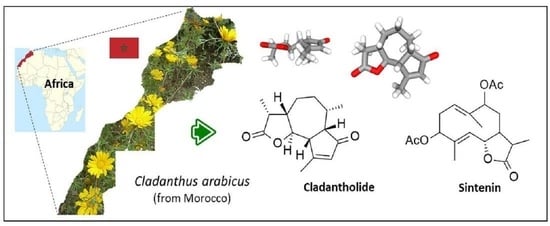
The Medicinal Moroccan Plant Cladanthus arabicus as a Prominent Source of Sesquiterpenes Cladantholide and Sintenin
Abstract
:1. Introduction
2. Use of C. arabicus in Traditional Medicine
3. Pharmacological Activities of C. arabicus Extracts
4. Phytochemical Analysis
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Bellioua, S.; Amari, S.; Warda, K.; Aghraz, A.; Dilagui, I.; Ouhaddou, S.; Sissi, S.; Bekkouche, K.; Larhsini, M.; Markouk, M. Chemical profile, antioxidant and antimicrobial effects of essential oil from the Moroccan endemic plant Cladanthus scariosus (L.). J. Essent. Oil Res. 2022, 34, 394–404. [Google Scholar] [CrossRef]
- El Hafidi, S.; Bakhy, K.; Ouhssine, M.; Benzakour, A.; Khamar, H.; Casanova, J.; Paoli, M.; Tomi, F. Composition and Chemical Variability of the Essential Oil from Aerial Parts of Cladanthus scariosus, an Endemic Species to the Moroccan High Atlas. Chem. Biodivers. 2023, 20, e202201022. [Google Scholar] [CrossRef]
- Elouaddari, A.; El Amrani, A.; JamalEddine, J. Intraspecific variability of the essential oil of Cladanthus mixtus from Morocco. Nat. Prod. Commun. 2014, 9, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Benmerache, A.; Alabdul Magid, A.; Kabouche, A.; Harakat, D.; Voutquenne-Nazabadioko, L.; Kabouche, Z. 6‴-O-acetylisospinosin, a new C-glycosylflavone and known compounds from the aerial parts of Cladanthus mixtus. Nat. Prod. Res. 2020, 34, 2887–2893. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Erbiai, E.H.; Charfi, S.; Pinto, E.; Castillo, M.E.C.; Hernández-Ruiz, J.; Cano, A.; Badoc, A.; Lamarti, A.; Esteves da Silva, J.C.G.; et al. Chemical Characterization and Several Bioactivities of Cladanthus mixtus from Morocco. Molecules 2023, 28, 3196. [Google Scholar] [CrossRef] [PubMed]
- Elouaddari, A.; El Amrani, A.; Eddine, J.; Correia, A.I.D.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C. Yield and chemical composition of the essential oil of Moroccan chamomile [Cladanthus mixtus (L.) Chevall.] growing wild at different sites in Morocco. Flavour Fragance J. 2013, 28, 360–366. [Google Scholar] [CrossRef]
- Ghanimi, R.; Ouhammou, A.; Babahmad, R.A.; Cherkaoui, M. A Quantitative Study on the Ethnobotanical Knowledge about Wild Edible Plants among the Population of Messiwa. Ethiop. J. Health Sci. 2022, 32, 1237–1244. [Google Scholar] [PubMed]
- Ghanimi, R.; Ouhammou, A.; Ahouach, A.; Cherkaoui, M. Ethnobotanical study on wild edible plants traditionally used by Messiwa people, Morocco. J. Ethnobiol. Ethnomed. 2022, 18, 16. [Google Scholar] [CrossRef]
- Ait Baamrane, M.A.; Shehzad, W.; Ouhammou, A.; Abbad, A.; Naimi, M.; Coissac, E.; Taberlet, P.; Znari, M. Assessment of the food habits of the Moroccan dorcas gazelle in M’Sabih Talaa, west central Morocco, using the trnL approach. PLoS ONE 2012, 7, e35643. [Google Scholar] [CrossRef]
- Blajan, L.; Lasnami, K. Nutrition et pathologie du dromadaire. Options Méditerr. 1989, 2, 131–139. [Google Scholar]
- Kumar, P.; Kumar, R. Rural Health Scenario—Role of family medicine: Academy of Family Physicians of India Position Paper. J. Fam. Med. Prim. Care 2018, 7, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Kolié, D.; Van De Pas, R.; Codjia, L.; Zurn, P. Increasing the availability of health workers in rural sub-Saharan Africa: A scoping review of rural pipeline programmes. Hum. Resour. Health 2023, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- WHO Established the Global Center for Traditional Medicine in India. Available online: https://www.who.int/news/item/25-03-2022-who-establishes-the-global-centre-for-traditional-medicine-in-india (accessed on 30 October 2023).
