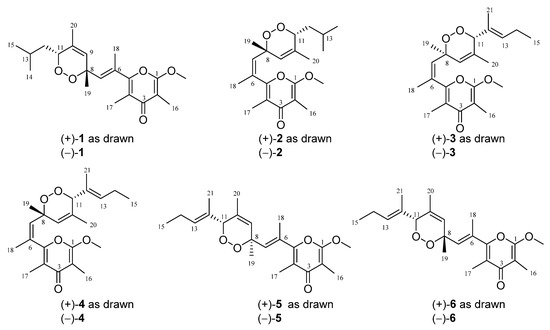
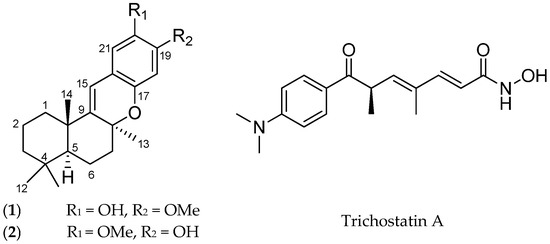
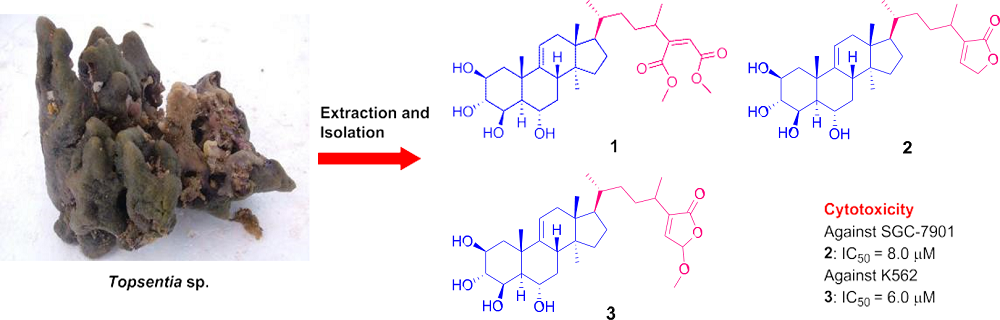
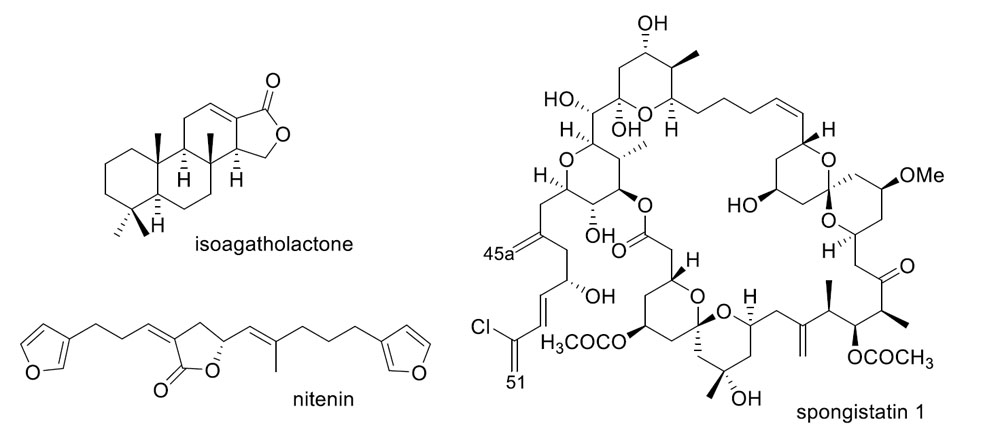
