Morphological Relationships between the Cholinergic and Somatostatin-28(1-12) Systems in the Alpaca (Lama pacos) Brainstem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Processing
2.3. Immunohistochemistry
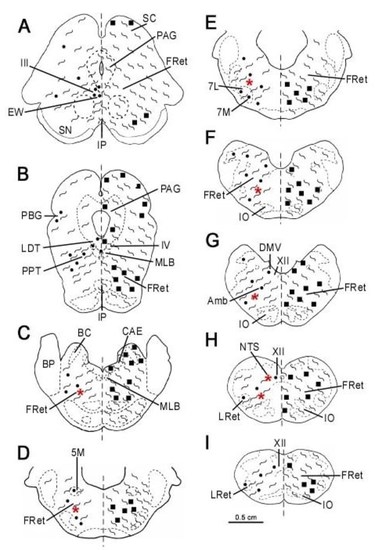
2.4. Mapping
3. Results
3.1. Single Immunolabeling for Som-28(1-12) and ChAT
3.2. Colocalization of Som-28(1-12) and ChAT in Cell Bodies
3.3. Fibrilar Immunolabeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| III | nucleus of oculomotor nerve (III cranial nerve) |
| IV | nucleus of the trochlear nerve (IV cranial nerve) |
| 5M | motor trigeminal nucleus |
| 5SL | laminar spinal trigeminal nucleus |
| 5SP | spinal trigeminal nucleus |
| VI | abducens nerve (VI cranial nerve) |
| 7L | facial nucleus, lateral division |
| 7M | facial nucleus, medial division |
| XII | nucleus of the hypoglossal nerve (XII cranial nerve) |
| Amb | nucleus ambiguus |
| BC | brachium conjunctivum |
| BCL | marginal nucleus of the brachium conjunctivum, lateral division |
| BCM | marginal nucleus of the brachium conjunctivum, medial division |
| CAE | locus coeruleus |
| ChAT | choline acetyl transferase |
| Cu | cuneate nucleus |
| CX | external cuneate nucleus |
| DMV | dorsal motor nucleus of the vagus nerve |
| DRN | dorsal raphe nucleus |
| EW | nucleus of Edinger–Westphal |
| FRet | reticular formation |
| Gr | gracile nucleus |
| IC | inferior colliculus |
| IO | inferior olive |
| IP | interpeduncular nucleus |
| LDT | laterodorsal tegmental nucleus (Ch6 cholinergic cell group) |
| LRet | lateral reticular nucleus |
| MLF | medial longitudinal fascicle |
| NR | red nucleus |
| NTS | nucleus of the solitary tract |
| P | pyramidal tract |
| PAG | periaqueductal gray |
| PBG | parabigeminal nucleus (Ch8 cholinergic cell group) |
| Ped | cerebral peduncle |
| PG | pontine gray |
| PGL | pontine gray, lateral division |
| PGM | pontine gray, medial division |
| PH | nucleus praepositus hypoglossi |
| PPT | pedunculopontine tegmental nucleus (Ch5 cholinergic cell group) |
| S | solitary tract |
| SC | superior colliculus |
| SNC | substantia nigra, pars compacta |
| SNR | substantia nigra, pars reticulata |
| SO | superior colliculus |
| Som-28(1-12) | somatostatin-28 (1-12) |
| T | nucleus of the trapezoid body |
| TB | trapezoid body |
| TDC | dorsal tegmental nucleus, central division |
| TDP | dorsal tegmental nucleus, pericentral division |
| TRC | tegmental reticular nucleus, central division |
| Ves | vestibular nucleus |
References
- Davimes, J.G.; Alagaili, A.N.; Bennett, N.C.; Mohammed, O.B.; Bhagwandin, A.; Manger, P.R.; Gravett, N. Neurochemical organization and morphology of the sleep related nuclei in the brain of the Arabian oryx, Oryx leucoryx. J. Chem. Neuroanat. 2017, 81, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.A.; Karlsson, K.A.; Patzke, N.; Spocter, M.A.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the minke whale (Balaenoptera acutorostrata). J. Comp. Neurol. 2015, 524, 2018–2035. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.A.; Patzke, N.; Spocter, M.A.; Bertelsen, M.F.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the river hippopotamus (Hippopotamus amphibius): A most inusual Certiodactyl species. J. Comp. Neurol. 2015, 524, 2036–2058. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.A.; Patzke, N.; Spocter, M.A.; Siegel, J.M.; Manger, P.R. Organization of the sleep-related neural systems in the brain of the harbour porpoise (Phocoena phocoena). J. Comp. Neurol. 2016, 524, 1999–2017. [Google Scholar] [CrossRef] [PubMed]
