Journal Description
Liquids
Liquids
is an international, peer-reviewed, open access journal on all aspects of liquid material research published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within AGRIS, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 28 days after submission; acceptance to publication is undertaken in 4.9 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
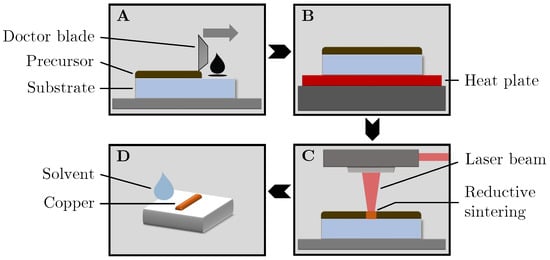
Rheological Investigation of Highly Filled Copper(II) Oxide Nanosuspensions to Optimize Precursor Particle Content in Reductive Laser-Sintering
Liquids 2024, 4(2), 382-392; https://doi.org/10.3390/liquids4020019 (registering DOI) - 24 Apr 2024
Abstract
►
Show Figures
In this article, the particle concentration of finely dispersed copper(II) oxide nanosuspensions as precursors for reductive laser sintering (RLS) is optimized on the basis of rheological investigations. For this metallization process, a smooth, homogeneous and defect-free precursor layer is a prerequisite for adherent
[...] Read more.
In this article, the particle concentration of finely dispersed copper(II) oxide nanosuspensions as precursors for reductive laser sintering (RLS) is optimized on the basis of rheological investigations. For this metallization process, a smooth, homogeneous and defect-free precursor layer is a prerequisite for adherent and reproducible copper structures. The knowledge of the rheological properties of an ink is crucial for the selection of a suitable coating technology as well as for the adjustment of the ink formulation. Different dilutions of the nanosuspension were examined for their rheological behavior by recording flow curves. A strong shear thinning behavior was found and the viscosity decreases exponentially with increasing dilution. The viscoelastic behavior was investigated by a simulated doctor blade coating process using three-interval thixotropy tests. An overshoot in viscosity is observed, which decreases with increasing thinning of the precursor. As a comparison to these results, doctor blade coating of planar glass and polymer substrates was performed to prepare precursor layers for reductive laser sintering. Surface morphology measurements of the resulting coatings using laser scanning microscopy and rheological tests show that homogeneous precursor layers with constant thickness can be produced at a particle–solvent ratio of 1.33. A too-high particle content results in an irregular coating layer with deep grooves and a peak-to-valley height
Open AccessReview
Solvent Replacement Strategies for Processing Pharmaceuticals and Bio-Related Compounds—A Review
by
Jia Lin Lee, Gun Hean Chong, Masaki Ota, Haixin Guo and Richard Lee Smith, Jr.
Liquids 2024, 4(2), 352-381; https://doi.org/10.3390/liquids4020018 - 09 Apr 2024
Abstract
An overview of solvent replacement strategies shows that there is great progress in green chemistry for replacing hazardous di-polar aprotic solvents, such as N,N-dimethylformamide (DMF), 1-methyl-2-pyrrolidinone (NMP), and 1,4-dioxane (DI), used in processing active industrial ingredients (APIs). In synthetic chemistry, alcohols, carbonates, ethers,
[...] Read more.
An overview of solvent replacement strategies shows that there is great progress in green chemistry for replacing hazardous di-polar aprotic solvents, such as N,N-dimethylformamide (DMF), 1-methyl-2-pyrrolidinone (NMP), and 1,4-dioxane (DI), used in processing active industrial ingredients (APIs). In synthetic chemistry, alcohols, carbonates, ethers, eucalyptol, glycols, furans, ketones, cycloalkanones, lactones, pyrrolidinone or solvent mixtures, 2-methyl tetrahydrofuran in methanol, HCl in cyclopentyl methyl ether, or trifluoroacetic acid in propylene carbonate or surfactant water (no organic solvents) are suggested replacement solvents. For the replacement of dichloromethane (DCM) used in chromatography, ethyl acetate ethanol or 2-propanol in heptanes, with or without acetic acid or ammonium hydroxide additives, are suggested, along with methanol acetic acid in ethyl acetate or methyl tert-butyl ether, ethyl acetate in ethanol in cyclohexane, CO2-ethyl acetate, CO2-methanol, CO2-acetone, and CO2-isopropanol. Supercritical CO2 (scCO2) can be used to replace many organic solvents used in processing materials from natural sources. Vegetable, drupe, legume, and seed oils used as co-extractants (mixed with substrate before extraction) can be used to replace the typical organic co-solvents (ethanol, acetone) used in scCO2 extraction. Mixed solvents consisting of a hydrogen bond donor (HBD) solvent and a hydrogen bond acceptor (HBA) are not addressed in GSK or CHEM21 solvent replacement guides. Published data for 100 water-soluble and water-insoluble APIs in mono-solvents show polarity ranges appropriate for the processing of APIs with mixed solvents. When water is used, possible HBA candidate solvents are acetone, acetic acid, acetonitrile, ethanol, methanol, 2-methyl tetrahydrofuran, 2,2,5,5-tetramethyloxolane, dimethylisosorbide, Cyrene, Cygnet 0.0, or diformylxylose. When alcohol is used, possible HBA candidates are cyclopentanone, esters, lactone, eucalytol, MeSesamol, or diformylxylose. HBA—HBA mixed solvents, such as Cyrene—Cygnet 0.0, could provide interesting new combinations. Solubility parameters, Reichardt polarity, Kamlet—Taft parameters, and linear solvation energy relationships provide practical ways for identifying mixed solvents applicable to API systems.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
Figure 1
Open AccessArticle
Frustrated-Laser-Induced Thermal Starting Plumes in Fresh and Salt Water
by
Johnathan Biebighauser, Johan Dominguez Lopez, Krys Strand, Mark W. Gealy and Darin J. Ulness
Liquids 2024, 4(2), 332-351; https://doi.org/10.3390/liquids4020017 - 08 Apr 2024
Abstract
The results of a photothermal spectroscopy technique that effectively images convective and conductive heat flow in liquids via a thermal lensing effect are described. Pure water; sodium chloride solutions at salinities of approximately 5, 15, 25, and 35 g/kg; and an artificial seawater
[...] Read more.