- Šantić, Ž.; Pravdić, N.; Bevanda, M.; Galić, K. The historical use of medicinal plants in traditional and scientific medicine. Psychiatr. Danub. 2017, 29, 787–792. [Google Scholar] [PubMed]
- Cingi, C.; Bayar Muluk, N.; Tezol, A.; Çukurova, I. Efficacy of traditional herbal formulas on human immunity. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 27–40. [Google Scholar] [PubMed]
- Kurek, M.; Benaida-Debbache, N.; Elez Garofulić, I.; Galić, K.; Avallone, S.; Voilley, A.; Waché, Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants 2022, 11, 742. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Liu, Y.; Li, S.H. The untapped potential of plant sesterterpenoids: Chemistry, biological activities and biosynthesis. Nat. Prod. Rep. 2021, 38, 2293–2314. [Google Scholar] [CrossRef]
- Calixto, J.B.; Beirith, A.; Ferreira, J.; Santos, A.R.; Filho, V.C.; Yunes, R.A. Naturally occurring antinociceptive substances from plants. Phytother. Res. 2000, 14, 401–418. [Google Scholar] [CrossRef]
- Dewanjee, S.; Sohel, M.; Hossain, M.S.; Ansari, F.; Islam, M.T.; Sultana, F.; Al Mamun, A.; Islam, M.M.; Amin, M.N. A comprehensive review on clinically proven natural products in the management of nerve pain, with mechanistic insights. Heliyon 2023, 9, e15346. [Google Scholar] [CrossRef]
- Kooti, W.; Servatyari, K.; Behzadifar, M.; Asadi-Samani, M.; Sadeghi, F.; Nouri, B.; Zare Marzouni, H. Effective Medicinal Plant in Cancer Treatment, Part 2: Review Study. J. Evid. Based Complement. Altern. Med. 2017, 22, 982–995. [Google Scholar] [CrossRef]
- Aiello, P.; Sharghi, M.; Mansourkhani, S.M.; Ardekan, A.P.; Jouybari, L.; Daraei, N.; Peiro, K.; Mohamadian, S.; Rezaei, M.; Heidari, M.; et al. Medicinal Plants in the Prevention and Treatment of Colon Cancer. Oxid. Med. Cell Longev. 2019, 2019, 2075614. [Google Scholar] [CrossRef]
- Menezes, R.; Foito, A.; Jardim, C.; Costa, I.; Garcia, G.; Rosado-Ramos, R.; Freitag, S.; Alexander, C.J.; Outeiro, T.F.; Stewart, D.; et al. Bioprospection of Natural Sources of Polyphenols with Therapeutic Potential for Redox-Related Diseases. Antioxidants 2020, 9, 789. [Google Scholar] [CrossRef]
- Rosa, M.N.; E Silva, L.R.V.; Longato, G.B.; Evangelista, A.F.; Gomes, I.N.F.; Alves, A.L.V.; de Oliveira, B.G.; Pinto, F.E.; Romão, W.; de Rezende, A.R.; et al. Bioprospecting of Natural Compounds from Brazilian Cerrado Biome Plants in Human Cervical Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 3383. [Google Scholar] [CrossRef]
- Rani, D.M.; Wongso, H.; Purwoko, R.Y.; Winarto, N.B.; Shalas, A.F.; Triatmoko, B.; Pratama, A.N.W.; Keller, P.A.; Nugraha, A.S. Anti-cancer bioprospecting on medicinal plants from Indonesia: A review. Phytochemistry 2023, 216, 113881. [Google Scholar] [CrossRef]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Ethnopharmacological review of medicinal plants used to manage diabetes in Morocco. Clin. Phytosci. 2020, 6, 18. [Google Scholar] [CrossRef]
- Benkhnigue, O.; Ben Akka, F.; Salhi, S.; Fadli, M.; Douira, A.; Zidane, L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc). J. Anim. Plant Sci. 2014, 23, 3539–3568. [Google Scholar]
- Belhaj, S.; Chaachouay, N.; Zidane, L. Ethnobotanical and toxicology study of medicinal plants used for the treatment of diabetes in the High Atlas Central of Morocco. J. Pharm. Pharmacog. Res. 2021, 9, 619–662. [Google Scholar] [CrossRef]
- Benkhnigue, O.; Chaachouay, N.; Khamar, H.; El Azzouzi, F.; Douira, A.; Zidane, L. Ethnobotanical and ethnopharmacological study of medicinal plants used in the treatment of anemia in the region of Haouz-Rehamna (Morocco). J. Pharm. Pharmacogn. Res. 2022, 10, 279–302. [Google Scholar] [CrossRef]