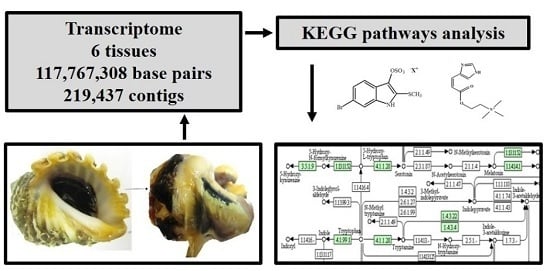
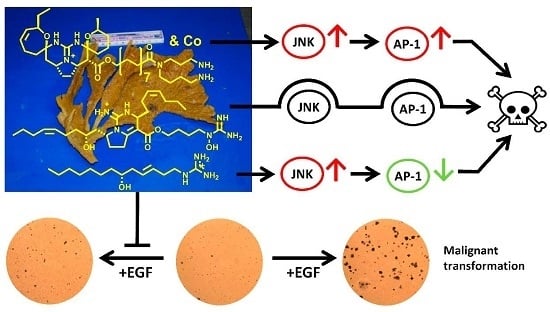
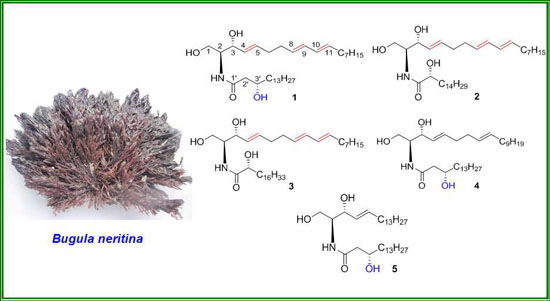
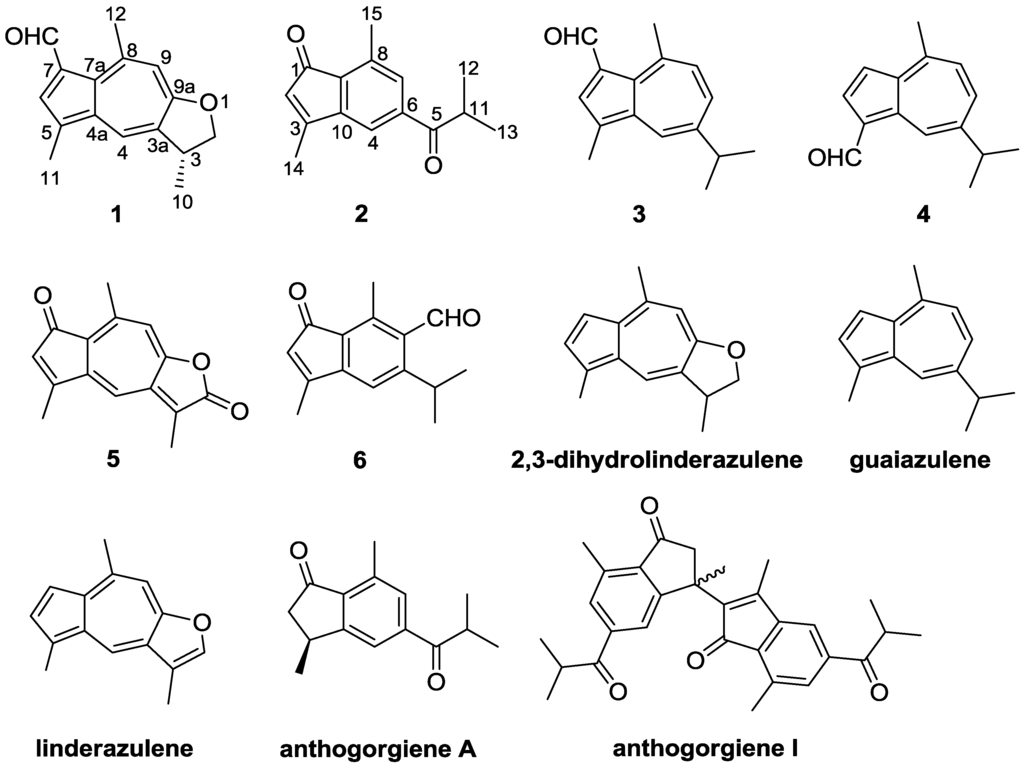