The results of a photothermal spectroscopy technique that effectively images convective and conductive heat flow in liquids via a thermal lensing effect are described. Pure water; sodium chloride solutions at salinities of approximately 5, 15, 25, and 35 g/kg; and an artificial seawater of 35 g/kg were studied across a range of temperatures. This system was studied because of the importance of thermal pluming in seawater. ‘Frustrated’ thermal starting plumes were observed near the temperature of maximum density. The physical characteristics of these thermal starting plumes are reported.
Full article
(This article belongs to the Special Issue Energy Transfer in Liquids)
►▼
Show Figures
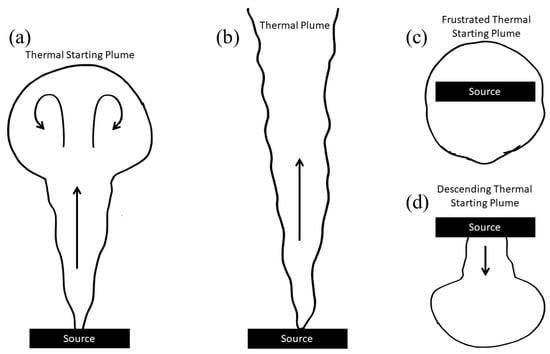
Figure 1
Open AccessArticle
An Ab Initio Investigation of the Hydration of Antimony(III)
by
Cory C. Pye and Champika Mahesh Gunasekara
Liquids 2024, 4(2), 322-331; https://doi.org/10.3390/liquids4020016 - 01 Apr 2024
Abstract
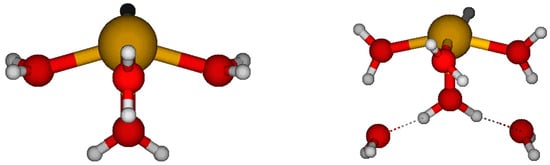
The energies, structures, and vibrational frequencies of [Sb(H2O)n]3+, n = 0–9, 18 have been calculated at the Hartree–Fock and second-order Møller–Plesset levels of theory using the CEP, LANL2, and SDD effective core potentials in combination with their
[...] Read more.
The energies, structures, and vibrational frequencies of [Sb(H2O)n]3+, n = 0–9, 18 have been calculated at the Hartree–Fock and second-order Møller–Plesset levels of theory using the CEP, LANL2, and SDD effective core potentials in combination with their associated basis sets, or with the 6-31G* and 6-31+G* basis sets. The metal–oxygen distances and totally symmetric stretching frequency of the aqua ions were compared with each other and with related crystal structure measurements where available.
Full article
(This article belongs to the Special Issue Hydration of Ions in Aqueous Solution)
►▼
Show Figures
Graphical abstract
Open AccessArticle
Conventional and Green Rubber Plasticizers Classified through Nile Red [E(NR)] and Reichardt’s Polarity Scale [ET(30)]
by
Franco Cataldo
Liquids 2024, 4(2), 305-321; https://doi.org/10.3390/liquids4020015 - 31 Mar 2024
Abstract
After a survey on polymer plasticization theories and conventional criteria to evaluate polymer–plasticizer compatibility through the solubility parameter, an attempt to create a polymer–plasticizer polarity scale through solvatochromic dyes has been made. Since Reichardt’s ET(30) dye is insoluble in rubber hydrocarbon
[...] Read more.
After a survey on polymer plasticization theories and conventional criteria to evaluate polymer–plasticizer compatibility through the solubility parameter, an attempt to create a polymer–plasticizer polarity scale through solvatochromic dyes has been made. Since Reichardt’s ET(30) dye is insoluble in rubber hydrocarbon polymers like polyisoprene, polybutadiene and styrene–butadiene copolymers and is not useful for the evaluation of the hydrocarbons and ester plasticizers, the Nile Red solvatochromic dye was instead used extensively and successfully for this class of compounds. A total of 53 different compounds were evaluated with the Nile Red dye and wherever possible also with Reichardt’s ET(33) dye. A very good correlation was then found between the Nile Red scale E(NR) and Reichardt’s ET(30) scale for this class of compounds focusing on diene rubbers and their typical hydrocarbons and new ester plasticizers. Furthermore, the E(NR) scale also shows a reasonable correlation with the total solubility parameter calculated according to the Van Krevelen method. Based on the above results, some conclusion was made about the compatibility between the diene rubbers and the conventional plasticizers, as well as a new and green plasticizer proposed for the rubber compounds.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
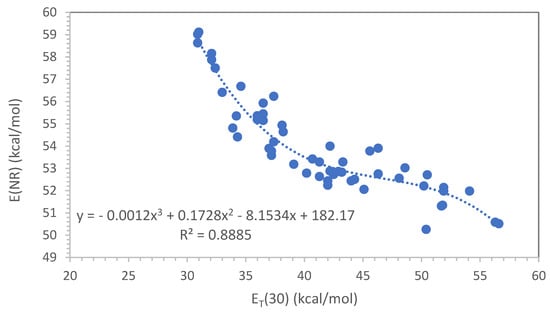
Figure 1
Open AccessArticle
Exploring Solvation Properties of Protic Ionic Liquids by Employing Solvatochromic Dyes and Molecular Dynamics Simulation Analysis
by
Stuart J. Brown, Andrew J. Christofferson, Calum J. Drummond, Qi Han and Tamar L. Greaves
Liquids 2024, 4(1), 288-304; https://doi.org/10.3390/liquids4010014 - 20 Mar 2024
Abstract
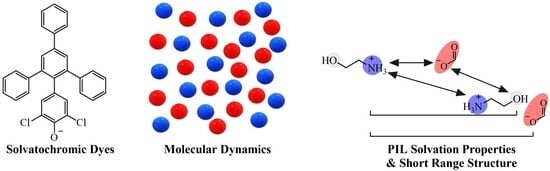
Solvation properties are key for understanding the interactions between solvents and solutes, making them critical for optimizing chemical synthesis and biochemical applications. Designable solvents for targeted optimization of these end-uses could, therefore, play a big role in the future of the relevant industries.