- Ouhaddou, H.; Alaoui, A.; Laaribya, S.; Ayan, S. Ethnobotanical survey of medicinal plants used for treating diabetes in Agadir Ida Outanane region, Southwestern Morocco. Arab. J. Med. Aromat. Plants 2020, 6, 72–86. [Google Scholar]
- Katiri, A.; Karkaoui, M.; Msanda, F.; Boubaker, H. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes in the Tizi n’ Test Region (Taroudant Province, Morocco). J. Pharmacogn. Nat. Prod. 2017, 3, 1000130. [Google Scholar] [CrossRef]
- Chaachouay, N.; Benkhnigue, O.; Fadli, M.; El Ibaoui, H.; Zidane, L. Ethnobotanical and ethnopharmacological studies of medicinal and aromatic plants used in the treatment of metabolic diseases in the Moroccan Rif. Heliyon 2019, 5, e02191. [Google Scholar] [CrossRef] [PubMed]
- Naceiri Mrabti, H.; Bouyahya, A.; Naceiri Mrabti, N.; Jaradat, N.; Doudach, L.; Faouzi, M.E.A. Ethnobotanical Survey of Medicinal Plants Used by Traditional Healers to Treat Diabetes in the Taza Region of Morocco. eCAM 2021, 2021, 5515634. [Google Scholar] [CrossRef]
- Daoudi, A.; Bammou, M.; Zarkani, S.; Slimani, I.; Ibijbijen, J.; Nassiri, L. Ethnobotanical study of medicinal flora in rural municipality of Aguelmouss—Khenifra province—(Morocco). Phytothérapie 2016, 14, 220–228. [Google Scholar] [CrossRef]
- El Hanbali, F.; Mellouki, F.; Akssira, M.; El hassani, B.; Blázquez, M.A.; Boira, H. Composition and Antibacterial Activity of Essential Oils of Cladanthus arabicus Cass. (Asteraceae). J. Essent. Oil Bear. Plants 2005, 8, 213–217. [Google Scholar] [CrossRef]
- Aghraz, A.; Wanner, J.; Schmidt, E.; Aitdra, L.; Aitsidibrahim, M.; Tabanca, N.; Ali, A.; Nafis, A.; Hassani, L.; Markouk, M.; et al. Chemical Composition, in vitro Antioxidant, Antimicrobial and Insecticidal Activities of Essential Oil from Cladanthus arabicus. J. Essent. Oil Bear. Plants 2017, 20, 601–609. [Google Scholar] [CrossRef]
- Aghraz, A.; Benameur, Q.; Gervasi, T.; Ait Dra, L.; Ben-Mahdi, M.H.; Larhsini, M.; Markouk, M.; Cicero, N. Antibacterial activity of Cladanthus arabicus and Bubonium imbricatum essential oils alone and in combination with conventional antibiotics against Enterobacteriaceae isolates. Lett. Appl. Microbiol. 2018, 67, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Mziouid, A.; Chebli, B.; Berrabah, M.; Chebli, H.; Heimeur, N.; Bounimi, S.; Mayad, E.H. Phytochemical screening and antioxidant activity of four Moroccan aromatic plant methanolic extracts and essential oils. Arab. J. Med. Aromat. Plants 2022, 8, 117–132. [Google Scholar]
- Dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol-a Sesquiterpene Isolated From Casearia sylvestris (Salicaceae)-Displayed In Vitro Activity and Causes Hyperpolarization of the Membrane Potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127, Corrigendum in Front. Pharmacol. 2022, 13, 865432. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Chen, R.; Zhou, Z.; Liu, R.; Wen, J. Efficacy and safety of caffeic acid tablets in the treatment of thrombocytopenia: A systematic review and meta-analysis. Medicine 2023, 102, 35353. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory effect of caffeic acid in alleviating diabetes and associated complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef] [PubMed]
