1. Introduction
Entropic forces are dominant in condensed matter systems such as colloids, polymers, and even biological systems. These forces originate from the collective tendency of a system to maximize its entropy and without any apparent underlying potential. Consequently, they are considered “effective” or “emergent” forces of the physical system. These forces have been observed to manifest as an attractive interaction between particles in suspensions of macromolecules despite the absence of any energetic interaction between the suspended particles. Their magnitude seems to depend on the concentration of the solution and the shape and size of macromolecules and co-solutes. They have been found to have a significant effect on the phase and the structure of the solutions of macromolecules [
1] and to be particularly stronger in suspensions containing charged species [
2]. Depletion forces have been described in many other systems. They are considered to be a subset of a more general set of interactions known as “structural forces” [
3].
An observation of the effect of entropic forces on the structure and the phase of a thermodynamic system was reported, perhaps for the first time, in 1939 [
1]. It was observed that a stable dispersion of an elastomeric polymer (rubber latex) undergoes a phase separation upon mixing with certain hydrophilic colloids. No explanation of the possible mechanism for the induced phase transition was provided at that time. The earliest formal description of the underlying mechanism for the emergence of entropic forces in solutions of macromolecules was provided by Sho Asakura and Fumio Oosawa [
4]. The entropic force in such systems is known as the Asakura–Oosawa depletion interaction [
5].
The Asakura–Oosawa model [
5] describes the behavior of a colloid suspension and presents a formal derivation of the interactions between particles in a suspension of macromolecules in the presence of co-solutes. The model derives the Asakura–Oosawa depletion interaction as an attractive force resulting from the balance between the gain of translational entropy by the small molecules in the system due to an increase in the excluded volume and the loss of translational entropy by the macromolecules due to the flocculation of the suspension [
5]. The larger magnitude of the gain in translational entropy by the small colloids originates the entropic force. Since the Asakura–Oosawa model considers all the solutes hard spheres, it lacks any energetic contribution. Consequently, the effective force that is obtained is considered entirely entropic. A virial formulation of the Asakura–Oosawa effective force, recently reported [
2], confirmed that the Asakura-Oosawa force, in its original formulation, involving just pair-wise interactions, is exact for small-size ratios between the small colloids and the macromolecules.
The driving force for any process taking place in a thermodynamic system is the chemical potential. In an isothermal system, the chemical potential results from changes in the Helmholtz free energy and any energetic contribution in those systems results from changes in the configurational space. These changes are entirely determined by the potential energy. Therefore, for any force to be effective in such conditions, it must be strong enough to overcome the actual interaction potential in the thermodynamic system.
In the formalism of classical statistical mechanics, the entropy (
S) of a system described by coordinates
q and canonical conjugate
p in the phase space (
) is given as a function of the probability density function
as [
6]
where
is the Boltzmann’s constant. The probability distribution function (pdf) is given by
where
,
T is the temperature,
is the system’s energy, and
is the partition function. For a canonical ensemble, the entropy can be expressed as
where
is the ensemble-average energy given by
By splitting the total energy (
) into kinetic (
) and potential (
) energies as
the partition function can be factorized as
where
are, respectively, the kinetic and spatial partition functions. This allows for us to write the probability distribution function as
where
are, respectively, the kinetic and the spatial distribution functions. Spatial entropy can now be obtained integrating over the configuration phase space
and for the ensemble
where
is the ensemble-average potential energy. Since the dependence on kinetic energy is factored out, the spatial entropy does not depend on the temperature, only on the spatial coordinates. Therefore, for an isothermal restructuring process, the total entropy change in the system is
The work associated with the structural changes in the system can be obtained as
This formalism can be used to analyze isothermal processes. In such processes, all changes take place in the configurational space. Consequently, the Helmholtz free energy can be expressed as
where
is the change in internal potential energy and
is the change in configurational entropy.
This work investigates the structural changes in confined liquids that have been attributed to entropic forces. The distribution function for non-interacting, weakly interacting, and strongly interacting systems under confinement are determined and examined in order to gain insight into the impact of entropic forces on these systems.
3. Discussion
All calculated distribution functions show similar behavior. Moving from the center of the region toward the walls, or to the edges in the case of the pins-on-a-rope system, the probability increases from the bulk value at the center and oscillates forming layers of increased probability in the vicinity of the walls. The increase in probability density is significantly less pronounced for the case of liquid NaCl.