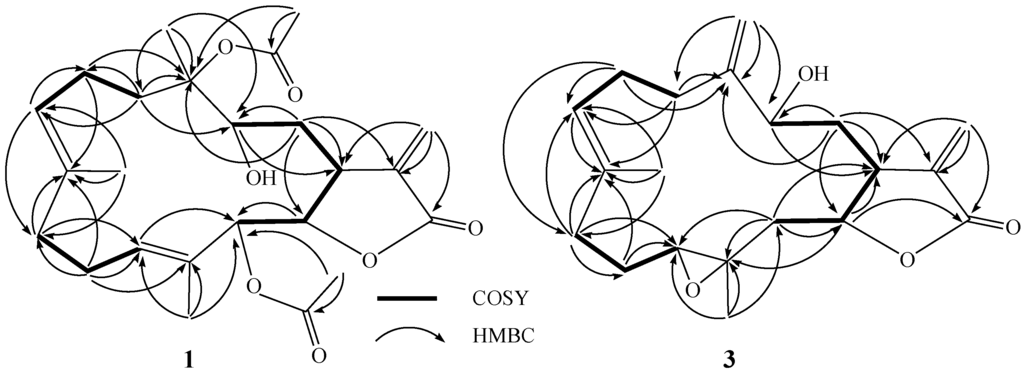
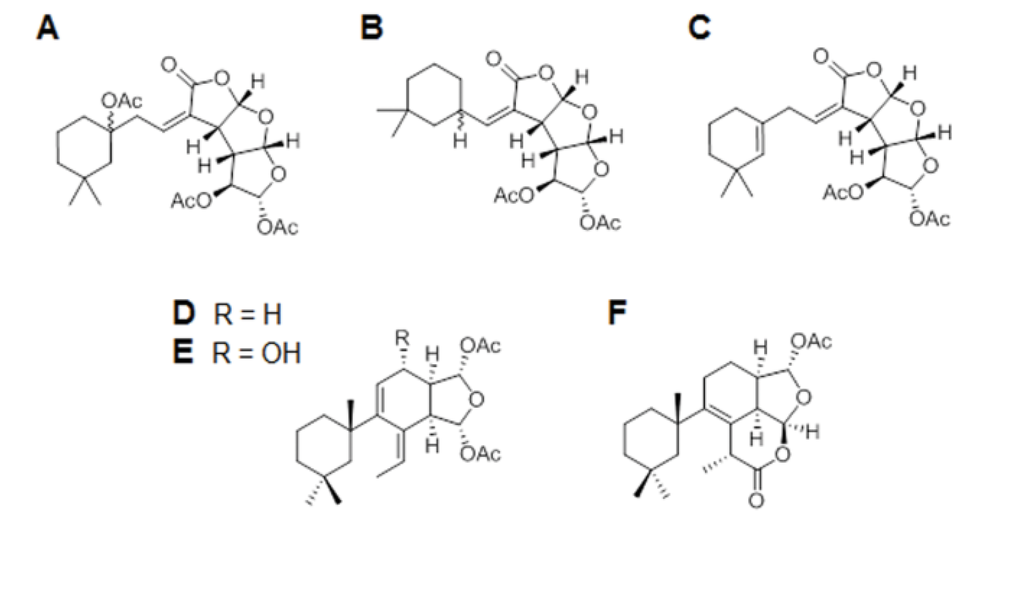
Bioactive Compounds from Marine Invertebrates
A topical collection in Marine Drugs (ISSN 1660-3397).