- Malungo, I.B.; Gravett, N.; Bhagwandin, A.; Davimes, J.G.; Manger, P.R. A preliminary description of the sleep-related neural systems in the brain of the blue wildebeest, Connochaetes taurinus. Anat. Rec. 2020, 303, 1977–1997. [Google Scholar] [CrossRef]
- De Souza, E.; Yi, P.; Aguilar, L.A.; Coveñas, R.; Lerma, L.; Andrade, R.; Mangas, A.; Narváez, J.A. Mapping of leucine-encephalin in the alpaca (Lama pacos) brainstem. In Focus on Neuropeptide Research; Coveñas, R., Mangas, A., Narváez, J.A., Eds.; Transworld Research Network: Trivandrum, India, 2007; pp. 103–113. [Google Scholar]
- De Souza, E.; Coveñas, R.; Yi, P.; Aguilar, L.A.; Lerma, L.; Andrade, R.; Mangas, A.; Díaz-Cabiale, Z.; Narváez, J.A. Mapping of CGRP in the alpaca (Lama pacos) brainstem. J. Chem. Neuroanat. 2008, 35, 346–355. [Google Scholar] [CrossRef]
- De Souza, E.; Sánchez, M.L.; Aguilar, L.A.; Díaz-Cabiale, Z.; Narváez, J.A.; Coveñas, R. Mapping of Somatostatin-28(1-12) in the alpaca (Lama pacos) brainstem. Microsc. Res. Tech. 2015, 78, 363–374. [Google Scholar] [CrossRef]
- Marcos, P.; Arroyo-Jiménez, M.M.; Lozano, G.; Aguilar, L.A.; Coveñas, R. Mapping of tyrosine hydroxylase in the alpaca (Lama pacos) brainstem and colocalization with CGRP. J. Chem. Neuroanat. 2011, 41, 63–72. [Google Scholar] [CrossRef]
- Coveñas, R.; Mangas, A.; Medina, L.E.; Sánchez, M.L.; Aguilar, L.A.; Díaz-Cabiale, Z.; Narváez, J.A. Mapping of somatostatin-28 (1-12) in the alpaca diencephalon. J. Chem. Neuroanat. 2011, 42, 89–98. [Google Scholar] [CrossRef]
- Marcos, P.; Arroyo-Jiménez, M.M.; Lozano, G.; González-Fuentes, J.; Lagartos-Donate, M.J.; Aguilar, L.A.; Coveñas, R. Mapping of tyrosine hydroxylase in the diencephalon of alpaca (Lama pacos) and co-distribution with somatostatin-28 (1-12). J. Chem. Neuroanat. 2013, 50–51, 66–74. [Google Scholar] [CrossRef]
- Marcos, P.; Coveñas, R. Immunohistochemical study of the brainstem cholinergic system in the alpaca (Lama pacos) and colocalization with CGRP. Eur. J. Histochem. 2021, 65, 3266. [Google Scholar] [CrossRef] [PubMed]
- Manger, P.R. Establishing order at the systems level in mammalian brain evolution. Brain Res. Bull. 2005, 66, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Reichlin, S. Somatostatin. In Brain Peptides; Krieger, D.T., Browstein, M.J., Martin, J.B., Eds.; John Wiley and Sons: New York, NY, USA, 1983; pp. 711–752. [Google Scholar]
- Benoit, R.; Ling, N.; Bakhit, C.; Morrison, J.H.; Alford, B.; Guillemin, R. Somatostatin-28(1-12)-like immunoreactivity in the rat. Endocrinology 1982, 111, 2149–2151. [Google Scholar] [CrossRef] [PubMed]
- Spary, E.J.; Maqbool, A.; Batten, T.F.C. Expression and localization of somatostatin receptor subtypes sst1-sst5 in areas of the rat medulla oblongata involved in autonomic regulation. J. Chem. Neuroanat. 2008, 35, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Sutin, E.L.; Jacobowitz, D.M. Immunocytochemical localization of peptides and other neurochemical in the rat laterodorsal tegmental nucleus and adjacent area. J. Comp. Neurol. 1988, 270, 243–270. [Google Scholar] [CrossRef]
