A Comprehensive Analysis of Microflora and Metabolites in the Development of Ulcerative Colitis into Colorectal Cancer Based on the Lung–Gut Correlation Theory
Abstract
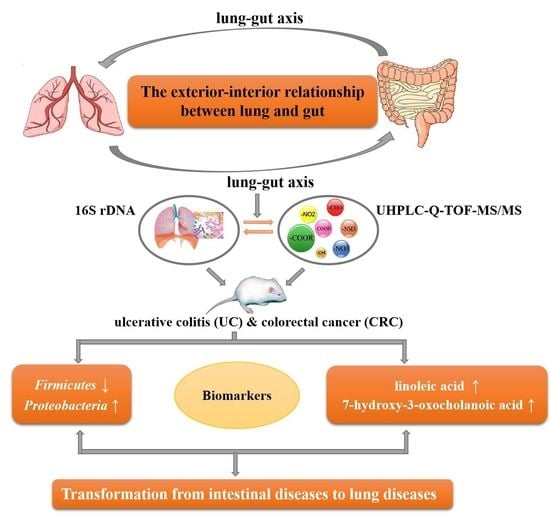
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animal and Modeling Process
2.3. Sample Collection and Preparation
2.4. Nontargeted Metabolomics by UHPLC–Q-TOF-MS/MS
2.5. Microbial DNA Extraction and Sequencing
2.6. Statistical Analysis of Data
3. Results
3.1. Metabolomics Data Analysis
3.2. Intestinal Microflora Analysis
3.3. Correlation between the Metabolite and Pulmonary–Intestinal Microecology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, R.; Wang, K.; Gao, L.; Gao, W. TIMP1 Is a Potential Key Gene Associated With The Pathogenesis And Prognosis of Ulcerative Colitis-Associated Colorectal Cancer. Onco Targets Ther. 2019, 12, 8895–8904. [Google Scholar] [CrossRef] [PubMed]
- Bopanna, S.; Ananthakrishnan, A.N.; Kedia, S.; Yajnik, V.; Ahuja, V. Risk of colorectal cancer in Asian patients with ulcerative colitis: A systematic review and meta-analysis. Lancet Gastroenterol. 2017, 2, 269–276. [Google Scholar]
- Testa, U.; Pelosi, E.; Castelli, G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med. Sci. 2018, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Chen, S.Y.; Lin, C.C. Theoretical basis, application, reliability, and sample size estimates of a Meridian Energy Analysis Device for Traditional Chinese Medicine Research. Clinics 2017, 72, 254–257. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhuang, Y. Modern interpretation of “exterior-interior correlation between lung and large intestine” theory in acute and critical cases. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2020, 32, 1040–1044. [Google Scholar]
- Zhong, X.G.; Zheng, F.J.; Li, Y.H.; Xu, H.; Wang, Q.; Liu, Y.C.; Liu, M.; Wu, R.H.; Gao, Y.S.; Zhang, S.J.; et al. Specific Link between Lung and Large Intestine: A New Perspective on Neuropeptide Secretion in Lung with Herbal Laxative Stimulation. Evid. Based Complement. Alternat. Med. 2013, 2013, 547837. [Google Scholar] [CrossRef]
- Yin, L.M.; Zhang, G.Q.; Yan, X.K.; Wang, Y.; Xu, Y.D.; Yang, Y.Q. An In Vivo and In Vitro Evaluation of the Mutual Interactions between the Lung and the Large Intestine. Evid.-Based Complement. Alternat. 2013, 2013, 695641. [Google Scholar] [CrossRef]
- Labarca, G.; Drake, L.; Horta, G.; Jantz, M.A.; Mehta, H.J.; Fernandez-Bussy, S.; Folch, E.; Majid, A.; Picco, M. Association between inflammatory bowel disease and chronic obstructive pulmonary disease: A systematic review and meta-analysis. BMC Pulm. Med. 2019, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Han, X.; Zhou, T. A case report and literature review of small intestinal metastasis of large cell lung cancer. Zhongguo Fei Ai Za Zhi 2010, 13, 655–658. [Google Scholar]