[...] Read more.
Solvation properties are key for understanding the interactions between solvents and solutes, making them critical for optimizing chemical synthesis and biochemical applications. Designable solvents for targeted optimization of these end-uses could, therefore, play a big role in the future of the relevant industries. The tailorable nature of protic ionic liquids (PILs) as designable solvents makes them ideal candidates. By alteration of their constituent structural groups, their solvation properties can be tuned as required. The solvation properties are determined by the polar and non-polar interactions of the PIL, but they remain relatively unknown for PILs as compared to aprotic ILs and their characterization is non-trivial. Here, we use solvatochromic dyes as probe molecules to investigate the solvation properties of nine previously uncharacterized alkyl- and dialkylammonium PILs. These properties include the Kamlet–Aboud–Taft (KAT) parameters: π* (dipolarity/polarizability), α (H-bond acidity) and β (H-bond basicity), along with the ET(30) scale (electrophilicity/polarizability). We then used molecular dynamics simulations to calculate the radial distribution functions (RDF) of 21 PILs, which were correlated to their solvation properties and liquid nanostructure. It was identified that the hydroxyl groups on the PIL cation increase α, π* and ET(30), and correspondingly increase the cation–anion distance in their RDF plots. The hydroxyl group, therefore, reduces the strength of the ionic interaction but increases the polarizability of the ions. An increase in the alkyl chain length on the cation led to a decrease in the distances between cations, while also increasing the β value. The effect of the anion on the PIL solvation properties was found to be variable, with the nitrate anion greatly increasing π*, α and anion–anion distances. The research presented herein advances the understanding of PIL structure–property relationships while also showcasing the complimentary use of molecular dynamics simulations and solvatochromic analysis together.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
Graphical abstract
Open AccessCommunication
The Photophysics of Diphenyl Polyenes Analyzed by Their Solvatochromism
by
Javier Catalán and Henning Hopf
Liquids 2024, 4(1), 278-287; https://doi.org/10.3390/liquids4010013 - 13 Mar 2024
Abstract
The solvent-dependent intensity changes in the first UV/Vis absorption band of the three polyenes DPH, DPHb, and ttbP3 dissolved in a hydrocarbon solvent with temperature allow for the conclusion that, at temperatures above 233 K, the two phenyl groups of DPH are rotated
[...] Read more.
The solvent-dependent intensity changes in the first UV/Vis absorption band of the three polyenes DPH, DPHb, and ttbP3 dissolved in a hydrocarbon solvent with temperature allow for the conclusion that, at temperatures above 233 K, the two phenyl groups of DPH are rotated out-of-plane to yield a non-coplanar molecular structure. This leads to the conclusion that DPH becomes increasingly less coplanar as the temperature rises above 233 K. When the phenyl groups rotate out-of-plane, the polarizability decreases, and the energy of the first electronic transition increases by an extra value. Therefore, below 233 K, the correlation lines between the absorption energy of the 0–0 component of the UV/Vis absorption band and the solvent polarizability, as measured by the SP values, show bilinear behavior. The unexpected behavior shown by DPH dissolved in tetrachloro- and dichloromethane is discussed. We dedicate this research as a tribute to the very important contribution to the solvent effect made by Prof. Christian Reichardt and also to his generous and altruistic scientific help that he has always shown.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
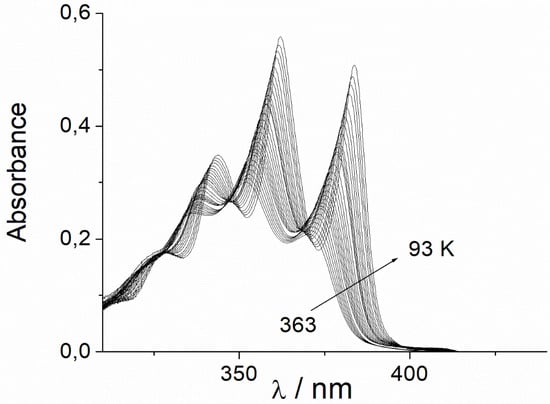
Figure 1
Open AccessArticle
Automated Equations of State Tuning Workflow Using Global Optimization and Physical Constraints
by
Eirini Maria Kanakaki and Vassilis Gaganis
Liquids 2024, 4(1), 261-277; https://doi.org/10.3390/liquids4010012 - 06 Mar 2024
Abstract
A computational model that can accurately describe the thermodynamics of a hydrocarbon system and its properties under various conditions is a prerequisite for running reservoir and pipeline simulations. Cubic Equations of State (EoS) are mathematical tools used to model the phase and volumetric
[...] Read more.