Viewed by 630611Editor
Topical Collection Information
Dear Colleagues,
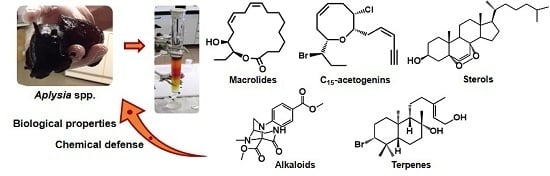
All major lineages of invertebrates evolved in the oceans, and as such, the marine environment harbors the largest diversity of invertebrate phyla and species. As part of the struggle for survival, all extant marine invertebrate species occupy a unique niche within the marine environment, with specific adaptations to withstand a wide range of abiotic and biotic pressures. Many of these marine invertebrates are sessile or slow moving, and lack physical defense structures to protect against potential predators and competitors. They all also lack adaptive immunity against pathogens and parasites, despite being constantly bathed in microorganisms, and thus rely entirely on effective innate immune systems to keep themselves free of infection. To compensate for these apparent deficiencies, marine invertebrates have developed an arsenal of bioactive secondary metabolites. In addition to these chemically mediated defense interactions, some marine invertebrates use water soluble secondary metabolites for chemical communication (pheromones, settlement cues) and neurotoxins (in venoms) to paralyze or kill their prey.
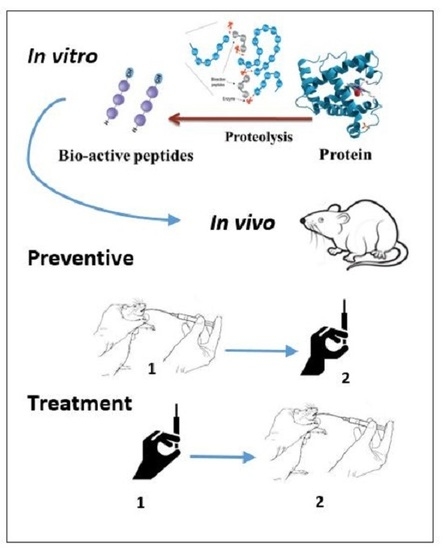
Many of these intrinsically biologically active compounds produced by marine invertebrates provide useful leads for pharmaceutical, nutraceutical, and other industrial (e.g., anti-fouling) development. However, sustainable production is often limited by molecular complexity, which can limit economical chemical synthesis. Further insight into the ecology of the source species is required, including knowledge of the biosynthetic origin of the bioactive compounds, so as to distinguish innately synthesized, dietary derived or symbiotic microbial sources for sustainable culture. Investigation into the diversity and function of marine invertebrate secondary metabolites is also a vital step towards developing a comprehensive understanding of how chemicals might help structure marine populations, communities, and ecosystems.
In this collection, we hope to explore all aspects of bioactive secondary metabolism in marine invertebrates, including the chemical diversity within certain invertebrate taxa, chemical ecology research aimed at elucidating the natural function of bioactive secondary metabolites, and the neuroecology of marine natural products, as well as bioactivity profiles, biosynthesis, and/or biodistributional studies on specific marine invertebrate natural products. We would also be interested in highlighting recent innovative research on the sustainable production, biomedical or industrial, of marine invertebrate natural products, or research into the traditional use of marine invertebrates that produce bioactive compounds. We welcome the submission of comprehensive/mini reviews, original research articles, and communications.
As guest editor, I invite you to contribute to the Marine Drugs collection on “Bioactive Compounds from Marine Invertebrates”.
