Feature Papers in Journal of Molecular Pathology
Share This Topical Collection
Editors
 Prof. Dr. Giancarlo Troncone
Prof. Dr. Giancarlo Troncone
 Prof. Dr. Giancarlo Troncone
Prof. Dr. Giancarlo Troncone
E-Mail
Website
Guest Editor
Department of Public Health, University of Naples Federico II, 80131 Naples, Italy
Interests: molecular cytopathology; lung cancer; cytopathology; next-generation sequencing; thyroid neoplasms; liquid biopsy; immunotherapy
Special Issues, Collections and Topics in MDPI journals
 Dr. Pasquale Pisapia
Dr. Pasquale Pisapia
 Dr. Pasquale Pisapia
Dr. Pasquale Pisapia
E-Mail
Website
Guest Editor
Department of Public Health, University of Naples Federico II, Naples, Italy
Interests: molecular pathology; predictive molecular pathology in solid tumors; next-generation sequencing; liquid biopsies; biomarkers; target therapies; immunotherapy
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topical Collection “Feature Papers in Journal of Molecular Pathology” aims to collect high-quality research articles, review articles, and communications in all the fields of molecular pathology research and routine practice application. Since the aim of this Topical Collection is to illustrate, through selected works, frontier research in molecular pathology, we encourage Editorial Board Members of Journal of Molecular Pathology to contribute papers reflecting the latest progress in their research field, and welcome relevant experts and colleagues to do so as well. Please kindly note that only invited papers can be published online once accepted in this collection.
Topics include, but are not limited to, the following:
- Technological advances in the field of genomics, proteomics, and metabolomics.
- Predictive and prognostic biomarkers;
- Translational studies involving the adoption of novel approaches;
- Novel sources of tumoral nucleic acids (liquid biopsies).
Prof. Dr. Giancarlo Troncone
Dr. Pasquale Pisapia
Guest Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Journal of Molecular Pathology is an international peer-reviewed open access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript.
The Article Processing Charge (APC) for publication in this open access journal is 1000 CHF (Swiss Francs).
Submitted papers should be well formatted and use good English. Authors may use MDPI's
English editing service prior to publication or during author revisions.
Keywords
- predictive biomarkers
- prognostic biomarkers
- next generation technologies
- molecular pathology
- target therapies
- liquid biopsies
- immunotherapy
- tissue-based analysis
- solid tumors
- molecular techniques
Published Papers (6 papers)
Open AccessReview
STING-Associated Vasculopathy with Onset in Infancy: A Review Focusing on Pathophysiology and Treatment Options
by
Konstantinos Drougkas, Roubini Smerla, Charalampos Skarlis and Clio P. Mavragani
Viewed by 1199
Abstract
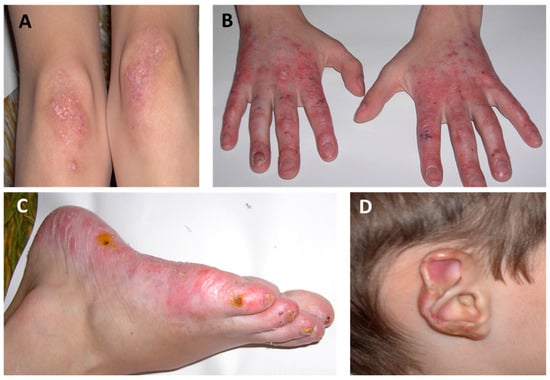
STING-associated vasculopathy with onset in infancy (SAVI) is a rare type Ι interferonopathy caused by gain of function mutations in an encoding stimulator of interferon genes (STING) protein 1. SAVI is characterized by neonatal or infantile-onset systemic inflammation, mainly affecting peripheral cutaneous blood
[...] Read more.