A computational model that can accurately describe the thermodynamics of a hydrocarbon system and its properties under various conditions is a prerequisite for running reservoir and pipeline simulations. Cubic Equations of State (EoS) are mathematical tools used to model the phase and volumetric behavior of reservoir fluids when compositional effects need to be considered. To anticipate uncertainty and enhance the quality of their predictions, EoS models must be adjusted to adequately match the available lab-measured PVT values. This task is challenging given that there are many potential tuning parameters, thus leading to various tuning results of questionable validity. In this paper, we present an automated EoS tuning workflow that employs a Generalized Pattern Search (GPS) optimizer for efficient tuning of a cubic EoS model. Specifically, we focus on the Peng–Robinson (PR) model, which is the oil and gas industry standard, to accurately capture the behavior of diverse multicomponent, complex hydrocarbon mixtures encountered in subsurface reservoirs. This approach surpasses the limitations of conventional gradient-based (GB) methods, which are susceptible to getting trapped in local optima. The proposed technique also allows physical constraints to be imposed on the optimization procedure. A gas condensate and an H2S-rich oil were used to demonstrate the effectiveness of the GPS algorithm in finding an optimized solution for high-dimensional search spaces, and its superiority over conventional gradient-based optimization was confirmed by automatically tracking globally optimal and physically sound solutions.
Full article
(This article belongs to the Section Physics of Liquids)
►▼
Show Figures
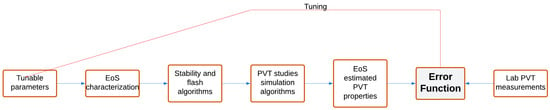
Figure 1
Open AccessArticle
Calculation of the Three Partition Coefficients logPow, logKoa and logKaw of Organic Molecules at Standard Conditions at Once by Means of a Generally Applicable Group-Additivity Method
by
Rudolf Naef and William E. Acree, Jr.
Liquids 2024, 4(1), 231-260; https://doi.org/10.3390/liquids4010011 - 01 Mar 2024
Abstract
Assessment of the environmental impact of organic chemicals has become an important subject in chemical science. Efficient quantitative descriptors of their impact are their partition coefficients logPow, logKoa and logKaw. We present a group-additivity method that has proven
[...] Read more.
Assessment of the environmental impact of organic chemicals has become an important subject in chemical science. Efficient quantitative descriptors of their impact are their partition coefficients logPow, logKoa and logKaw. We present a group-additivity method that has proven its versatility for the reliable prediction of many other molecular descriptors for the calculation of the first two partition coefficients and indirectly of the third with high dependability. Based on the experimental logPow data of 3332 molecules and the experimental logKoa data of 1900 molecules at 298.15 K, the respective partition coefficients have been calculated with a cross-validated standard deviation S of only 0.42 and 0.48 log units and a goodness of fit Q2 of 0.9599 and 0.9717, respectively, in a range of ca. 17 log units for both descriptors. The third partition coefficient logKaw has been derived from the calculated values of the former two descriptors and compared with the experimentally determined logKaw value of 1937 molecules, yielding a standard deviation σ of 0.67 log units and a correlation coefficient R2 of 0.9467. This approach enabled the quick calculation of 29,462 logPow, 27,069 logKoa and 26,220 logKaw values for the more than 37,100 molecules of ChemBrain’s database available to the public.
Full article
(This article belongs to the Special Issue Recent Advances in the Behavior of Liquids in Honor of Prof. Dr. William Acree Jr.)
►▼
Show Figures
Figure 1
Open AccessReview
Polarity of Organic Solvent/Water Mixtures Measured with Reichardt’s B30 and Related Solvatochromic Probes—A Critical Review
by
Stefan Spange
Liquids 2024, 4(1), 191-230; https://doi.org/10.3390/liquids4010010 - 17 Feb 2024
Abstract
The UV/Vis absorption energies (νmax) of different solvatochromic probes measured in co-solvent/water mixtures are re-analyzed as a function of the average molar concentration (Nav) of the solvent composition compared to the use of the mole fraction. The
[...] Read more.