The strong layering effect observed for the one-dimensional, non-interacting system of the pins on the rope has been described previously [
13]. The increased probability on both edges has been attributed to an attractive entropic force created by the inaccessible space at both ends of the line. This force has been termed an “effective” force. That is, it is not generated by any underlying potential. From the point of view of pure statistics, the final configuration of the system corresponds to the distribution function of a single particle that translates into a configuration that provides the maximum accessible space for all the other particles. This configuration has the highest probability [
14]. In such a configuration, each individual particle is attracted to the edges and particles are attracted to each other. Similar results have been obtained for three-dimensional systems composed of non-interacting hard spheres [
15].
In the case of the interacting, three-dimensional systems of argon, nbatf2 and liquid NaCl, the distribution functions show the formation of layers, with increased probability, parallel to the confining walls. The density of the layers decreases down to the bulk density of the particular liquid when moving from the walls to the middle point between them. In spite of the significant differences in structural complexity and interaction potentials, the three systems display remarkably similar density distribution functions. All these distribution functions are also very similar to those of the one-dimensional, non-interacting system of pins on a rope. It is also noticeable that the distribution function for liquid NaCl, although it displays the same oscillations and increases probability in the vicinity of the walls as the others, the amplitude of the oscillations is much lower than that of all of the other systems. This layering effect due to confinement has been previously obtained for simple [
8,
15] and complex [
16] liquids, and it is believed to be understood qualitatively [
17]. Namely, the excluded volume at the edges of the system attracts the liquid particles increasing the density in these regions. This attraction is postulated to be an entropic force [
13]. Once periodic boundary conditions are restored in all directions, the density of the entire system becomes uniform with a magnitude equal to the bulk density of the liquid.
In all the studied liquids, the distribution functions show that the atoms and molecules are attracted to the walls which is reminiscent of what is observed in the non-interacting system of pins on a rope. However, in the liquid systems, the particles exist on an energy landscape. Therefore, in order to effect change in the system, the entropic forces must overcome the energetic cost of redistributing the particles. In an isothermal process, this cost is given by the change in potential energy.
For the pins-on-the-rope system, the probability distribution shown in
Figure 1 can be interpreted as a Boltzmann distribution with a potential which gives rise to an entropic force that attracts the pins to the wall and increases the probability in that region. The gradient for that force is the tendency of the system to increase its entropy [
18]. Thus,
Equation (
19) is the force for the macroscopic state
and
is the change in
S with respect to the macroscopic state.
This is the only force acting in the pins-on-the-rope system since the there is no interaction potential. The magnitude of this force can be estimated from Equation (
11) as
A similar calculation can be performed for all three-dimensional confined liquids, argon, nbatf2, and NaCl, by using the density distribution function calculated along the
z axis and perpendicular to the plane of the confining walls. This density distribution function is calculated for slabs that are parallel to the confining walls and have a thickness of
. Because of the symmetry of the systems, which have periodic boundary conditions in the
x and
y directions but not in the
z direction, this density function is equivalent to the linear density along the
z direction in the pins-on-the-rope system. On performing the calculations for the magnitude of the entropic force, using mass density (
) units or number density (
) makes no difference since the slope (
) of the curve is the same in both cases because the shape of the curves is identical. For unit consistency, number density units are used in Equation (
20). The magnitude of the entropic force was estimated as the average slope along the entire range of the
z-coordinate.
Table 2 shows the magnitude of the entropic forces. For thermodynamic systems, the force was estimated using Equation (
20) and the respective distribution function along the
z-axis as shown in
Figure 3,
Figure 6 and
Figure 7. The strength of this force appears similar in all the studied systems. This is mainly due to the fact that this force is the slope of the curve
and small but steep changes in the distribution function can contribute substantially to its magnitude. However, the effectiveness of the force seems to diminish in the case of liquid NaCl.
Liquid argon is the thermodynamic system that more closely resembles the pins-on-the-rope system. Since its interaction potential is extremely weak, the energetic cost for the restructuring of the liquid is effectively zero. Both liquid argon and the pins-on-a-rope system adopt the most statistically favored configuration.