- Keely, S.; Talley, N.J.; Hansbro, P.M. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol. 2012, 5, 7–18. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Kumamoto, Y.; Peaper, D.R.; Ho, J.H.; Murray, T.S.; Iwasaki, A. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc. Natl. Acad. Sci. USA 2011, 108, 5354–5359. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Jiang, Z.Y.; Sun, Y.F.; Yu, B.; Chen, J.; Dai, C.Q.; Wu, X.L.; Tang, X.L.; Chen, X.Y. Microbiota Regulates the TLR7 Signaling Pathway Against Respiratory Tract Influenza A Virus Infection. Curr. Microbiol. 2013, 67, 414–422. [Google Scholar] [CrossRef]
- Chakradhar, S. A curious connection: Teasing apart the link between gut microbes and lung disease. Nat. Med. 2017, 23, 402–404. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Misra, B.B.; Liang, L.; Bi, D.X.; Weng, W.H.; Wu, W.; Cai, S.J.; Qin, H.L.; Goel, A.; Li, X.X.; et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019, 9, 4101–4114. [Google Scholar] [CrossRef]
- Liu, T.H.; Zhang, C.Y.; Din, A.U.; Li, N.; Wang, Q.; Yu, J.Z.; Xu, Z.Y.; Li, C.X.; Zhang, X.M.; Yuan, J.L.; et al. Bacterial association and comparison between lung and intestine in rats. Biosci. Rep. 2020, 40, BSR20191570. [Google Scholar] [CrossRef]
- Barcik, W.; Boutin, R.C.T.; Sokolowska, M.; Finlay, B.B. The Role of Lung and Gut Microbiota in the Pathology of Asthma. Immunity 2020, 52, 241–255. [Google Scholar] [CrossRef]
- Wypych, T.P.; Wickramasinghe, L.C.; Marsland, B.J. The influence of the microbiome on respiratory health. Nat. Immunol. 2019, 20, 1279–1290. [Google Scholar] [CrossRef]
- Tamburini, S.; Clemente, J.C. Gut microbiota: Neonatal gut microbiota induces lung immunity against pneumonia. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 263–264. [Google Scholar] [CrossRef]
- Hu, Y.F.; Feng, Y.Q.; Wu, J.N.; Liu, F.; Zhang, Z.G.; Hao, Y.N.; Liang, S.H.; Li, B.X.; Li, J.; Lv, N.; et al. The Gut Microbiome Signatures Discriminate Healthy from Pulmonary Tuberculosis Patients. Front. Cell. Infect. Microbiol. 2019, 9, 90. [Google Scholar] [CrossRef]
- Li, B.; Yin, G.F.; Wang, Y.L.; Tan, Y.M.; Huang, C.L.; Fan, X.M. Impact of fecal microbiota transplantation on TGF-beta1/Smads/ERK signaling pathway of endotoxic acute lung injury in rats. 3 Biotech 2020, 10, 52. [Google Scholar] [CrossRef]
- Tang, Q.; Cang, S.; Jiao, J.; Rong, W.; Xu, H.; Bi, K.; Li, Q.; Liu, R. Integrated study of metabolomics and gut metabolic activity from ulcerative colitis to colorectal cancer: The combined action of disordered gut microbiota and linoleic acid metabolic pathway might fuel cancer. J. Chromatogr. A 2020, 1629, 461503. [Google Scholar] [CrossRef] [PubMed]
- Romagnolo, D.F.; Donovan, M.G.; Doetschman, T.C.; Selmin, O.I. n-6 Linoleic Acid Induces Epigenetics Alterations Associated with Colonic Inflammation and Cancer. Nutrients 2019, 11, 171. [Google Scholar] [CrossRef] [PubMed]