The UV/Vis absorption energies (νmax) of different solvatochromic probes measured in co-solvent/water mixtures are re-analyzed as a function of the average molar concentration (Nav) of the solvent composition compared to the use of the mole fraction. The empirical ET(30) parameter of Reichardt’s dye B30 is the focus of the analysis. The Marcus classification of aqueous solvent mixtures is a useful guide for co-solvent selection. Methanol, ethanol, 1,2-ethanediol, 2-propanol, 2-methyl-2-propanol, 2-butoxyethanol, formamide, N-methylformamide (NMF), N,N-dimethylformamide (DMF), N-formylmorpholine (NFM), 1,4-dioxane and DMSO were considered as co-solvents. The ET(30) values of the binary solvent mixtures are discussed in relation to the physical properties of the co-solvent/water mixtures in terms of quantitative composition, refractive index, thermodynamics of the mixture and the non-uniformity of the mixture. Significant linear dependencies of ET(30) as a function of Nav can be demonstrated for formamide/water, 1,2-ethanediol/water, NMF/water and DMSO/water mixtures over the entire compositional range. These mixtures belong to the group of solvents that do not enhance the water structure according to the Marcus classification. The influence of the solvent microstructure on the non-linearity ET(30) as a function of Nav is particularly clear for alcohol/water mixtures with an enhanced water structure.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
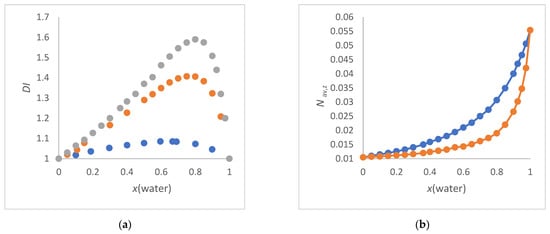
Figure 1
Open AccessArticle
Solvatochromic and Computational Study of Some Cycloimmonium Ylids
by
Daniela Babusca, Andrei Vleoanga and Dana Ortansa Dorohoi
Liquids 2024, 4(1), 171-190; https://doi.org/10.3390/liquids4010009 - 12 Feb 2024
Abstract
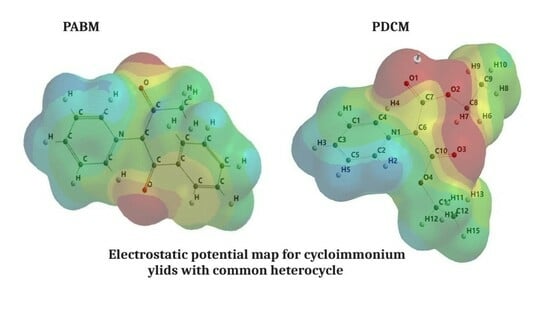
This article contains a comparative spectral analysis corroborated with the quantum mechanical computations of four cycloimmonium ylids. The spectral shift of the visible electronic absorption band of the studied molecules in 20 solvents with different empirical parameters is expressed by linear multi-parametric dependences
[...] Read more.
This article contains a comparative spectral analysis corroborated with the quantum mechanical computations of four cycloimmonium ylids. The spectral shift of the visible electronic absorption band of the studied molecules in 20 solvents with different empirical parameters is expressed by linear multi-parametric dependences that emphasize the intramolecular charge transfer (ICT) process. The nature of molecular interactions and their contribution to the spectral shift of the visible ICT band of solutes are also established in this manuscript. The results of the statistical analysis are used to estimate the cycloimmonium ylids’ excited dipole moment by the variational method, using the hypothesis of McRae. The importance of the structure of both the heterocycle and carbanion substituents to the stability and reactivity of the studied cycloimmonium ylids is underlined by the quantum mechanical computations of the molecular descriptors.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
Graphical abstract
Open AccessCommunication
Solvent Polarity/Polarizability Parameters: A Study of Catalan’s SPPN, Using Computationally Derived Molecular Properties, and Comparison with π* and ET(30)
by
W. Earle Waghorne
Liquids 2024, 4(1), 163-170; https://doi.org/10.3390/liquids4010008 - 08 Feb 2024
Abstract
Catalan’s SPPN, a measure of solvent polarity/polarizability has been analysed in terms of molecular properties derived from computational chemistry. The results show that SPPN correlates positively with the molecular dipole moment and quadrupolar amplitude and negatively with the molecular polarizability.
[...] Read more.
Catalan’s SPPN, a measure of solvent polarity/polarizability has been analysed in terms of molecular properties derived from computational chemistry. The results show that SPPN correlates positively with the molecular dipole moment and quadrupolar amplitude and negatively with the molecular polarizability. These correlations are shared with Kamet and Taft’s π* and Reichardt and Dimroth’s ET(30). Thus, one can associate the solvent polarity with non-specific interactions involving the permanent charges on solvent molecules. It is also noted that the opposite correlations, all three parameters increasing with increasing solvent polarity but decreasing with increasing solvent polarizability, creates an ambiguity in their use, for example, in linear free energy relationships.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
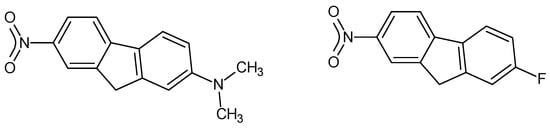
Figure 1
Open AccessArticle
Use of DFT Calculations as a Tool for Designing New Solvatochromic Probes for Biological Applications
by
Cynthia M. Dupureur
Liquids 2024, 4(1), 148-162; https://doi.org/10.3390/liquids4010007 - 04 Feb 2024
Abstract
The intramolecular charge transfer behavior of push–pull dyes is the origin of their sensitivity to environment. Such compounds are of interest as probes for bioimaging and as biosensors to monitor cellular dynamics and molecular interactions. Those that are solvatochromic are of particular interest
[...] Read more.
The intramolecular charge transfer behavior of push–pull dyes is the origin of their sensitivity to environment. Such compounds are of interest as probes for bioimaging and as biosensors to monitor cellular dynamics and molecular interactions. Those that are solvatochromic are of particular interest in studies of lipid dynamics and heterogeneity. The development of new solvatochromic probes has been driven largely by the need to tune desirable properties such as solubility, emission wavelength, or the targeting of a particular cellular structure. DFT calculations are often used to characterize these dyes. However, if a correlation between computed (dipole moment) and experimentally measured solvatochromic behavior can be established, they can also be used as a design tool that is accessible to students. Here, we examine this correlation and include case studies of the effects of probe modifications and conformation on dipole moments within families of solvatochromic probes. Indeed, the ground state dipole moment, an easily computed parameter, is correlated with experimental solvatochromic behavior and can be used in the design of new environment-sensitive probes before committing resources to synthesis.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
Figure 1
Open AccessArticle
Evaluation of Thermodynamic and Kinetic Contributions to Over-Extraction of Extractables by Nonpolar Organic Solvents in Comparison to Lipids in Exhaustive and Exaggerated Extractions of Medical Devices Based on Abraham Solvation Model and Solvent–Material Interactions Using Low-Density Polyethylene as a Representative Material
by
Jianwei Li
Liquids 2024, 4(1), 117-147; https://doi.org/10.3390/liquids4010006 - 23 Jan 2024
Abstract
The thermodynamic and kinetic contributions to the over-extraction of extractables by nonpolar organic solvents relative to biological lipids in exhaustive and exaggerated extractions of medical devices are studied based on the Abraham solvation model and solvent–material interactions, using low-density polyethylene (LDPE) as an
[...] Read more.