- Okpara, E.S.; Adedara, I.A.; Guo, X.; Klos, M.L.; Farombi, E.O.; Han, S. Molecular mechanisms associated with the chemoprotective role of protocatechuic acid and its potential benefits in the amelioration of doxorubicin-induced cardiotoxicity: A review. Toxicol. Rep. 2022, 9, 1713–1724. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef]
- Aghraz, A.; Albergamo, A.; Benameur, Q.; Salvo, A.; Larhsini, M.; Markouk, M.; Gervasi, T.; Cicero, N. Polyphenols contents, heavy metals analysis and in vitro antibacterial activity of extracts from Cladanthus arabicus and Bubonium imbricatum of Moroccan Origin. Nat. Prod. Res. 2020, 34, 63–70. [Google Scholar] [CrossRef]
- Aghraz, A.; Gonçalves, S.; Rodríguez-Solana, R.; Ait Dra, L.; Di Stefano, V.; Dugo, G.; Cicero, N.; Larhsini, M.; Markouk, M.; Romano, A. Antioxidant activity and enzymes inhibitory properties of several extracts from two Moroccan Asteraceae species. S. Afr. J. Bot. 2018, 118, 58–64. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Danikiewicz, W.; Gumulka, M.; Pankowska, E.; Krajewski, J.; Grabarczyk, H.; Wichlacz, M. Sesquiterpenes of Cladanthus arabicus. Phytochemistry 1993, 34, 1639–1641. [Google Scholar] [CrossRef]
- Monde, K.; Oya, T.; Takasugi, M.; Shirata, A. A guaianolide phytoalexin, cichoralexin, from Cichorium intybus. Phytochemistry 1990, 29, 3449–3451. [Google Scholar] [CrossRef]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Chávez-Silva, F.; Navarrete-Vázquez, G.; León-Rivera, I.; Perea-Arango, I.; Villalobos-Molina, R.; Ibarra-Barajas, M. Tracheal relaxation through calcium channel blockade of Achillea millefolium hexanic extract and its main bioactive compounds. J. Ethnopharmacol. 2020, 253, 112643. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Frattaruolo, L.; Haworth, I.; Brindisi, M.; El-magboub, A.; Ferrario, A.; Gomer, C.; Aiello, F.; Adams, J.D. Naturally occurring sesquiterpene lactones and their semi-synthetic derivatives modulate PGE2 levels by decreasing COX2 activity and expression. Heliyon 2019, 5, e01366, Corrigendum in Heliyon 2019, 5, e01513. [Google Scholar] [CrossRef] [PubMed]
- Khazneh, E.; Hřibová, P.; Hošek, J.; Suchý, P.; Kollár, P.; Pražanová, G.; Muselík, J.; Hanaková, Z.; Václavík, J.; Miłek, M.; et al. The Chemical Composition of Achillea wilhelmsii C. Koch and Its Desirable Effects on Hyperglycemia, Inflammatory Mediators and Hypercholesterolemia as Risk Factors for Cardiometabolic Disease. Molecules 2016, 21, 404. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Li, X.F.; Jin, M.J.; Li, Y.; Wu, Y.L.; Jin, Q.; Zhang, Y.; Li, X.; Jiang, M.; Cui, B.W.; et al. Leucodin attenuates inflammatory response in macrophages and lipid accumulation in steatotic hepatocytes via P2x7 receptor pathway: A potential role in alcoholic liver disease. Biomed. Pharmacother. 2018, 107, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Arias-Durán, L.; Estrada-Soto, S.; Hernández-Morales, M.; Millán-Pacheco, C.; Navarrete-Vázquez, G.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Almanza-Pérez, J.C. Antihypertensive and vasorelaxant effect of leucodin and achillin isolated from Achillea millefolium through calcium channel blockade and NO production: In vivo, functional ex vivo and in silico studies. J. Ethnopharmacol. 2021, 273, 113948. [Google Scholar] [CrossRef] [PubMed]