- Tjonneland, A.; Overvad, K.; Bergmann, M.M.; Boeing, H.; Nagel, G.; Linseisen, J.; Hallmans, G.; Palmqvist, R.; Sjodin, H.; Hagglund, G.; et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: A nested case-control study within a European prospective cohort study. Gut 2009, 58, 1606–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildreth, K.; Kodani, S.D.; Hammock, B.D.; Zhao, L. Cytochrome P450-derived linoleic acid metabolites EpOMEs and DiHOMEs: A review of recent studies. J. Nutr. Biochem. 2020, 86, 108484. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Singh, S.; Leishangthem, G.D.; Aich, J.; Kumar, M.; Khanna, K.; Singh, V.P.; et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci. Rep. 2013, 3, 1349. [Google Scholar] [CrossRef]
- Pamungkas, A.D.; Medriano, C.A.; Sim, E.; Lee, S.; Park, Y.H. A pilot study identifying a potential plasma biomarker for determining EGFR mutations in exons 19 or 21 in lung cancer patients. Mol. Med. Rep. 2017, 15, 4155–4161. [Google Scholar] [CrossRef]
- Patil, M.D.; Bhaumik, J.; Babykutty, S.; Banerjee, U.C.; Fukumura, D. Arginine dependence of tumor cells: Targeting a chink in cancer’s armor. Oncogene 2016, 35, 4957–4972. [Google Scholar] [CrossRef]
- Chen, C.L.; Hsu, S.C.; Ann, D.K.; Yen, Y.; Kung, H.J. Arginine Signaling and Cancer Metabolism. Cancers 2021, 13, 3541. [Google Scholar] [CrossRef]
- Geiger, R.; Rieckmann, J.C.; Wolf, T.; Basso, C.; Feng, Y.; Fuhrer, T.; Kogadeeva, M.; Picotti, P.; Meissner, F.; Mann, M.; et al. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell 2016, 167, 829–842.e13. [Google Scholar] [CrossRef]
- Xiong, L.; Teng, J.L.; Botelho, M.G.; Lo, R.C.; Lau, S.K.; Woo, P.C. Arginine Metabolism in Bacterial Pathogenesis and Cancer Therapy. Int. J. Mol. Sci. 2016, 17, 363. [Google Scholar] [CrossRef]
- de Souza, H.S.P. Purinergic Signalling in Inflammatory Bowel Diseases. Purinerg. Signal. 2018, 14, S19. [Google Scholar]
- Yin, J.; Ren, W.; Huang, X.; Deng, J.; Li, T.; Yin, Y. Potential Mechanisms Connecting Purine Metabolism and Cancer Therapy. Front. Immunol. 2018, 9, 1697. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Rhee, C.K.; Kim, K.; Ra, S.W.; Lee, J.H.; Jung, K.S.; Yoo, K.H.; Kim, Y.I.; Kim, D.K. Prescription Status and Clinical Outcomes of Methylxanthines and Leukotriene Receptor Antagonists in Mild-to-Moderate Chronic Obstructive Pulmonary Disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 2639–2647. [Google Scholar] [CrossRef] [PubMed]
- Sarria, B.; Gomez-Juaristi, M.; Martinez Lopez, S.; Garcia Cordero, J.; Bravo, L.; Mateos Briz, M.R. Cocoa colonic phenolic metabolites are related to HDL-cholesterol raising effects and methylxanthine metabolites and insoluble dietary fibre to anti-inflammatory and hypoglycemic effects in humans. PeerJ 2020, 8, e9953. [Google Scholar] [CrossRef]