The thermodynamic and kinetic contributions to the over-extraction of extractables by nonpolar organic solvents relative to biological lipids in exhaustive and exaggerated extractions of medical devices are studied based on the Abraham solvation model and solvent–material interactions, using low-density polyethylene (LDPE) as an exemplary material. The thermodynamic effect is evaluated by the partition constant of extractables between LDPE and extraction solvents, hexane and lipids, defined as the concentration in the polymer phase divided by the concentration in the solvent phase. The Abraham solvation model is used to correlate the measured LDPE-lipid partition constant (
(This article belongs to the Special Issue Recent Advances in the Behavior of Liquids in Honor of Prof. Dr. William Acree Jr.)
►▼
Show Figures
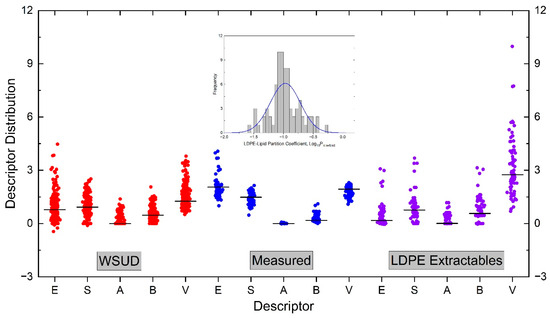
Figure 1
Open AccessPerspective
Polarity of Aqueous Solutions
by
Pedro P. Madeira, Luisa A. Ferreira, Vladimir N. Uversky and Boris Y. Zaslavsky
Liquids 2024, 4(1), 107-116; https://doi.org/10.3390/liquids4010005 - 12 Jan 2024
Abstract
This short review describes the expansion of the solvatochromic approach utilizing water-soluble solvatochromic dyes to the analysis of solvent features of aqueous media in solutions of various compounds. These solvent features (polarity/dipolarity, hydrogen bond donor ability (HBD acidity), and hydrogen bond acceptor ability
[...] Read more.
This short review describes the expansion of the solvatochromic approach utilizing water-soluble solvatochromic dyes to the analysis of solvent features of aqueous media in solutions of various compounds. These solvent features (polarity/dipolarity, hydrogen bond donor ability (HBD acidity), and hydrogen bond acceptor ability (HBA basicity)) vary depending on the nature and concentration of a solute. Furthermore, the solvent features of water (the solvent dipolarity/polarizability and hydrogen bond donor ability) in solutions of various compounds describe multiple physicochemical properties of these solutions (such as the solubility of various compounds in aqueous solutions, salting-out and salting-in constants for polar organic compounds in the presence of different inorganic salts, as well as water activity, osmotic coefficients, surface tension, viscosity, and the relative permittivity of aqueous solutions of different individual compounds) and are likely related to changes in the arrangement of hydrogen bonds of water in these solutions.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
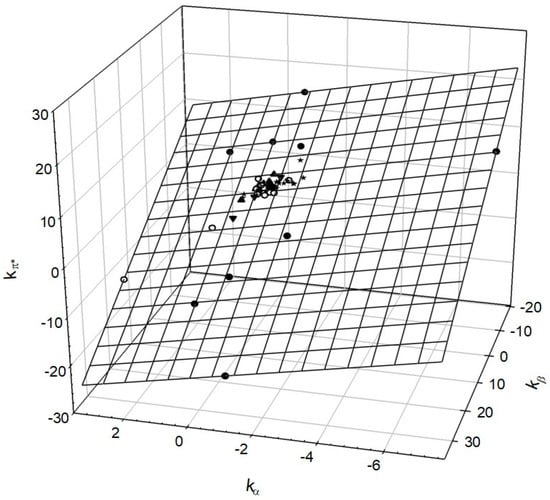
Figure 1
Open AccessArticle
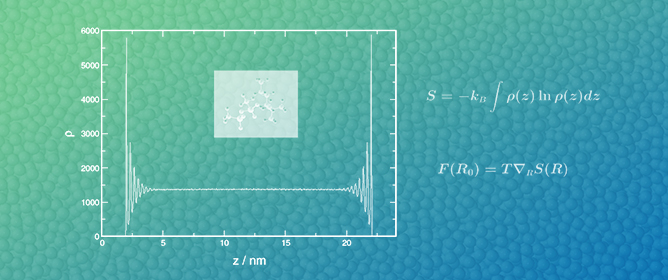
The Spatial Entropy of Confined Liquids
by
Henry J. Castejón
Liquids 2024, 4(1), 95-106; https://doi.org/10.3390/liquids4010004 - 08 Jan 2024
Abstract
►▼
Show Figures
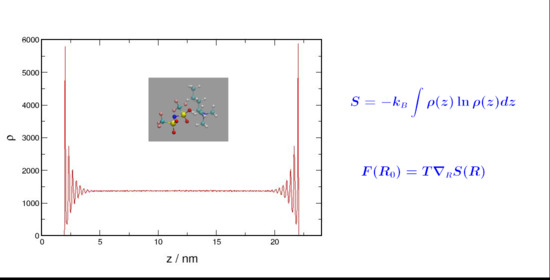
Molecular dynamics simulations have been used to investigate the structural changes in confined liquids. The density distribution functions for weakly and strongly interacting liquids were determined and compared to those of a non-interacting system in order to assess the impact of the entropic
[...] Read more.