- Wang, Y.T.; Neuman, R.S.; Bieger, D. Somatostatin inhibits nicotinic cholinoceptor mediated-excitation in rat ambigual motoneurons in vitro. Neurosci. Lett. 1991, 123, 236–239. [Google Scholar] [CrossRef]
- Wang, Y.T.; Zhang, M.; Neuman, R.S.; Bieger, D. Somatostatin regulates excitatory amino acid receptor-mediated fast excitatory postsynaptic potential components in vagal motoneurons. Neuroscience 1993, 53, 7–9. [Google Scholar] [CrossRef]
- Batten, T.F.C. Immunolocalization of putative neurotransmitters innervating autonomic regulating neurons of cat ventral medulla. Brain Res. Bull. 1995, 37, 487–506. [Google Scholar] [CrossRef]
- Matsuoka, N.; Yamazaki, M.; Yamaguchi, I. Changes in brain somatostatin in memory-deficient rats: Comparison with cholinergic markers. Neuroscience 1995, 66, 617–626. [Google Scholar] [CrossRef]
- Marcos, P.; Corio, M.; Dubourg, P.; Tramu, G. Reciprocal synaptic connections between neurotensin- and tyrosine hydroxylase-immunoreactive neurons in the mediobasal hypothalamus of the guinea pig. Brain. Res. 1996, 715, 63–70. [Google Scholar] [CrossRef]
- Jasper, A.L.; Ajmone-Marsan, C. A Stereotaxic Atlas of the Diencephalon of the Cat; National Research Council of Canada: Ottawa, ON, Canada, 1966. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Badlangana, N.L.; Bhagwandin, A.; Fuxe, K.; Manger, P.R. Observations on the giraffe central nervous system related to the corticospinal tract, motor cortex and spinal cord: What difference does a long neck make? Neuroscience 2007, 148, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Bux, F.; Bhagwandin, A.; Fuxe, K.; Manger, P.R. Organization of cholinergic, putative catecholaminergic and serotonergic nuclei in the diencephalon, midbrain and pons of sub-adult male giraffes. J. Chem. Neuroanat. 2010, 39, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Dell, L.A.; Gruger, J.L.; Bhagwandin, A.; Jillani, N.E.; Pettigrew, J.D.; Manger, P.R. Nuclear organization of cholinergic, putative catecholaminergic, and serotonergic systems in the brains of two megachiropteran species. J. Chem. Neuroanat. 2010, 40, 177–195. [Google Scholar] [CrossRef] [PubMed]
- Houser, C.R.; Crawford, G.D.; Barber, R.P.; Salvaterra, P.M.; Vaughn, J.E. Organization and morphological characteristics of cholinergic neurons: An immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983, 266, 97–119. [Google Scholar] [CrossRef]
- Kimura, H.; Maeda, T. Aminergic and cholinergic systems in the dorsolateral pontine tegmentum. Brain Res. Bull. 1982, 9, 493–499. [Google Scholar] [CrossRef]
- Levey, A.I.; Wainer, B.H.; Mufson, E.J.; Mesulam, M.M. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience 1983, 9, 9–22. [Google Scholar] [CrossRef]
- Manaye, K.F.; Zweig, R.; Wu, D.; Hersh, L.B.; de Lacalle, S.; Saper, C.B.; German, D.C. Quantification of cholinergic and select non-cholinergic mesopontine neuronal populations in the human brain. Neuroscience 1999, 89, 759–770. [Google Scholar] [CrossRef]
- Marcos, P.; Coveñas, R. Neuroanatomical relationship between the cholinergic and tachykininergic systems in the adult human brainstem: An immunohistochemical study. J. Chem. Neuroanat. 2019, 102, 101701. [Google Scholar] [CrossRef]
- Mesulam, M.M. Cholinergic pathways and the ascending reticular activating system of the human brain. Ann. N. Y. Acad. Sci. 1995, 757, 169–179. [Google Scholar] [CrossRef]