- Ryu, H.M.; Kim, Y.J.; Oh, E.J.; Oh, S.H.; Choi, J.Y.; Cho, J.H.; Kim, C.D.; Park, S.H.; Kim, Y.L. Hypoxanthine induces cholesterol accumulation and incites atherosclerosis in apolipoprotein E-deficient mice and cells. J. Cell. Mol. Med. 2016, 20, 2160–2172. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.; Yin, L.; Du, Y.; Zhang, S.; Suo, J. Association of serum levels of deoxyribose 1-phosphate and S-lactoylglutathione with neoadjuvant chemotherapy sensitivity in patients with gastric cancer: A metabolomics study. Oncol. Lett. 2020, 19, 2231–2242. [Google Scholar] [CrossRef]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef]
- Brown, I.A.M.; Gulbransen, B.D. The antioxidant glutathione protects against enteric neuron death in situ, but its depletion is protective during colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G39–G52. [Google Scholar] [CrossRef]
- Xin, X.F.; Dai, W.; Wu, J.; Fang, L.P.; Zhao, M.; Zhang, P.P.; Chen, M. Mechanism of intestinal mucosal barrier dysfunction in a rat model of chronic obstructive pulmonary disease: An observational study. Exp. Ther. Med. 2016, 12, 1331–1336. [Google Scholar] [CrossRef]
- Segal, L.N.; Clemente, J.C.; Tsay, J.C.J.; Koralov, S.B.; Keller, B.C.; Wu, B.G.; Li, Y.H.; Shen, N.; Ghedin, E.; Morris, A.; et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat. Microbiol. 2016, 1, 16031. [Google Scholar] [CrossRef]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef] [Green Version]




| No. | t(R) | ESI Mode | m/z | Identification | Fomular | VIP1 | VIP2 | Fold1 | Fold2 | Source | Change Trend | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UC/Con. | CRC/Con. | CRC/UC | |||||||||||
| 1 | 3.1 | + | 153.1354 | 3-Hydroxyanthranilic acid | C7H7NO3 | 1.29 | 3.55 | BALF UC | ↓ | ||||
| 2 | 3.3 | + | 166.1374 | 3-Methylxanthine | C6H6N4O2 | 1.69 | 1.74 | 7.02 | 12.95 | BALF UC&CRC | ↓ | ↓ | ↓ |
| 3 | 9.5 | + | 197.1879 | L-Dopa | C8H15N5O | 1.49 | 0.43 | BALF UC | ↑ | ||||
| 4 | 6.3 | + | 282.4614 | Oleic acid | C18H34O2 | 1.25 | 0.16 | BALF UC | ↑ | ||||
| 5 | 9.1 | + | 364.4758 | Tetrahydrocortisone | C21H32O5 | 1.30 | 1.71 | 3.66 | 6.35 | BALF UC&CRC | ↓ | ↓ | ↓ |
| 6 | 3.1 | + | 365.3900 | Isopentenyladenine-9-N-glucoside | C16H23N5O5 | 1.84 | 68.03 | BALF CRC | ↓ | ||||
| 7 | 6.3 | + | 387.5140 | 4-Hydroxytamoxifen | C26H29NO2 | 1.25 | 0.08 | BALF UC | ↑ | ||||
| 8 | 6.4 | + | 432.2784 | N-Stearoyl phenylalanine | C27H45NO3 | 1.22 | 0.06 | BALF UC | ↑ | ||||
| 9 | 11.5 | + | 495.6301 | LysoPC(16:0/0:0) | C24H50NO7P | 1.02 | 0.45 | BALF UC | ↑ | ||||
| 10 | 6.4 | + | 515.7030 | Taurocholic acid | C26H45NO7S | 1.60 | 3.85 | BALF UC | ↓ | ||||
| 11 | 12.1 | − | 201.2628 | N-Acetylaminooctanoic acid | C10H19NO3 | 1.13 | 1.69 | 8.30 | 5.09 | BALF UC&CRC | ↓ | ↓ | ↓ |
| 12 | 3.0 | − | 218.2518 | N-Acetylserotonin | C12H14N2O2 | 1.55 | 8.30 | BALF CRC | ↑ | ||||
| 13 | 11.3 | − | 258.3538 | Tetradecanedioic acid | C14H26O4 | 1.37 | 0.19 | BALF CRC | ↑ | ||||
| 14 | 10.8 | − | 278.4296 | alpha-Linolenic acid | C18H30O2 | 1.04 | 0.31 | BALF CRC | ↑ | ||||
