Interspecific Interactions between Protozoan Parasites and their Hosts and Neighbors
A special issue of Tropical Medicine and Infectious Disease (ISSN 2414-6366). This special issue belongs to the section "Infectious Diseases".
Deadline for manuscript submissions: closed (27 October 2023) | Viewed by 2601
Special Issue Editors
Interests: cell biology; protozoology; immunology; neutrophil biology; toxoplasmosis; Toxoplasma gondii; host–pathogen interaction
Special Issue Information
Dear Colleagues,
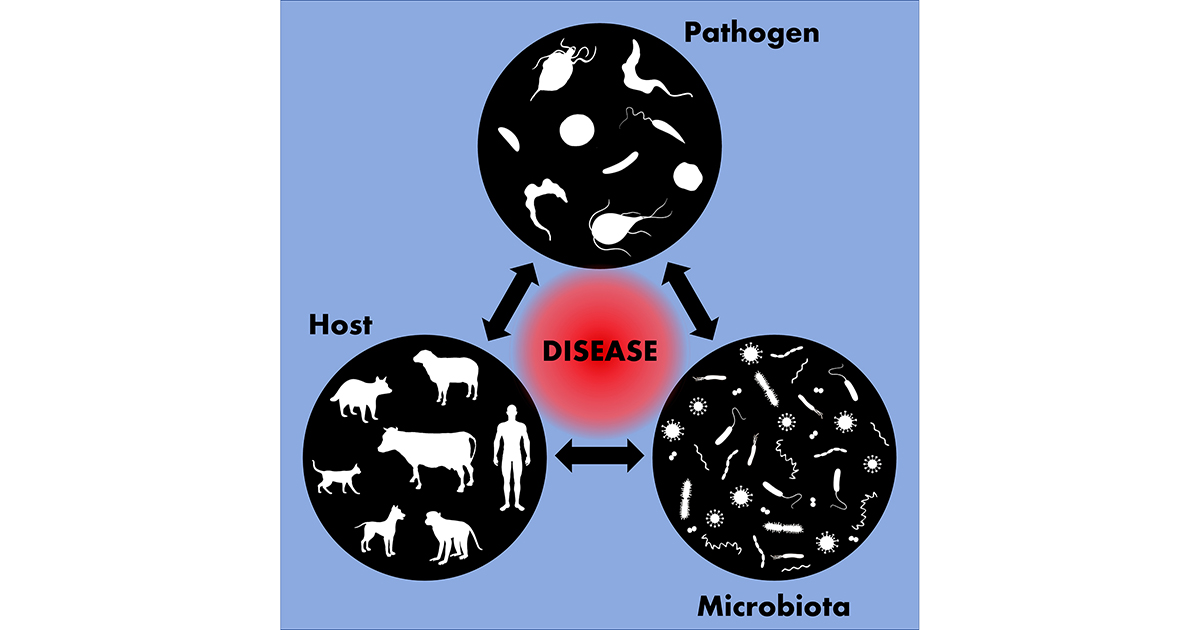
Protozoan parasites include pathogens of medical and veterinary importance that, although largely neglected, lead to important human and animal health problems. Together, parasites such as Giardia, Entamoeba, Trichomonas, Toxoplasma, Plasmodium, Trypanosoma and Leishmania affect billions of people worldwide, leading to thousands of deaths annually. In addition, they can infect domestic animals, including companion animals and livestock, and lead to various diseases and reproductive complications, causing psychological trauma to pet families and great economic losses to the world livestock market.
To be successful in infecting their hosts, these parasites must reach the target tissue and deal with natural barriers such as the host's immune cells and various other microorganisms they encounter during their journey. Interactions with such agents can be beneficial or harmful for the protozoan itself, and the balance between parasite virulence, host resistance and the influence of the local microbiota will determine the fate of the infection and the outcome of the disease.
The host–parasite–microbiota interactions are complex; if, on the one hand, protozoan infections affect the composition of the mucosal microbiota, on the other hand, specific components of the microbiota can affect the parasitic infection and the progression of the diseases caused by them. Therefore, understanding the biology, ultrastructure and dynamics of these host–parasite, parasite–microbiota and host–parasite–microbiota relationships can favor the development of therapeutic strategies to combat these life-threatening pathogenic protozoa.
This Special Issue will cover various aspects of the host–parasite–microbiota interplay under three main topics: (1) the relationship between protozoan parasites and host cells (e.g., cellular and molecular biology of protozoan interactions with target tissues/cells, interactions with the host immune system, and the life cycle during host colonization); (2) parasitic protozoa and their relationship with the microbiological world (fungi, viruses, bacteria and other protozoa); and (3) the relationship between protozoan parasites and the niche where they live within the host (e.g., gut or vaginal microbiota).
Dr. Rafael Mariante Meyer
Dr. Victor Do Valle Pereira Midlej
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Tropical Medicine and Infectious Disease is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- protozoan parasites
- host–pathogen interaction
- microbiota
- intestinal protozoa
- trichomonads
- trypanosomatids
- Apicomplexa