- Tschiggerl, C.; Bucar, F. Guaianolides and volatile compounds in chamomile tea. Plant Foods Human Nutr. 2012, 67, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Csupor-Löffler, B.; Hajdú, Z.; Zupkó, I.; Réthy, B.; Falkay, G.; Forgo, P.; Hohmann, J. Antiproliferative effect of flavonoids and sesquiterpenoids from Achillea millefolium s.l. on cultured human tumour cell lines. Phytother. Res. 2009, 23, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Trifunović, S.; Isaković, A.M.; Isaković, A.; Vučković, I.; Mandić, B.; Novaković, M.; Vajs, V.; Milosavljević, S.; Trajković, V. Isolation, characterization, and in vitro cytotoxicity of new sesquiterpenoids from Achillea clavennae. Planta Med. 2014, 80, 297–305. [Google Scholar] [CrossRef]
- Gören, N.; Oksüz, S.; Ulubelen, A. A sesquiterpene lactone, sintenin, from Achillea sintenisii. Phytochemistry 1988, 27, 2346–2347. [Google Scholar] [CrossRef]
- Bruno, M.; Rosselli, S.; Raccuglia, R.A.; Maggio, A.; Senatore, F.; Arnold, N.A.; Griffin, C.A.; Herz, W. Terpenoids and Flavones from Achillea falcata (Asteraceae). Rev. Soc. Quim. México 2003, 47, 130–131. [Google Scholar]
- Hatam, N.A.R.; Yousif, N.J.; Porzel, A.; Seifert, K. Sesquiterpene lactones from Achillea micrantha. Phytochemistry 1992, 31, 2160–2162. [Google Scholar] [CrossRef]
- Korkmaz, B.; Renda, G.; Erik, İ.; Kılıç, G.; Coşkunçelebi, K.; Yaylı, N. Two new dihydroisocoumarins and terpenoids from Scorzonera longiana Sümbül an endemic species to Turkey and their antimicrobial activity. Nat. Prod. Res. 2023, 37, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Yoon, C.H.; Sung, Y.S.; Kim, Y.K.; Yun, M.; Kim, S. Total Synthesis of (+)-Cladantholide and (−)-Estafiatin: 5-Exo,7-Endo Radical Cyclization Strategy for the Construction of Guaianolide Skeleton. J. Am. Chem. Soc. 1997, 119, 8391–8392. [Google Scholar] [CrossRef]
- Schall, A.; Reiser, O. Synthesis of Biologically Active Guaianolides with a trans-Annulated Lactone Moiety. Eur. J. Org. Chem. 2008, 14, 2353–2364. [Google Scholar] [CrossRef]
- Fernandes, R.A.; Moharana, S.; Khatun, G.N. Recent advances in the syntheses of guaianolides. Org. Biomol. Chem. 2023, 21, 6652–6670. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Shalaby, N.M.; Hamed, M.A.; El-Rigal, N.S.; Al-Ghamdi, S.N.; Bouajila, J. Phytochemical composition, protective and therapeutic effect on gastric ulcer and α-amylase inhibitory activity of Achillea biebersteinii Afan. Arch. Pharmacal Res. 2016, 39, 10–20. [Google Scholar] [CrossRef]
- Bailly, C.; Vergoten, G. Japonicone A and related dimeric sesquiterpene lactones: Molecular targets and mechanisms of anticancer activity. Inflamm. Res. 2022, 71, 267–276. [Google Scholar] [CrossRef]
- Migheli, R.; Virdis, P.; Galleri, G.; Arru, C.; Lostia, G.; Coradduzza, D.; Muroni, M.R.; Pintore, G.; Podda, L.; Fozza, C.; et al. Antineoplastic Properties by Proapoptotic Mechanisms Induction of Inula viscosa and Its Sesquiterpene Lactones Tomentosin and Inuviscolide. Biomedicines 2022, 10, 2739. [Google Scholar] [CrossRef] [PubMed]
- Fadul, E.; Nizamani, A.; Rasheed, S.; Adhikari, A.; Yousuf, S.; Parveen, S.; Gören, N.; Alhazmi, H.A.; Choudhary, M.I.; Khalid, A. Anti-glycating and anti-oxidant compounds from traditionally used anti-diabetic plant Geigeria alata (DC) Oliv. & Hiern. Nat. Prod. Res. 2020, 34, 2456–2464. [Google Scholar] [PubMed]
- Hu, L.H.; Zou, H.B.; Gong, J.X.; Li, H.B.; Yang, L.X.; Cheng, W.; Zhou, C.X.; Bai, H.; Guéritte, F.; Zhao, Y. Synthesis and biological evaluation of a natural ester sintenin and its synthetic analogues. J. Nat. Prod. 2005, 68, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.X.; Zhang, L.J.; Huang, K.X.; Li, X.K.; Hu, L.H.; Wang, X.Y.; Stockigt, J.; Zhao, Y. Antioxidant and neuroprotective effects of synthesized sintenin derivatives. J. Enzym. Inhib. Med. Chem. 2009, 24, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Wichlacz, M.; Daniewski, W.M.; Danikiewicz, W.; Gumulka, M.; Drozdz, B.; Grabarczyk, H. Constituents of Cladanthus Arabicus. Pol. J. Chem. 1994, 68, 2147–2152. [Google Scholar]
