E.; Suzen, S.; Saso, L. The Interaction of Flavonols with Membrane Components: Potential Effect on Antioxidant Activity. J. Membr. Biol. 2020, 253, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Hendrich, A.B. Flavonoid-membrane interactions: Possible consequences for biological effects of some polyphenolic compounds1. Acta Pharmacol. Sin. 2006, 27, 27–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dzydzan, O.; Brodyak, I.; Sokół-Łętowska, A.; Kucharska, A.Z.; Sybirna, N. Loganic Acid, an Iridoid Glycoside Extracted from Cornus mas L. Fruits, Reduces of Carbonyl/Oxidative Stress Biomarkers in Plasma and Restores Antioxidant Balance in Leukocytes of Rats with Streptozotocin-Induced Diabetes Mellitus. Life 2020, 10, 349. [Google Scholar] [CrossRef]
- Minatel, I.O.; Borges, C.V.; Ferreira, M.I.; Gomez, H.A.G.; Chen, C.-Y.O.; Lima, G.P.P. Phenolic Compounds: Functional Properties, Impact of Processing and Bioavailability. In Phenolic Compounds; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; InTech: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [Green Version]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea Var. Kamtschatica sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-X.; Liu, C.-T.; Liu, Q.-B.; Ren, J.; Li, L.-Z.; Huang, X.-X.; Wang, Z.-Z.; Song, S.-J. Iridoid glycosides from the flower buds of Lonicera japonica and their nitric oxide production and α-glucosidase inhibitory activities. J. Funct. Foods 2015, 18, 512–519. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohnishi, T. The Significance of the Study about the Biological Effects of Solar Ultraviolet Radiation using the Exposed Facility on the International Space Station. Biol. Sci. Space 2004, 18, 255–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strugała, P.; Cyboran-Mikołajczyk, S.; Dudra, A.; Mizgier, P.; Kucharska, A.Z.; Olejniczak, T.; Gabrielska, J. Biological Activity of Japanese Quince Extract and Its Interactions with Lipids, Erythrocyte Membrane, and Human Albumin. J. Membr. Biol. 2016, 249, 393–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, M.; García, A.F.; de Madariaga, M.A.; Sagristá, M.L.; Casadó, F.J.; Mora, M. Interaction of tocopherols and phenolic compounds with membrane lipid components: Evaluation of their antioxidant activity in a liposomal model system. Life Sci. 2003, 72, 2337–2360. [Google Scholar] [CrossRef]
- Fadel, O.; EL Kirat, K.; Morandat, S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blagojević, B.; Agić, D.; Serra, A.T.; Matić, S.; Matovina, M.; Bijelić, S.; Popović, B.M. An in vitro and in silico evaluation of bioactive potential of cornelian cherry (Cornus mas L.) extracts rich in polyphenols and iridoids. Food Chem. 2020, 335, 127619. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panahi, F.; Yousefi, R.; Mehraban, M.H.; Khalafi-Nezhad, A. Synthesis of new pyrimidine-fused derivatives as potent and selective antidiabetic α-glucosidase inhibitors. Carbohydr. Res. 2013, 380, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ogunwande, I.; Matsui, T.; Fujise, T.; Matsumoto, K. ALPHA.-Glucosidase Inhibitory Profile of Nigerian Medicinal Plants in Immobilized Assay System. Food Sci. Technol. Res. 2007, 13, 169–172. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, J.; Mizuhata, K.; Sato, E.; Nishioka, T.; Aoyama, Y.; Kasai, T. 6-Hydroxyflavonoids as α-Glucosidase Inhibitors from Marjoram (Origanum majorana) Leaves. Biosci. Biotechnol. Biochem. 2003, 67, 445–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasouli, H.; Hosseini-Ghazvini, S.M.; Adibi, H.; Khodarahmi, R. Differential α-amylase/α-glucosidase inhibitory activities of plant-derived phenolic compounds: A virtual screening perspective for the treatment of obesity and diabetes. Food Funct. 2017, 8, 1942–1954. [Google Scholar] [CrossRef] [PubMed]
- Ning, Z.-W.; Zhai, L.-X.; Huang, T.; Peng, J.; Hu, D.; Xiao, H.-T.; Wen, B.; Lin, C.-Y.; Zhao, L.; Bian, Z.-X. Identification of α-glucosidase inhibitors from Cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019, 10, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase inhibition by flavonoids: An in vitro and in silico structure–activity relationship study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef] [Green Version]