STING-associated vasculopathy with onset in infancy (SAVI) is a rare type Ι interferonopathy caused by gain of function mutations in an encoding stimulator of interferon genes (STING) protein 1. SAVI is characterized by neonatal or infantile-onset systemic inflammation, mainly affecting peripheral cutaneous blood vessels, skin, and lungs. The main disease manifestations include recurrent febrile episodes, cough, dyspnea, and failure to thrive, in association with progressive interstitial lung disease, polyarthritis, and cold-induced red violet plaques or papules on fingers, knees, toes, heels, nasal tip, and ears that can lead to distal ulcerations, skin necrosis, tissue loss, and autoamputation. For the management of SAVI, JAK inhibitors can be a valuable therapeutic intervention that hampers disease progression, while conventional immunosuppressive treatments have shown minimal efficacy. This review aims to describe the underlying pathophysiologic mechanisms of SAVI, highlighting the main clinical manifestations and discussing the current treatment approaches.
Full article
►▼
Show Figures
Open AccessReview
The Potential Role of Histone Modifications in Glioblastoma Therapy: Review Article
by
Mohammed A. Azab
Viewed by 1884
Abstract
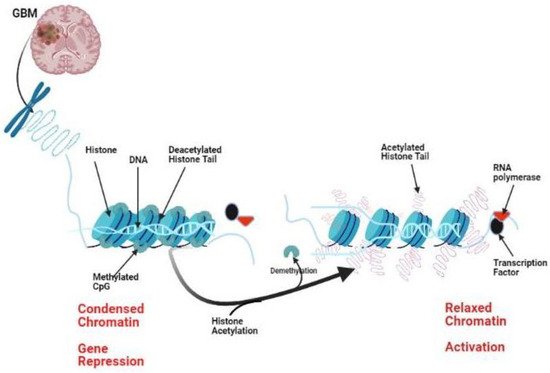
Glioblastoma (GBM) is considered the most aggressive primary brain tumor. Recurrence after treatment is a significant problem with a failed response to optimal therapies. The recurrence of GBM is linked to different cellular and molecular pathways. Not only genetics are involved in gliomagenesis,
[...] Read more.
Glioblastoma (GBM) is considered the most aggressive primary brain tumor. Recurrence after treatment is a significant problem with a failed response to optimal therapies. The recurrence of GBM is linked to different cellular and molecular pathways. Not only genetics are involved in gliomagenesis, but also epigenetics. Histone modulation through acetylation, phosphorylation, ubiquitination, and methylation can regulate gene expression and may play a role in the pathogenesis of GBM. Preclinical and clinical studies currently target epigenetic enzymes in gliomas, including a new generation of histone deacetylase (HDAC) inhibitors. Herein, I tried to highlight current research in glioma epigenetics, focusing on the culprit of histone modifications and the use of HDAC target therapies as a possible treatment line for glioblastoma.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
BRAF and MLH1 Analysis Algorithm for the Evaluation of Lynch Syndrome Risk in Colorectal Carcinoma Patients: Evidence-Based Data from the Analysis of 100 Consecutive Cases
by
Thais Maloberti, Antonio De Leo, Viviana Sanza, Lidia Merlo, Michela Visani, Giorgia Acquaviva, Sara Coluccelli, Annalisa Altimari, Elisa Gruppioni, Stefano Zagnoni, Daniela Turchetti, Sara Miccoli, Michelangelo Fiorentino, Antonietta D’Errico, Dario de Biase and Giovanni Tallini
Cited by 1 | Viewed by 3415
Abstract
Several causes may lead to CRC, either extrinsic (sporadic forms) or genetic (hereditary forms), such as Lynch syndrome (LS). Most sporadic deficient mismatch repair (dMMR) CRC cases are characterized by the methylation of the
MLH1 promoter gene and/or
BRAF gene mutations. Usually, the
[...] Read more.