- Ebrahim, W.; Aly, A.H.; Mándi, A.; Wray, V.; Essassi el, M.; Ouchbani, T.; Bouhfid, R.; Lin, W.; Proksch, P.; Kurtán, T.; et al. O-heterocyclic embeurekols from Embellisia eureka, an endophyte of Cladanthus arabicus. Chirality 2013, 25, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, W.; Aly, A.H.; Wray, V.; Mándi, A.; Teiten, M.H.; Gaascht, F.; Orlikova, B.; Kassack, M.U.; Lin, W.; Diederich, M.; et al. Embellicines A and B: Absolute configuration and NF-κB transcriptional inhibitory activity. J. Med. Chem. 2013, 56, 2991–2999. [Google Scholar] [CrossRef]
- Özçınar, Ö.; Özgür, T.; Yusufoglu, H.; Kivçak, B.; Bedir, E. Biotransformation of Neoruscogenin by the Endophytic Fungus Alternaria eureka. J. Nat. Prod. 2018, 81, 1357–1367. [Google Scholar] [CrossRef]
- Duman, S.; Ekiz, G.; Yılmaz, S.; Yusufoglu, H.; Ballar Kırmızıbayrak, P.; Bedir, E. Telomerase activators from 20(27)-octanor-cycloastragenol via biotransformation by the fungal endophytes. Bioorg. Chem. 2021, 109, 104708. [Google Scholar] [CrossRef]
- Küçüksolak, M.; Üner, G.; Ballar Kırmızıbayrak, P.; Bedir, E. Neuroprotective metabolites via fungal biotransformation of a novel sapogenin, cyclocephagenol. Sci. Rep. 2022, 12, 18481. [Google Scholar] [CrossRef]
- Ouchbani, T.; Janati Idriss, F.E.; Bouhfid, R.; Proksch, P.; Essassi, E.M. Neonectria Macrodidyma and Embellisia eureka, two novel producers of brefeldin A and 3,4-dihydro-3,4-8-trihydroxy’1[2H]-naphthalenone. J. Maroc. Chim. Hétérocycl. 2012, 11, 23–35. [Google Scholar]
- Gremaud, G.; Tabacchi, R. Relationship between the fungus Ceratocystis fimbriata coffea and the canker disease of the coffee tree. Phytochemistry 1996, 42, 1547–1549. [Google Scholar] [CrossRef]
- Okole, B.N.; Schulz, F.A. Selection of Mycosphaerella fijiensis-resistant cell lines from micro-cross sections of banana and plantain. Plant Cell Rep. 1997, 16, 339–343. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef]
- Garg, M.; Chaudhary, S.K.; Goyal, A.; Sarup, P.; Kumari, S.; Garg, N.; Vaid, L.; Shiveena, B. Comprehensive review on therapeutic and phytochemical exploration of diosmetin: A promising moiety. Phytomed. Plus 2022, 2, 100179. [Google Scholar] [CrossRef]
- Gong, X.; Xiong, L.; Caihong, B.; Zhang, B. Diosmetin ameliorate type 2 diabetic mellitus by up-regulating Corynebacterium glutamicum to regulate IRS/PI3K/AKT-mediated glucose metabolism disorder in KK-Ay mice. Phytomedicine 2021, 87, 153582. [Google Scholar] [CrossRef]
- Angamuthu, H.; Ramachandrane, M. Investigations on the structural, vibrational, computational, and molecular docking studies on potential antidiabetic chemical agent Diosmetin. J. Mol. Recogn. 2020, 33, e2819. [Google Scholar] [CrossRef]
- Comakli, V.; Adem, S.; Oztekin, A.; Demirdag, R. Screening inhibitory effects of selected flavonoids on human recombinant aldose reductase enzyme: In vitro and in silico study. Arch. Physiol. Biochem. 2022, 128, 1368–1374. [Google Scholar] [CrossRef]
- Valdés, B. Early botanical exploration of the Maghreb. Flora Mediterr. 2021, 31, 5–18. [Google Scholar]
- Tanji, A.; Ait Lhaj, A. Weeds of barley and wheat in Souss-Massa region. Rev. Maroc. Protect Plants 2010, 1, 11–23. [Google Scholar]