- Motts, S.D.; Slusarczyk, A.S.; Sowick, C.S.; Schofield, B.R. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience 2008, 154, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Oda, Y. Choline acetyltransferase: The structure, distribution and pathologic changes in the central nervous system. Pathol. Int. 1999, 49, 921–937. [Google Scholar] [CrossRef]
- Sakai, K.; Luppi, P.H.; Salvert, D.; Kimura, H.; Maeda, T.; Jouvet, M. Localization of cholinergic neurons in the cat lower brainstem. CR Acad. Sci. III 1986, 303, 317–324. [Google Scholar]
- Tago, H.; McGeer, P.L.; McGeer, E.G.; Akiyama, H.; Hersh, L.B. Distribution of choline acetyltransferase immunopositive structures in the rat brainstem. Brain Res. 1989, 495, 271–297. [Google Scholar] [CrossRef]
- Yasuhara, O.; Aimi, Y.; Matsuo, A.; Kimura, H. Distribution of a splice variant of choline acetyltransferase in the trigeminal ganglion and brainstem of the rat: Comparison with calcitonin gene-related peptide and substance P. J. Comp. Neurol. 2008, 509, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.M.; Geula, C.; Bothwell, M.A.; Hersh, L.B. Human reticular formation: Cholinergic neurons of the pedunculopontine and laterodorsal tegmental nuclei and some cytochemical comparisons to forebrain cholinergic neurons. J. Comp. Neurol. 1989, 283, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Ajiki, K.; Matsuura, J.; Misawa, H. Localization of two cholinergic markers, choline acetyltransferase and vesicular acetylcholine transporter in the central nervous system of the rat: In situ hybridization histochemistry and immunohistochemistry. J. Chem. Neuroanat. 1997, 13, 23–39. [Google Scholar] [CrossRef]
- Zaborsky, L. Afferent connections of the forebrain cholinergic projection neurons, with special reference to monoaminergic and peptidergic fibers. Experientia 1989, 57, 12–32. [Google Scholar] [CrossRef]
- Maley, B.E. Immunohistochemical localization of neuropeptides and neurotransmitters in the nucleus solitarius. Chem. Senses 1996, 21, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Cebada-Sánchez, S.; Insausti, R.; González-Fuentes, J.; Arroyo-Jiménez, M.M.; Rivas-Infante, E.; Lagartos, M.J.; Martínez-Ruiz, J.; Lozano, G.; Marcos, P. Distribution of peptidergic populations in the human dentate gyrus (Somatostatin [SOM-28, SOM-12] and neuropeptide Y [NPY]) during postnatal development. Cell Tissue Res. 2014, 358, 25–41. [Google Scholar] [CrossRef]
- Gillis, R.A.; Dezfuli, G.; Bellusci, L.; Vicini, S.; Sahibzada, N. Brainstem neuronal circuitries controlling gastric tonic and phasic contractions: A review. Cell. Mol. Neurobiol. 2022, 42, 333–360. [Google Scholar] [CrossRef]
- Jacquin, T.; Champagnat, J.; Madamba, S.; Denavit-Saubié, M.; Siggins, G.R. Somatostatin depresses excitability in neurons of the solitary tract complex through hyperpolarization and augmentation of IM, a non-inactivating voltage-dependent outward current blocked by muscarinic agonists. Proc. Natl. Acad. Sci. USA 1988, 85, 948–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, E.T.; Sawchenko, P.E. Central neural control of esophageal motility: A review. Disphagia 1990, 5, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Pagani, F.D.; Norman, W.P.; Gillis, R.A. Medullary parasympathetic projections innervate specific sites in the feline stomach. Gastroenterology 1988, 95, 277–288. [Google Scholar] [CrossRef]