| 15 | 14.6 | − | 280.4455 | (9E,11E)-Octadecadienoic acid | C18H32O2 | 1.80 | 3.23 | 4.30 | 13428.75 | BALF UC&CRC | ↓ | ↓ | ↓ |
| 16 | 6.6 | − | 283.2407 | Guanosine | C10H13N5O5 | 1.16 | 0.23 | BALF CRC | ↑ | ||||
| 17 | 15.4 | − | 282.4614 | Oleic acid | C18H34O2 | 2.39 | 0.01 | BALF UC | ↑ | ||||
| 18 | 16.5 | − | 284.4772 | Stearic acid | C18H36O2 | 2.67 | 0.01 | BALF UC | ↑ | ||||
| 19 | 16.5 | − | 286.4070 | Hexadecanedioic acid | C16H30O4 | 1.86 | 10.59 | BALF UC | ↓ | ||||
| 20 | 16.0 | − | 308.4986 | Eicosadienoic acid | C20H36O2 | 1.76 | 0.01 | BALF UC | ↑ | ||||
| 21 | 19.1 | − | 354.6101 | Tricosanoic acid | C23H46O2 | 1.34 | 5.07 | BALF UC | ↓ | ||||
| 22 | 19.6 | − | 368.6367 | Tetracosanoic acid | C24H48O2 | 2.10 | 0.17 | BALF CRC | ↑ | ||||
| 23 | 14.3 | − | 376.5726 | 12b-Hydroxy-5b-cholanoic acid | C24H40O3 | 2.24 | 2.31 | 0.01 | 0.01 | BALF UC&CRC | ↑ | ↑ | ↑ |
| 24 | 3.1 | − | 379.3860 | S-Lactoylglutathione | C13H21N3O8S | 1.33 | 2.36 | 6.47 | 192.22 | BALF UC&CRC | ↓ | ↓ | ↓ |
| 25 | 9.2 | − | 390.5561 | 7-Hydroxy-3-oxocholanoic acid | C24H38O4 | 1.65 | 0.01 | BALF UC | ↑ | ||||
| 26 | 2.3 | + | 158.1519 | Succinylacetone | C7H10O4 | 1.63 | 101.76 | Lung CRC | ↓ | ||||
| 27 | 2.1 | + | 174.2010 | L-Arginine | C6H14N4O2 | 1.18 | 1.19 | 10.08 | 15.72 | Lung UC&CRC | ↓ | ↓ | ↓ |
| 28 | 4.8 | + | 224.2133 | Hydroxykynurenine | C10H12N2O4 | 1.07 | 0.23 | Lung CRC | ↑ | ||||
| 29 | 2.3 | + | 252.2300 | Deoxyinosine | C10H12N4O4 | 1.07 | 1.33 | 0.04 | 0.04 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 30 | 8.0 | + | 281.2719 | Oleamide | C18H35NO | 1.38 | 2.14 | 0.01 | 0.01 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 31 | 12.6 | + | 301.5078 | Sphinganine | C18H39NO2 | 1.93 | 0.01 | Lung UC | ↑ | ||||
| 32 | 11.8 | + | 317.5072 | Phytosphingosine | C18H39NO3 | 2.04 | 2.34 | 0.01 | 0.01 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 33 | 7.8 | + | 364.4758 | Tetrahydrocortisone | C21H32O5 | 1.25 | 0.03 | Lung UC | ↑ | ||||
| 34 | 10.9 | + | 366.3700 | Tryptophan 2-C-mannoside | C17H22N2O7 | 1.21 | 0.02 | Lung CRC | ↑ | ||||
| 35 | 5.3 | + | 475.5558 | LysoPE (18:3(6Z,9Z,12Z)/0:0) | C23H42NO7P | 1.35 | 1.44 | 0.03 | 0.14 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 36 | 9.6 | + | 501.2855 | LysoPE (20:4(5Z,8Z,11Z,14Z)/0:0) | C25H44NO7P | 1.52 | 1.93 | 0.01 | 0.01 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 37 | 5.2 | + | 519.3325 | LysoPC (18:2(9Z,12Z)/0:0) | C26H50NO7P | 1.39 | 1.74 | 0.05 | 0.04 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 38 | 5.3 | + | 607.4577 | LysoPC(24:0/0:0) | C32H66NO7P | 1.57 | 0.01 | Lung UC | ↑ | ||||
| 39 | 2.4 | − | 152.1109 | Xanthine | C5H4N4O2 | 1.91 | 456.69 | Lung CRC | ↓ | ||||
| 40 | 2.4 | − | 153.1784 | Dopamine | C8H11NO2 | 1.33 | 17.24 | Lung CRC | ↓ | ||||
| 41 | 9.6 | − | 173.2096 | N-Acetylleucine | C8H15NO3 | 1.27 | 4.90 | Lung CRC | ↓ | ||||