- Szczepaniak, O.; Cielecka-Piontek, J.; Kobus-Cisowska, J. Hypoglycaemic, antioxidative and phytochemical evaluation of Cornus mas varieties. Eur. Food Res. Technol. 2020, 247, 183–191. [Google Scholar] [CrossRef]
- David, L.; Danciu, V.; Moldovan, B.; Filip, A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants 2019, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Asaduzzaman; Uddin, J.; Kader, M.; Alam, A.K.; Rahman, A.A.; Rashid, M.; Kato, K.; Tanaka, T.; Takeda, M.; Sadik, G. In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: Implications for the treatment of Alzheimer’s disease. Psychogeriatrics 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Sundaramoorthy, P.M.K.; Packiam, K.K. In vitro enzyme inhibitory and cytotoxic studies with Evolvulus alsinoides (Linn.) Linn. Leaf extract: A plant from Ayurveda recognized as Dasapushpam for the management of Alzheimer’s disease and diabetes mellitus. BMC Complement. Med. Ther. 2020, 20, 1–12. [Google Scholar] [CrossRef]
- Wang, Z.; Tu, Z.; Xie, X.; Cui, H.; Kong, K.; Zhang, L. Perilla frutescens Leaf Extract and Fractions: Polyphenol Composition, Antioxidant, Enzymes (α-Glucosidase, Acetylcholinesterase, and Tyrosinase) Inhibitory, Anticancer, and Antidiabetic Activities. Foods 2021, 10, 315. [Google Scholar] [CrossRef] [PubMed]
- Gutierres, J.M.; Carvalho, F.; Schetinger, M.R.C.; Agostinho, P.; Marisco, P.C.; Vieira, J.M.; Rosa, M.M.; Bohnert, C.; Rubin, M.; Morsch, V.M.; et al. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int. J. Dev. Neurosci. 2013, 33, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase Activities of Selected Polyphenols—A Short Report. Pol. J. Food Nutr. Sci. 2014, 64, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Papandreou, M.A.; Dimakopoulou, A.; Linardaki, Z.I.; Cordopatis, P.; Klimis-Zacas, D.; Margarity, M.; Lamari, F.N. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behav. Brain Res. 2009, 198, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Boespflug, E.L.; Fleck, D.E.; Stein, A.L.; Wightman, J.D.; Shidler, M.D.; Sadat-Hossieny, S. Concord Grape Juice Supplementation and Neurocognitive Function in Human Aging. J. Agric. Food Chem. 2012, 60, 5736–5742. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.; Charlton, K.; Roodenrys, S.; Batterham, M.; Potter, J.; Traynor, V.; Gilbert, H.; Morgan, O.; Richards, R. Consumption of anthocyanin-rich cherry juice for 12 weeks improves memory and cognition in older adults with mild-to-moderate dementia. Eur. J. Nutr. 2015, 56, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of Various Phenolic Acids and Flavonoid Derivatives for their Anticholinesterase Potential. Z. Naturforsch. C. J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Lin, X.; Zhang, J.; Chol, K.S.; Huang, W.; Yang, B.; He, Q.; Hu, Y. Design, synthesis and evaluation of flavonoid derivatives as potent AChE inhibitors. Bioorg. Med. Chem. 2009, 17, 6692–6698. [Google Scholar] [CrossRef] [PubMed]
- Codorniu-Hernández, E.; Rolo-Naranjo, A.; Montero-Cabrera, L.A. Theoretical affinity order among flavonoids and amino acid residues: An approach to understand flavonoid–protein interactions. J. Mol. Struct. THEOCHEM 2007, 819, 121–129. [Google Scholar] [CrossRef]
- Caraceni, P.; Tufoni, M.; Bonavita, M.E. Clinical use of albumin. Blood Transfus. 2013, 11, s18–s25. [Google Scholar] [CrossRef] [PubMed]
- Chaves, O.; Echevarria, Á; Esteves-Souza, A.; Maciel, M.; Netto-Ferreira, J. In vitro Analysis of the Interaction between Human Serum Albumin and Semi-Synthetic Clerodanes. J. Braz. Chem. Soc. 2018, 29, 1786–1795. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 5th ed.; Plenum Press: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Demchenko, P.D.A.P. Spectroscopic Properties of Protein Chromophores. In Ultraviolet Spectroscopy of Proteins; Springer: Berlin/Heidelberg, Germany, 1986; pp. 5–26. [Google Scholar] [CrossRef]
- Finding Binding Sites and Diffusion Limited Rates. Available online: https://neherlab.org/20171024_theoretical_biophysics.html (accessed on 24 October 2017).