Molecular dynamics simulations have been used to investigate the structural changes in confined liquids. The density distribution functions for weakly and strongly interacting liquids were determined and compared to those of a non-interacting system in order to assess the impact of the entropic forces on the equilibrium state of the systems. The effect of the entropic forces was assessed by quantifying the layering on the liquid structure upon confinement. The more pronounced layering obtained for weakly interacting and non-interacting systems indicated that entropic forces are more effective in these systems where an increase in the multiplicity of states does not require a prohibitively high cost in energy.
Full article
Graphical abstract
Open AccessReview
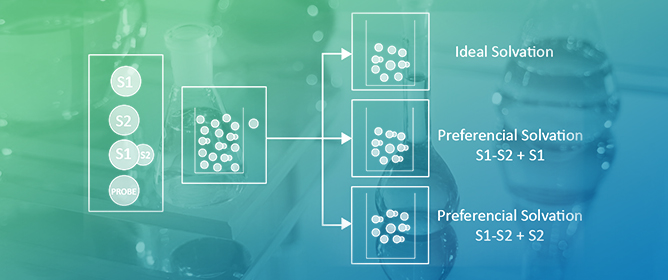
Solvatochromism in Solvent Mixtures: A Practical Solution for a Complex Problem
by
Omar A. El Seoud, Shirley Possidonio and Naved I. Malek
Liquids 2024, 4(1), 73-94; https://doi.org/10.3390/liquids4010003 - 03 Jan 2024
Abstract
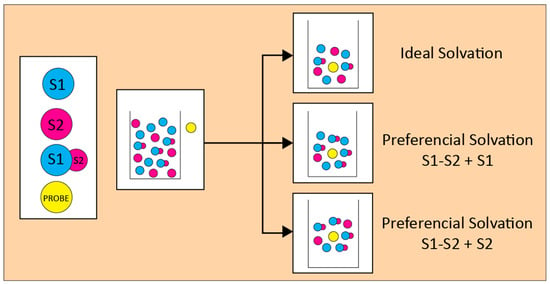
Many reactions are carried out in solvent mixtures, mainly because of practical reasons. For example, E2 eliminations are favored over SN2 substitutions in aqueous organic solvents because the bases are desolvated. This example raises the question: how do we chose binary
[...] Read more.
Many reactions are carried out in solvent mixtures, mainly because of practical reasons. For example, E2 eliminations are favored over SN2 substitutions in aqueous organic solvents because the bases are desolvated. This example raises the question: how do we chose binary solvents to favor reaction outcomes? This important question is deceptively simple because it requires that we understand the details of all interactions within the system. Solvatochromism (solvent-dependent color change of a substance) has contributed a great deal to answer this difficult question, because it gives information on the interactions between solvents, solute-solvent, and presumably transition state-solvent. This wealth of information is achieved by simple spectroscopic measurements of selected (solvatochromic) substances, or probes. An important outcome of solvatochromism is that the probe solvation layer composition is almost always different from that of bulk mixed solvent. In principle, this difference can be exploited to “tune” the composition of solvent mixture to favor the reaction outcome. This minireview addresses the use of solvatochromic probes to quantify solute-solvent interactions, leading to a better understanding of the complex effects of solvent mixtures on chemical phenomena. Because of their extensive use in chemistry, we focus on binary mixtures containing protic-, and protic-dipolar aprotic solvents.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
Graphical abstract
Open AccessReview
Colloid Chemistry of Fullerene Solutions: Aggregation and Coagulation
by
Nikolay O. Mchedlov-Petrossyan, Mykyta O. Marfunin and Nika N. Kriklya
Liquids 2024, 4(1), 32-72; https://doi.org/10.3390/liquids4010002 - 25 Dec 2023
Cited by 1
Abstract
This review article is devoted to the colloidal properties of fullerene solutions. According to generally accepted understandings, all solvents in relations to fullerenes are divided into “good”, “poor”, and “reactive”. We have consistently considered the state of fullerenes in these systems. In “good”,
[...] Read more.
This review article is devoted to the colloidal properties of fullerene solutions. According to generally accepted understandings, all solvents in relations to fullerenes are divided into “good”, “poor”, and “reactive”. We have consistently considered the state of fullerenes in these systems. In “good”, predominantly non-polar aromatic solvents and CS2, non-equilibrium dissolution methods lead to the formation of colloidal aggregates, whereas the utilization of equilibrium methods results in the formation of molecular solutions. The latter, however, have some unusual properties; new results considered in this review confirm previously expressed ideas about colloidal properties of these solutions. In “poor” (polar) solvents, lyophobic colloidal systems appear. Both “bottom-up” and “top-down” methods of preparation are well documented in the literature. However, N-methylpyrrolidine-2-one, DMSO, and DMF dissolve fullerenes quite easily and with less energy consumption. These solvents can be considered a subset of “poor” solvents that have some features of being “reactive” at the expense of basic properties. New data confirm that hydrosols of fullerenes are typical hydrophobic colloids that obey the Schulze–Hardy rule and other regularities in the presence of electrolytes. Organosols in acetonitrile and methanol are much less stable with respect to the effects of electrolytes. This allows us to assume a non-DLVO stabilizing factor in the hydrosols. Accordingly, a new estimate of the Hamaker constant of fullerene–fullerene interaction is proposed. In DMSO and DMF, the coagulation of fullerene sols is hindered due to strong solvation with these basic solvents.