For systems with a strong interaction potential, the entropic force expression must be reformulated to [
19]
where
is the change in the Helmholtz free energy with respect to macroscopic state
.
represents the force in macroscopic state
. A rigorous application of this equation requires the knowledge of the dependence of the internal potential energy on
z (
). However, an average approximate magnitude for this parameter can be obtained as the difference between the potential energy between the confined and the unconfined structures of the liquid. In isothermic processes, the contribution of internal energy comes solely from the potential energy. This change in potential energy represents the energetic cost of redistributing the particles in the system. It can be estimated as the difference in internal potential energy between the molecular configurations of the unconfined and the confined states (
).
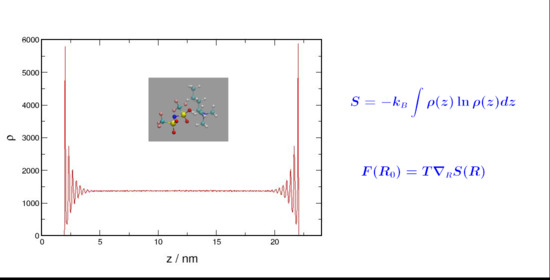
Because the nbatf2 molecule (see
Figure 4) distributes the ionic charges over a large volume reducing the strength of the electrostatic interaction, liquid nbatf2 has an interaction potential with an intermediate strength between that of the potential in argon and in liquid NaCl. Restructuring (layering) of the nbatf2 system takes place but it has an energetic cost that opposes the entropic force. This is shown in
Table 2. Because of the strength of the interaction potential in nbatf2, the energetic cost is affordable and nbatf2, like argon, displays significant layering upon confinement.
Although nbatf2 and NaCl are both ionic liquids, there is no dielectric screening in NaCl. Consequently, the interaction potential in NaCl is three times stronger than that in nbatf2. This strong potential increases the energetic cost of reorganizing the liquid and renders the entropic force ineffective in NaCl. This is demonstrated by the very small density oscillations observed in the probability distribution function for NaCl in
Figure 7 and the very low energetic cost of the redistribution of particles (see
Table 2) in the case of NaCl. The extremely low (almost zero) magnitude of this parameter indicates that there is not much redistribution of the system upon confinement due to the strong interaction potential.
Since distribution functions in the confined environment display oscillations around the bulk density, the best way to estimate the magnitude of the restructuring taking place in the liquid is to determine the standard deviation (
) of the normalized probability distribution function
. This was calculated as
The standard deviation of the local density from the bulk density of the liquid provides a measurement of the magnitude of the layering. In order to be able to compare the standard deviations for the different density distribution functions, the functions were normalized and their zeros were set to the respective bulk value of liquid density.
Table 2 shows the standard deviation for density distribution function
of the studied liquids. NaCl has the smallest deviation from bulk density. This supports the assertion that no restructuring is taking place in that liquid.
Although nbatf2 displays a seemingly small standard deviation with respect to bulk density, it is, however, thirty eight times larger than that of NaCl. This demonstrates the modulating effect that the molecular structure of nbatf2 (see
Figure 4) has on the interaction potential. The covalent part of the molecule screens the ionic charges and weakens the electrostatic interaction in a similar fashion to water in ionic aqueous solutions.
4. Concluding Remarks
In a non-interacting system, the configurational state that is observed is the one with the highest probability. The multiplicity of such a state increases its probability. On flipping a coin, all sequences have the same probability, but the heterogeneous sequences (those composed of heads and tails) have a higher multiplicity than the homogeneous sequences (those composed of only heads or only tails); consequently, the heterogeneous sequences are observed more frequently because there is no penalty when switching between these two types of sequences. The randomness in these systems maximizes the effect of entropy.
In a thermodynamic system, entropy dissipates energy by creating multiple states. If the average energy of these states is lower than the internal potential energy, an energy gradient is created which, in turn, gives rise to an entropic force. If the system can be disaggregated into multiple states of different but accessible energy, entropy plays a role. This process can be aided by steric interactions and crowding effects [
20], which facilitate the increase in the multiplicity of states as it can be inferred from its dominance in polymer solutions [
21], colloidal suspensions [
22], and even biological systems [
23]. However, if the energetic cost of disaggregation is prohibitive, the increase in multiplicity does not occur.
The quantitative assessment of the layering effect upon confinement for liquids with interaction potentials of different strength shows that the restructuring of the liquid is more pronounced for weakly interacting liquids. The layering effect diminishes as the strength of the interaction potential increases. The physical systems with weak interaction potentials appear to be more susceptible to entropic forces. All this suggests that, in the case of isothermic processes, the entropic forces may only be effective in systems with weak or moderately strong interaction potentials.