Several causes may lead to CRC, either extrinsic (sporadic forms) or genetic (hereditary forms), such as Lynch syndrome (LS). Most sporadic deficient mismatch repair (dMMR) CRC cases are characterized by the methylation of the
MLH1 promoter gene and/or
BRAF gene mutations. Usually, the first test performed is the mismatch repair deficiency analysis. If a tumor shows a dMMR,
BRAF mutations and then the
MLH1 promoter methylation status have to be assessed, according to the ACG/ASCO screening algorithm. In this study, 100 consecutive formalin-fixed and paraffin-embedded samples of dMMR CRC were analyzed for both
BRAF mutations and
MLH1 promoter methylation. A total of 47 (47%) samples were
BRAF p.V600E mutated, while
MLH1 promoter methylation was found in 77 cases (77.0%). The pipeline “BRAF-followed-by-MLH1-analysis” led to a total of 153 tests, while the sequence “MLH1-followed-by-BRAF-analysis” resulted in a total of 123 tests. This study highlights the importance of performing
MLH1 analysis in LS screening of
BRAF-WT specimens before addressing patients to genetic counseling. We show that
MLH1 analysis performs better as a first-line test in the screening of patients with LS risk than first-line
BRAF analysis. Our data indicate that analyzing
MLH1 methylation as a first-line test is more cost-effective.
Full article
►▼
Show Figures
Open AccessArticle
Use of the Biocartis Idylla™ Platform for the Detection of Epidermal Growth Factor Receptor, BRAF and KRAS Proto-Oncogene Mutations in Liquid-Based Cytology Specimens from Patients with Non-Small Cell Lung Carcinoma and Pancreatic Adenocarcinoma
by
Leonie Wheeldon, Mary Jones, Ben Probyn, Dushyant Shetty and James Garvican
Cited by 1 | Viewed by 2987
Abstract
The study aimed to demonstrate rapid and effective molecular testing on liquid-based cytology (LBC) samples for
EGFR,
KRAS and
BRAF mutations using the Biocartis Idylla™. Rapid on-site evaluation (ROSE) LBC samples for patients with non-small cell lung carcinoma (NSCLC) or pancreatic ductal
[...] Read more.
The study aimed to demonstrate rapid and effective molecular testing on liquid-based cytology (LBC) samples for
EGFR,
KRAS and
BRAF mutations using the Biocartis Idylla™. Rapid on-site evaluation (ROSE) LBC samples for patients with non-small cell lung carcinoma (NSCLC) or pancreatic ductal adenocarcinoma (PDAC) were tested for
EGFR,
KRAS and
BRAF mutations based on the relevance to tumour subtype. The quantification values (Cq values) and mutation detection status were compared between LBC samples and routine formalin-fixed paraffin-embedded (FFPE) clot samples. ROSE LBC samples (
n = 54) showed a higher yield of well-preserved tumour and wild type (WT) DNA, demonstrated by lower quantification cycles, no false positives or false negatives, and a higher sensitivity for low allele frequency mutations when compared with FFPE clot samples. The Biocartis Idylla™ provides highly sensitive, reliable and rapid testing for LBC samples for the detection of
EFGR and
KRAS mutations.
BRAF mutations were not detected in the participant cohort; however, all LBC WT
BRAF results correlated with the results from the FFPE clot samples. Access to rapid molecular testing using LBC samples can detect the most frequent driver mutations closer to the time of diagnosis, enabling the selection of the most effective first-line targeted therapy sooner, reducing delays or side effects from suboptimal treatments, patient anxiety and costs to healthcare systems, whilst improving patient outcomes.
Full article
►▼
Show Figures
Open AccessFeature PaperArticle
Evaluation of the TruSight Tumor 170 Assay and Its Value in Clinical Diagnostics
by
Carina Heydt, Roberto Pappesch, Katrin Stecker, Martin März and Sabine Merkelbach-Bruse
Viewed by 3308
Abstract
Background: Parallel sequencing technologies have become integrated into clinical practice. This study evaluated the TruSight Tumor 170 assay for the simultaneous detection of somatic gene mutations (SNPs and indels), gene fusions and CNVs, and its implementation into routine diagnostics. Methods: Forty-four formalin-fixed, paraffin-embedded
[...] Read more.