Dr. Kirsten Benkendorff
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Marine Drugs is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
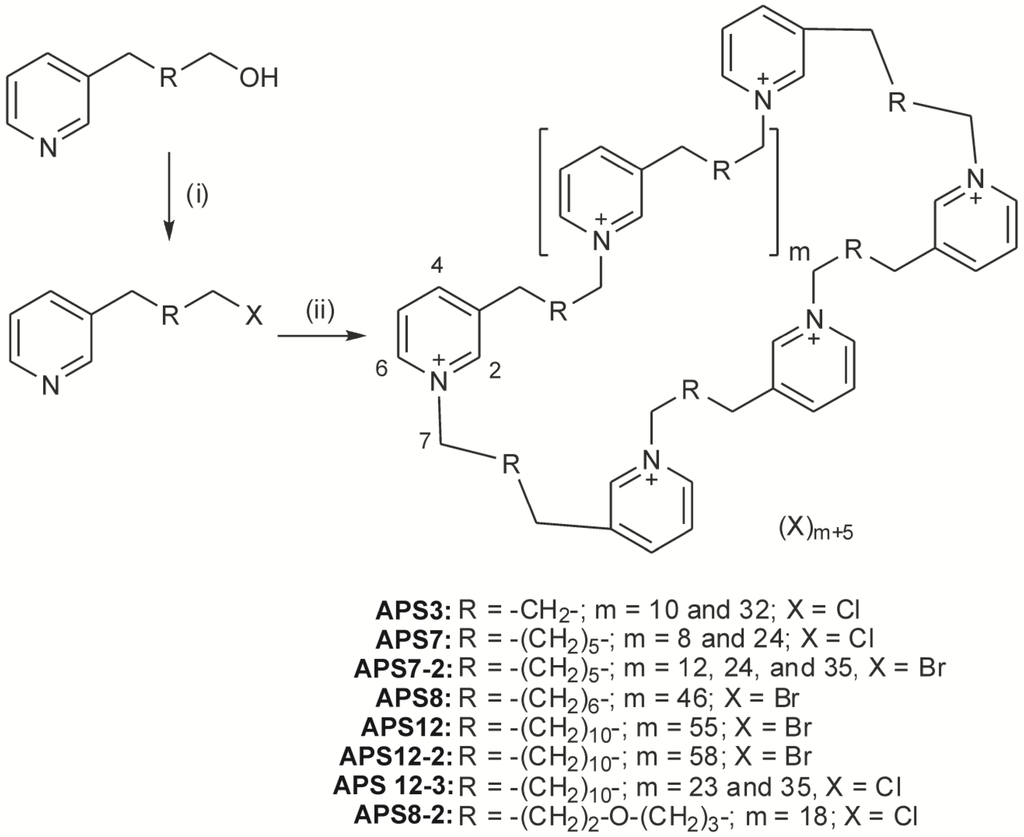
- ascidian secondary metabolites
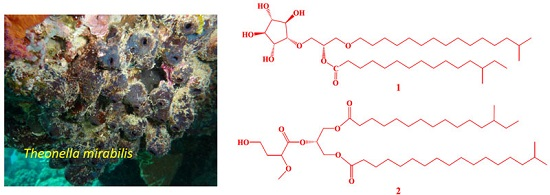
- sponge secondary metabolites
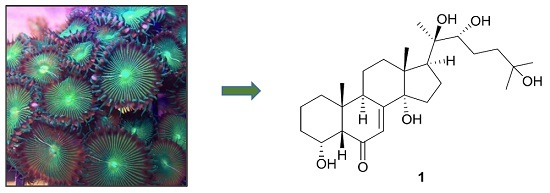
- cnidarian secondary metabolites
- mollusc secondary metabolites
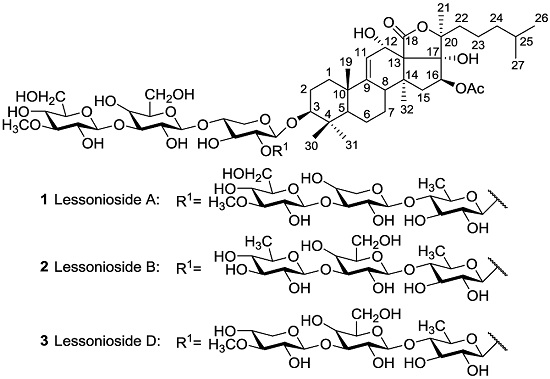
- echinoderm secondary metabolites
- chemical defense
- marine chemical ecology
- antimicrobial activity
- antiviral activity
- anti-inflammatory activity
- anticancer activity
- neurotoxin
- pheromone
- sustainable supply