- Ait-Sidi-Brahim, M.; Markouk, M.; Larhsini, M. Chapter 5. Moroccan Medicinal Plants as Anti-infective and Antioxidant Agents. In New Look to Phytomedicine. Advancements in Herbal Products as Novel Drug Leads; Academic Press: Cambridge, MA, USA, 2019; pp. 91–142. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Assaggaf, H.; Qasem, A.; El-Shemi, A.G.; Abdallah, E.M.; Mrabti, H.N.; Bouyahya, A. Antioxidant, Antidiabetic, and Antibacterial Potentials and Chemical Composition of Salvia officinalis and Mentha suaveolens Grown Wild in Morocco. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 2844880. [Google Scholar] [CrossRef]
- Han, M.; Lu, Y.; Tao, Y.; Zhang, X.; Dai, C.; Zhang, B.; Xu, H.; Li, J. Luteolin Protects Pancreatic β Cells against Apoptosis through Regulation of Autophagy and ROS Clearance. Pharmaceuticals 2023, 16, 975. [Google Scholar] [CrossRef]
- Shehnaz, S.I.; Roy, A.; Vijayaraghavan, R.; Sivanesan, S.; Pazhanivel, N. Modulation of PPAR-γ, SREBP-1c and inflammatory mediators by luteolin ameliorates β-cell dysfunction and renal damage in a rat model of type-2 diabetes mellitus. Mol. Biol. Rep. 2023, 50, 9129–9142. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tahilyani, V.; Patel, D.K. A review on pharmacological and analytical aspects of diosmetin: A concise report. Chin. J. Integr. Med. 2013, 19, 792–800. [Google Scholar] [CrossRef]
- El Alami, A.; Chait, A. Etude de l’alimentation du magot Macaca sylvanus dans le site touristique des cascades d’Ouzoud (Maroc). Rev. Primatol. 2016, 7, 2748. [Google Scholar] [CrossRef]
- Nouri, M.; Gonçalves, F.; Sousa, J.P.; Römbke, J.; Ksibi, M.; Pereira, R.; Haddioui, A. Metal and Phosphorus Uptake by Spontaneous Vegetation in an abandoned iron mine from a Semiarid Area in Center Morocco: Implications for Phytoextraction. Environ. Res. Eng. Manag. 2013, 2, 59–71. [Google Scholar] [CrossRef]
- Harras, N.; Lamarti, A. In Vitro Germination and Plantlet Establishment of Wild Chamomile of Morocco Cladanthus mixtus (L.) Oberpr. and Vogt. Am. J. Plant Sci. 2014, 5, 2623–2632. [Google Scholar] [CrossRef]
- El Hafidi, S.; Ouhssine, M.; Benzakour, A.; Gaboun, F.; Khamar, H.; Bakhy, K.; Homrani Bakali, A. Site effect on seed germination of two species of Cladanthus in Morocco. Afr. Mediterr. Agric. J. 2022, 137, 103–121. [Google Scholar]
- Tambewagh, U.U.; Kandhare, A.D.; Honmore, V.S.; Kadam, P.P.; Khedkar, V.M.; Bodhankar, S.L.; Rojatkar, S.R. Anti-inflammatory and antioxidant potential of Guaianolide isolated from Cyathocline purpurea: Role of COX-2 inhibition. Int. Immunopharmacol. 2017, 52, 110–118. [Google Scholar] [CrossRef]
- Adekenov, S. Syntheses Based on 3,4α-Epoxy-1,5,7α,6β(H)-guai-10(14),11(13)-dien-6,12-olide. Molecules 2022, 27, 1862. [Google Scholar] [CrossRef]
- Hu, Y.; Saito, Y.; Okamoto, Y.; Matsuo, Y.; Gong, X.; Tanaka, T. Chemical Compositions of Eupatorium heterophyllum Leaf Samples from Yunnan and Sichuan Provinces of China-Isolation of 13 New Sesquiterpene Lactones. Molecules 2023, 28, 5107. [Google Scholar] [CrossRef]
- Li, H.; Xu, N.; Li, J.; Aisa, H.A. Guaianolide-type sesquiterpene lactones from Achillea millefolium L. and their anti-inflammatory activity. Phytochemistry 2023, 216, 113894. [Google Scholar] [CrossRef]
- Wen, B.; Hexum, J.K.; Widen, J.C.; Harki, D.A.; Brummond, K.M. A redox economical synthesis of bioactive 6,12-guaianolides. Org. Lett. 2013, 15, 2644–2647. [Google Scholar] [CrossRef]
- Wells, S.M.; Brummond, K.M. Conditions for a Rh(I)-catalyzed [2 + 2 + 1] cycloaddition reaction with methyl substituted allenes and alkynes. Tetrahedron Lett. 2015, 56, 3546–3549. [Google Scholar] [CrossRef]