- Pal, S.; Saha, C. A review on structure–affinity relationship of dietary flavonoids with serum albumins. J. Biomol. Struct. Dyn. 2013, 32, 1132–1147. [Google Scholar] [CrossRef] [PubMed]
- Rimac, H.; Debeljak, Ž; Šakić, D.; Weitner, T.; Gabričević, M.; Vrček, V.; Zorc, B.; Bojić, M. Structural and electronic determinants of flavonoid binding to human serum albumin: An extensive ligand-based study. RSC Adv. 2016, 6, 75014–75022. [Google Scholar] [CrossRef] [Green Version]
- Strugała, P.; Tronina, T.; Huszcza, E.; Gabrielska, J. Bioactivity In Vitro of Quercetin Glycoside Obtained in Beauveria bassiana Culture and Its Interaction with Liposome Membranes. Molecules 2017, 22, 1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tronina, T.; Strugała, P.; Popłoński, J.; Włoch, A.; Sordon, S.; Bartmańska, A.; Huszcza, E. The Influence of Glycosylation of Natural and Synthetic Prenylated Flavonoids on Binding to Human Serum Albumin and Inhibition of Cyclooxygenases COX-1 and COX-2. Molecules 2017, 22, 1230. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Cao, H.; Wang, Y.; Zhao, J.; Wei, X. Glycosylation of Dietary Flavonoids Decreases the Affinities for Plasma Protein. J. Agric. Food Chem. 2009, 57, 6642–6648. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, C.; Guo, Y.; Yu, H.; Sun, M.-Z.; Greenaway, F. Comparative Binding Affinities of Flavonoid Phytochemicals with Bovine Serum Albumin. Iran J. Pharm. Res. 2014, 13, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Kanakis, C.; Tarantilis, P.; Polissiou, M.; Diamantoglou, S.; Tajmir-Riahi, H. Antioxidant flavonoids bind human serum albumin. J. Mol. Struct. 2006, 798, 69–74. [Google Scholar] [CrossRef]
- Shafreen, R.B.; Dymerski, T.; Namieśnik, J.; Jastrzębski, Z.; Vearasilp, S.; Gorinstein, S. Interaction of human serum albumin with volatiles and polyphenols from some berries. Food Hydrocoll. 2017, 72, 297–303. [Google Scholar] [CrossRef]
- Yousefi, R.; Taheri-Kafrani, A.; Nabavizadeh, S.M.; Pouryasin, Z.; Shahsavani, M.B.; Khoshaman, K.; Rashidi, M. The binding assessment with human serum albumin of novel six-coordinate Pt(IV) complexes, containing bidentate nitrogen donor/methyl ligands. Mol. Biol. Res. Commun. 2015, 4, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Gabrielska, J. Activity of blackcurrant and chokeberry extracts and two major cyanidin glycosides against lipid membrane oxidation and their binding nproperties to albumin. Acta Pol. Pharm. 2017, 74, 676–687. [Google Scholar] [PubMed]
- Gabrielska, J.; Oszmiański, J. Antioxidant Activity of Anthocyanin Glycoside Derivatives Evaluated by the Inhibition of Liposome Oxidation. Z. Naturforsch. C. J. Biosci. 2005, 60, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Strugała, P.; Dudra, A.; Gabrielska, J. Interaction between Mimic Lipid Membranes and Acylated and Nonacylated Cyanidin and Its Bioactivity. J. Agric. Food Chem. 2016, 64, 7414–7422. [Google Scholar] [CrossRef]
- Strugała, P.; Urbaniak, A.; Kuryś, P.; Włoch, A.; Kral, T.; Ugorski, M.; Hof, M.; Gabrielska, J. Antitumor and antioxidant activities of purple potato ethanolic extract and its interaction with liposomes, albumin and plasmid DNA. Food Funct. 2020, 12, 1271–1290. [Google Scholar] [CrossRef] [PubMed]