Full article
(This article belongs to the Special Issue Nanocarbon-Liquid Systems)
►▼
Show Figures
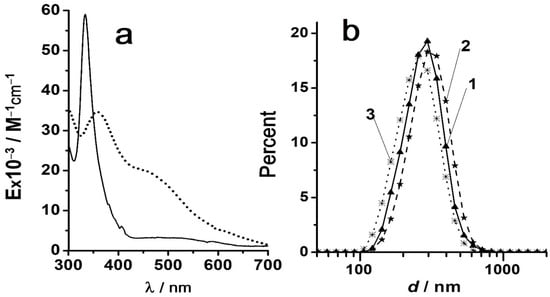
Figure 1
Open AccessReview
Inclusion Bodies in Ionic Liquids
by
András Szabadi, Robert Klausser, Oliver Spadiut and Christian Schröder
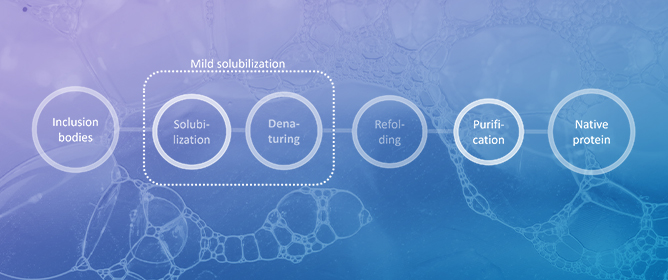
Liquids 2024, 4(1), 1-31; https://doi.org/10.3390/liquids4010001 - 22 Dec 2023
Abstract
The pivotal role of proteins in pharmaceuticals is challenged by stability issues, making the study of inclusion bodies—a source of insoluble protein aggregates—increasingly relevant. This review outlines the critical procedures in inclusion body processing, focusing on ’mild solubilization concepts’ and refolding methodologies. Attention
[...] Read more.
The pivotal role of proteins in pharmaceuticals is challenged by stability issues, making the study of inclusion bodies—a source of insoluble protein aggregates—increasingly relevant. This review outlines the critical procedures in inclusion body processing, focusing on ’mild solubilization concepts’ and refolding methodologies. Attention is afforded to the emerging role of ionic liquids with unique and tunable physicochemical properties in optimizing protein unfolding and refolding processes. The review critically assesses the existing literature at the intersection of inclusion bodies and ionic liquids, identifying recent advancements, potential applications, and avenues for future research. This comprehensive analysis aims to elucidate the complexities in efficient protein processing from inclusion bodies.
Full article
(This article belongs to the Section Chemical Physics of Liquids)
►▼
Show Figures
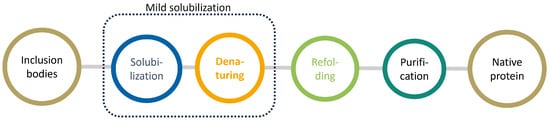
Figure 1
Open AccessCommunication
Prediction of Paracetamol Solubility in Binary Solvents Using Reichardt’s Polarity Parameter Combined Model
by
Elaheh Rahimpour and Abolghasem Jouyban
Liquids 2023, 3(4), 512-521; https://doi.org/10.3390/liquids3040032 - 14 Dec 2023
Abstract
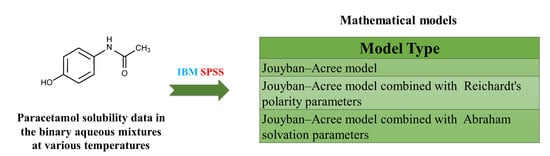
The objective of this research is to propose a general model utilizing the solvatochromic polarity of electronic transition energy (ET) of the Reichardt indicator to predict paracetamol solubility in the solvent mixtures. In order to model validation, the available ET (30) values of
[...] Read more.
The objective of this research is to propose a general model utilizing the solvatochromic polarity of electronic transition energy (ET) of the Reichardt indicator to predict paracetamol solubility in the solvent mixtures. In order to model validation, the available ET (30) values of nine aqueous mixtures obtained from existing literature sources were utilized. The trained model yielded a relatively accurate estimation of paracetamol solubility in the investigated systems.
Full article
(This article belongs to the Special Issue Solvatochromic Probes and Their Applications in Molecular Interaction Studies—a Themed Issue to Honor Professor Dr. Christian Reichardt)
►▼
Show Figures
Graphical abstract
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Conferences
Special Issues
Special Issue in
Liquids
Recent Advances in the Behavior of Liquids in Honor of Prof. Dr. William Acree Jr.
Guest Editors: William E. Acree, Jr., Juan SaavedraDeadline: 31 May 2024
Special Issue in
Liquids
Solubility and Solubilization of Drugs: Modeling and Thermodynamic Analysis
Guest Editor: Daniel Ricardo DelgadoDeadline: 31 July 2024
Special Issue in
Liquids
Nanocarbon-Liquid Systems
Guest Editor: Nikolay O. Mchedlov-PetrossyanDeadline: 31 August 2024
Topical Collections
Topical Collection in
Liquids
Feature Papers in Solutions and Liquid Mixtures Research
Collection Editors: Enrico Bodo, Federico Marini