| 42 | 4.8 | − | 205.2099 | Xanthurenic acid | C10H7NO4 | 1.55 | 1376.79 | Lung UC | ↓ | ||||
| 43 | 7.8 | − | 258.3538 | Tetradecanedioic acid | C14H26O4 | 1.40 | 0.01 | Lung UC | ↑ | ||||
| 44 | 7.0 | − | 267.2413 | Adenosine | C10H13N5O4 | 1.42 | 452.62 | Lung UC | ↓ | ||||
| 45 | 12.0 | − | 270.4507 | Heptadecanoic acid | C17H34O2 | 1.28 | 0.15 | Lung CRC | ↑ | ||||
| 46 | 11.6 | − | 272.4290 | 3-Hydroxyhexadecanoic acid | C16H32O3 | 1.93 | 0.01 | Lung CRC | ↑ | ||||
| 47 | 10.3 | − | 278.4296 | alpha-Linolenic acid | C18H30O2 | 1.57 | 1722.66 | Lung UC | ↓ | ||||
| 48 | 13.4 | − | 280.4455 | Linoleic acid | C18H32O2 | 1.33 | 1.76 | 0.05 | 0.04 | Lung UC&CRC | ↑ | ↑ | |
| 49 | 5.0 | − | 282.4614 | Oleic acid | C18H34O2 | 1.52 | 1017.46 | Lung UC | ↓ | ||||
| 50 | 3.7 | − | 284.2255 | Xanthosine | C10H12N4O6 | 1.03 | 6.26 | Lung CRC | ↓ | ||||
| 51 | 9.7 | − | 286.4070 | Hexadecanedioic acid | C16H30O4 | 1.10 | 0.28 | Lung CRC | ↑ | ||||
| 52 | 8.1 | − | 390.2770 | 7-Hydroxy-3-oxocholanoic acid | C24H38O4 | 1.43 | 1.61 | 0.01 | 0.12 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 53 | 9.7 | − | 392.5720 | Deoxycholic acid | C24H40O4 | 1.47 | 773.12 | Lung UC | ↓ | ||||
| 54 | 7.5 | − | 408.5714 | 3a,4b,12a-Trihydroxy-5b-cholanoic acid | C24H40O5 | 1.68 | 1.70 | 0.01 | 0.01 | Lung UC&CRC | ↑ | ↑ | ↑ |
| 55 | 20.2 | − | 691.9720 | PE (16:0/16:0) | C37H74NO8P | 1.07 | 0.01 | Lung CRC | ↑ | ||||
| Ulcerative Colitis | Colorectal Cancer | |
|---|---|---|
| Phylum | Firmicutes, Proteobacteria | Firmicutes, Proteobacteria |
| Class | Bacilli | Bacilli, Clostridia, Alphaproteobacteria |
| Gammaproteobacteria | ||
| Order | / | Corynebacteriales, Clostridiales, Rhizobiales, |
| Pasteurellales | ||
| Family | / | Corynebacteriaceae, Lachnospiraceae, Ruminococcaceae, Rhizobiaceae |
| Xanthobacteraceae, Pasteurellaceae, | ||
| Genus | / | Staphylococcus, Shinella, Bradyrhizobium, Rodentibacter |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Q.; Liu, R.; Chu, G.; Wang, Y.; Cui, H.; Zhang, T.; Bi, K.; Gao, P.; Song, Z.; Li, Q. A Comprehensive Analysis of Microflora and Metabolites in the Development of Ulcerative Colitis into Colorectal Cancer Based on the Lung–Gut Correlation Theory. Molecules 2022, 27, 5838. https://doi.org/10.3390/molecules27185838
Tang Q, Liu R, Chu G, Wang Y, Cui H, Zhang T, Bi K, Gao P, Song Z, Li Q. A Comprehensive Analysis of Microflora and Metabolites in the Development of Ulcerative Colitis into Colorectal Cancer Based on the Lung–Gut Correlation Theory. Molecules. 2022; 27(18):5838. https://doi.org/10.3390/molecules27185838
Chicago/Turabian StyleTang, Qi, Ran Liu, Ge Chu, Yue Wang, Haiyue Cui, Tongrui Zhang, Kaishun Bi, Peng Gao, Zonghua Song, and Qing Li. 2022. "A Comprehensive Analysis of Microflora and Metabolites in the Development of Ulcerative Colitis into Colorectal Cancer Based on the Lung–Gut Correlation Theory" Molecules 27, no. 18: 5838. https://doi.org/10.3390/molecules27185838