- Betigeri, S.; Thakur, A.; Raghavan, K. Use of 2,2?-Azobis(2-Amidinopropane) Dihydrochloride as a Reagent Tool for Evaluation of Oxidative Stability of Drugs. Pharm. Res. 2005, 22, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-M.; Jeong, Y.-K.; Wang, M.-H.; Lee, W.-Y.; Rhee, H.-I. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Houghton, P.; Soumyanath, A. α-Amylase inhibitory activity of some Malaysian plants used to treat diabetes; with particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry: International Edition, 7th ed.; W.H. Freeman: New York, NY, USA, 2017; pp. 202–212. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Jin, H.; Nguyen, T.; Go, M. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Properties of Functionalized Tetrahy droacridines and Related Analogs. Med. Chem. 2014, 4, 10. [Google Scholar] [CrossRef] [Green Version]







| Extracts | Inhibitory Potency of Cornelian Cherry Extracts (IC50, µg/mL) |
|---|---|
| RC | 25.68 ± 0.37 |
| YC | 28.46 ± 0.36 * |
| LA | 211.56 ± 3.84 ** |
| Acarbose # | 5.68 × 103 |
| Extracts | Concentration (mg/mL) | Inhibition (%) |
|---|---|---|
| RC | 1.0 | 70.1 ± 5.0 ** |
| YC | 1.0 | 58.4 ± 0.4 * |
| Neostigmine # | 5.1 × 10−5 | 50.0 ± 3.3 |
| Compound | T (K) | KSV (mL/g) | Kb (mL/g) | n | ∆G (kJ/g·mL−1) | ∆H (kJ/g·mL−1) | ∆S J/(g·mL−1·K) |
|---|---|---|---|---|---|---|---|
| RC | 295 | 30.91 × 103 | 3.705 × 104 | 1.05 | −25.72 | −24.88 | 30.04 |
| 300 | 29.51 × 103 | 2.985 × 104 | 0.99 | −25.67 | |||
| 305 | 28.78 × 103 | 2.505 × 104 | 0.93 | −25.64 | |||
| 310 | 27.82 × 103 | 2.128 × 104 | 0.87 | −25.63 | |||
| 315 | 27.04 × 103 | 1.933 × 104 | 0.83 | −25.81 | |||
| YC | 295 | 16.52 × 103 | 1.752 × 104 | 1.09 | −23.95 | −21.72 | 11.17 |
| 300 | 13.59 × 103 | 1.407 × 104 | 1.01 | −23.75 | |||
| 305 | 12.22 × 103 | 1.277 × 104 | 0.97 | −23.87 | |||
| 310 | 11.52 × 103 | 1.001 × 104 | 0.92 | −23.72 | |||
| 315 | 10.85 × 103 | 0.819 × 104 | 0.85 | −23.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzydzan, O.; Brodyak, I.; Strugała-Danak, P.; Strach, A.; Kucharska, A.Z.; Gabrielska, J.; Sybirna, N. Biological Activity of Extracts of Red and Yellow Fruits of Cornus mas L.—An In Vitro Evaluation of Antioxidant Activity, Inhibitory Activity against α-Glucosidase, Acetylcholinesterase, and Binding Capacity to Human Serum Albumin. Molecules 2022, 27, 2244. https://doi.org/10.3390/molecules27072244
Dzydzan O, Brodyak I, Strugała-Danak P, Strach A, Kucharska AZ, Gabrielska J, Sybirna N. Biological Activity of Extracts of Red and Yellow Fruits of Cornus mas L.—An In Vitro Evaluation of Antioxidant Activity, Inhibitory Activity against α-Glucosidase, Acetylcholinesterase, and Binding Capacity to Human Serum Albumin. Molecules. 2022; 27(7):2244. https://doi.org/10.3390/molecules27072244
Chicago/Turabian StyleDzydzan, Olha, Iryna Brodyak, Paulina Strugała-Danak, Angelika Strach, Alicja Z. Kucharska, Janina Gabrielska, and Natalia Sybirna. 2022. "Biological Activity of Extracts of Red and Yellow Fruits of Cornus mas L.—An In Vitro Evaluation of Antioxidant Activity, Inhibitory Activity against α-Glucosidase, Acetylcholinesterase, and Binding Capacity to Human Serum Albumin" Molecules 27, no. 7: 2244. https://doi.org/10.3390/molecules27072244