Background: Parallel sequencing technologies have become integrated into clinical practice. This study evaluated the TruSight Tumor 170 assay for the simultaneous detection of somatic gene mutations (SNPs and indels), gene fusions and CNVs, and its implementation into routine diagnostics. Methods: Forty-four formalin-fixed, paraffin-embedded tissue samples analyzed previously with validated methods were evaluated with the TruSight Tumor 170 assay (Illumina). For data analysis the TruSight Tumor 170 app, the BaseSpace Variant Interpreter (Illumina), and the Molecular Health Guide Software (Molecular Health) were used. Results: All somatic gene mutations were identified when covered by the assay. Two high-level
MET amplifications were detected by CNV analysis. Focal
MET amplifications with a copy number below 10 were not reliably detected at the DNA-level. Twenty-one of 31 fusions and splice variants were confirmed with the assay on the RNA-level. The remaining eight aberrations were incorrect by previous methods. In two cases, no splicing was observed. Conclusions: The TruSight Tumor 170 gives reliable results even if low DNA and RNA concentrations are applied in comparison to other methods and can be used in a routine workflow to detect somatic gene mutations, gene fusions, and splice variants. However, we were not able to detect most focal gene amplifications/deletions.
Full article
►▼
Show Figures
Open AccessReview
Molecular Testing and Treatment Strategies in RET-Rearranged NSCLC Patients: Stay on Target to Look Forward
by
Maria Lucia Reale, Valentina Bertaglia, Angela Listì, Silvia Novello and Francesco Passiglia
Cited by 4 | Viewed by 3682
Abstract
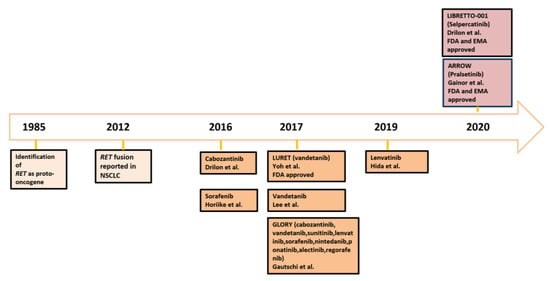
RET alterations are recognized as key oncogenic drivers in different cancer types, including non-small cell lung cancer (NSCLC). Multikinase inhibitors (MKIs) with anti-
RET activities resulted in variable efficacy with significant toxicities because of low target specificity. Selective RET kinase inhibitors, such as
[...] Read more.
RET alterations are recognized as key oncogenic drivers in different cancer types, including non-small cell lung cancer (NSCLC). Multikinase inhibitors (MKIs) with anti-
RET activities resulted in variable efficacy with significant toxicities because of low target specificity. Selective RET kinase inhibitors, such as pralsetinib and selepercatinib, demonstrated high efficacy and favorable tolerability in advanced
RET-rearranged NSCLC patients, leading to their introduction in the clinical setting. Among the different approaches available for the identification of
RET rearrangements, next-generation sequencing (NGS) assays present substantial advantages in terms of turnaround time and diagnostic accuracy, even if potentially limited by accessibility issues. The recent advent of novel effective targeted therapies raises several questions regarding the emergence of resistance mechanisms and the potential ways to prevent/overcome them. In this review, we discuss molecular testing and treatment strategies to manage
RET fusion positive NSCLC patients with a focus on resistance mechanisms and future perspectives in this rapidly evolving scenario.
Full article
►▼
Show Figures
Planned Papers
The below list represents only planned manuscripts. Some of these
manuscripts have not been received by the Editorial Office yet. Papers
submitted to MDPI journals are subject to peer-review.
Title: Evaluation of the TruSight Tumor 170 assay and its value in clinical diagnostics
Authors: Carina Heydt; Roberto Pappesch; Sabine Merkelbach-Bruse
Affiliation: Institute of Pathology, University Hospital Cologne, Cologne, Germany