- He, W.; Lai, R.; Lin, Q.; Huang, Y.; Wang, L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. BUON 2018, 23, 1679–1685. [Google Scholar]
- El Gaafary, M.; Morad, S.A.F.; Schmiech, M.; Syrovets, T.; Simmet, T. Arglabin, an EGFR receptor tyrosine kinase inhibitor, suppresses proliferation and induces apoptosis in prostate cancer cells. Biomed. Pharmacother. 2022, 156, 113873. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, L.; Wang, J.; Li, W.; Zhou, X.; Zhang, C.; Han, C. Arglabin regulates microglia polarization to relieve neuroinflammation in Alzheimer’s disease. J. Biochem. Mol. Toxicol. 2022, 36, e23045. [Google Scholar] [CrossRef]
- Mahalingam, D.; Peguero, J.; Cen, P.; Arora, S.P.; Sarantopoulos, J.; Rowe, J.; Allgood, V.; Tubb, B.; Campos, L. A Phase II, Multicenter, Single-Arm Study of Mipsagargin (G-202) as a Second-Line Therapy Following Sorafenib for Adult Patients with Progressive Advanced Hepatocellular Carcinoma. Cancers 2019, 11, 833. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Brennen, W.N.; Christensen, S.B.; Denmeade, S.R. Mipsagargin: The Beginning-Not the End-of Thapsigargin Prodrug-Based Cancer Therapeutics. Molecules 2021, 26, 7469. [Google Scholar] [CrossRef]
- Christensen, S.B.; Simonsen, H.T.; Engedal, N.; Nissen, P.; Møller, J.V.; Denmeade, S.R.; Isaacs, J.T. From Plant to Patient: Thapsigargin, a Tool for Understanding Natural Product Chemistry, Total Syntheses, Biosynthesis, Taxonomy, ATPases, Cell Death, and Drug Development. Prog. Chem. Org. Nat. Prod. 2021, 115, 59–114. [Google Scholar]
- Kim, D.Y.; Choi, B.Y. Costunolide-A Bioactive Sesquiterpene Lactone with Diverse Therapeutic Potential. Int. J. Mol. Sci. 2019, 20, 2926. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Marques, D.; Figueira, I.; Cankar, K.; Bosch, D.; Brito, M.A.; Dos Santos, C.N. Costunolide and parthenolide: Novel blood-brain barrier permeable sesquiterpene lactones to improve barrier tightness. Biomed. Pharmacother. 2023, 167, 115413. [Google Scholar] [CrossRef]
- Zhan, Z.Y.; Zhang, Z.H.; Yang, H.X.; Wu, Y.L.; Nan, J.X.; Lian, L.H. Potential skin health promoting benefits of costunolide: A therapeutic strategy to improve skin inflammation in imiquimod-induced psoriasis. Food Funct. 2023, 14, 2392–2403. [Google Scholar] [CrossRef]
- Wang, P.; Yang, H.; Lin, W.; Zhou, J.; Liu, Y.; Ma, L.; Li, M.; Hu, Y.; Yu, C.; Zhang, Y.; et al. Discovery of Novel Sesquiterpene Lactone Derivatives as Potent PKM2 Activators for the Treatment of Ulcerative Colitis. J. Med. Chem. 2023, 66, 5500–5523. [Google Scholar] [CrossRef]
- El Mihyaoui, A.; Charfi, S.; Erbiai, E.H.; Pereira, M.; Duarte, D.; Vale, N.; Candela Castillo, M.E.; Badoc, A.; Lamarti, A.; Esteves da Silva, J.C.G.; et al. Phytochemical Compounds and Anticancer Activity of Cladanthus mixtus Extracts from Northern Morocco. Cancers 2022, 15, 152. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouissane, L.; Bailly, C. The Medicinal Moroccan Plant Cladanthus arabicus as a Prominent Source of Sesquiterpenes Cladantholide and Sintenin. AppliedChem 2024, 4, 15-28. https://doi.org/10.3390/appliedchem4010002
Bouissane L, Bailly C. The Medicinal Moroccan Plant Cladanthus arabicus as a Prominent Source of Sesquiterpenes Cladantholide and Sintenin. AppliedChem. 2024; 4(1):15-28. https://doi.org/10.3390/appliedchem4010002
Chicago/Turabian StyleBouissane, Latifa, and Christian Bailly. 2024. "The Medicinal Moroccan Plant Cladanthus arabicus as a Prominent Source of Sesquiterpenes Cladantholide and Sintenin" AppliedChem 4, no. 1: 15-28. https://doi.org/10.3390/appliedchem4010002