- Norman, W.P.; Pagani, F.D.; Ormsbee, H.S., III; Kasbekar, D.K.; Gillis, R.A. Use of horseradish peroxidase to identify hindbrain sites that influence gastric motility in the cat. Gastroenterology 1985, 88, 701–705. [Google Scholar] [CrossRef]
- Moreira, T.S.; Sobrinho, C.R.; Falquetto, B.; Oliveira, L.M.; Lima, J.D.; Mulkey, D.K.; Takakura, A.C. The retrotrapezoid nucleus and the neuromodulation of breathing. J. Neurophysiol. 2021, 125, 699–719. [Google Scholar] [CrossRef]
- Tan, W.; Pagliardini, S.; Yang, P.; Janczewski, W.A.; Feldman, J.A. Projections of preBötzinger complex neurons in adult rats. J. Comp. Neurol. 2010, 518, 1862–1878. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.F.; Feldman, J.L. Efferent projections of excitatory and inhibitory preBötzinger Complex neurons. J. Comp. Neurol. 2018, 526, 1389–1402. [Google Scholar] [CrossRef]
- de Lecea, L.; Bourgin, P. Neuropeptide interactions and REM sleep: A role for urotensin II? Peptides 2008, 29, 845–851. [Google Scholar] [CrossRef]



| Som-28(1-12) | ChAT | Double-Labeling | |||
|---|---|---|---|---|---|
| NUCLEI | CB | F | CB | F | |
| III | − | + | + | + | |
| IV | − | + | + | + | |
| 5M | − | + | + | + | |
| 5SL | − | + | − | − | |
| 5SP | − | + | − | − | |
| VI | − | − | + | + | |
| 7L | − | + | + | + | |
| 7M | − | + | + | + | |
| XII | − | + | + | + | |
| Amb | + | + | + | + | + |
| BC | − | + | − | − | |
| BCL | + | + | − | − | |
| BCM | + | + | − | − | |
| CAE | − | + | − | − | |
| Cu | − | + | − | − | |
| CX | − | + | − | − | |
| DMV | + | + | + | + | |
| DRM | − | + | − | − | |
| EW | − | + | + | + | |
| FRet Mesencephalon | + | + | + | + | + |
| FRet Medulla | + | + | + | + | + |
| FRet Pons | + | + | + | + | + |
| Gr | − | + | − | − | |
| IC | + | + | − | − | |
| IO | − | + | − | − | |
| IP | + | + | − | + | |
| LDT | + | + | + | + | |
| LRet | − | + | − | + | |
| MLF | − | + | − | − | |
| NR | − | + | − | − | |
| NTS | + | + | + | + | + |
| P | − | + | − | − | |
| PAG | + | + | − | + | |
| PBG | + | + | + | + | |
| Ped | − | + | − | − | |
| PG | − | + | − | − | |
| PGL | − | + | − | − | |
| PGM | − | + | − | − | |
| PH | + | + | − | − | |
| PPT | + | + | + | + | |
| s | − | + | − | − | |
| SC | + | + | − | + | |
| SNC | + | + | − | + | |
| SNR | − | + | − | + | |
| SO | − | + | − | − | |
| T | + | + | − | − | |
| TB | − | + | − | − | |
| TDC | − | + | − | − | |
| TDP | + | + | − | − | |
| TRC | − | + | − | − | |
| Ves | + | + | − | − | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos, P.; Coveñas, R. Morphological Relationships between the Cholinergic and Somatostatin-28(1-12) Systems in the Alpaca (Lama pacos) Brainstem. Anatomia 2022, 1, 54-67. https://doi.org/10.3390/anatomia1010006
Marcos P, Coveñas R. Morphological Relationships between the Cholinergic and Somatostatin-28(1-12) Systems in the Alpaca (Lama pacos) Brainstem. Anatomia. 2022; 1(1):54-67. https://doi.org/10.3390/anatomia1010006
Chicago/Turabian StyleMarcos, Pilar, and Rafael Coveñas. 2022. "Morphological Relationships between the Cholinergic and Somatostatin-28(1-12) Systems in the Alpaca (Lama pacos) Brainstem" Anatomia 1, no. 1: 54-67. https://doi.org/10.3390/anatomia1010006