Stereoselective Synthesis and Isolation of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol
Abstract
:1. Introduction
2. Materials and Methods
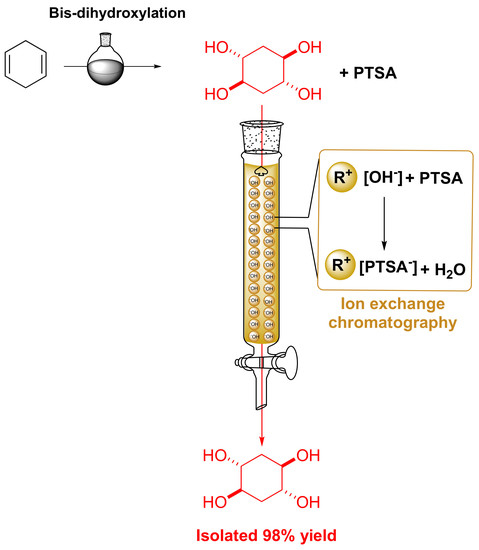
2.1. Synthesis of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol

2.2. Isolation of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol without the Use of Organic Solvents
2.3. Synthesis of (±)-trans,trans-Cyclohexane-1,2,4,5-tetrayltetraacetate

3. Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A




References
- Sun, Y.D.; Zhang, G.H.; Hawkes, C.A.; Shaw, J.E.; McLaurin, J.; Nitz, M. Synthesis of scyllo-inositol derivatives and their effects on amyloid beta peptide aggregation. Bioorg. Med. Chem. 2008, 16, 7177–7184. [Google Scholar] [CrossRef] [PubMed]
- Nyandoro, S.S.; Joseph, C.C.; Nkunya, M.H.; Hosea, K.M. New antimicrobial, mosquito larvicidal and other metabolites from two Artabotrys species. Nat. Prod. Res. 2013, 27, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Ogura, M.; Tsuda, S.; Maemoto, S.; Kutsuki, H.; Ohashi, T. High-yield Production of Optically Active 1,2-Diols from the Corresponding Racemates by Microbial Stereoinversion. Agric. Biol. Chem. Tokyo 1990, 54, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Kurbanoglu, N.I.; Celik, M.; Kilic, H.; Alp, C.; Sahin, E.; Balci, M. Stereospecific synthesis of a DL-gala-aminoquercitol derivative. Tetrahedron 2010, 66, 3485–3489. [Google Scholar] [CrossRef]
- Gogoi, P.; Sharma, S.D.; Konwar, D. SeO2/H2O2/H2O-dioxane: A new catalytic system for trans dihydroxylation of olefins. Lett. Org. Chem. 2007, 4, 249–252. [Google Scholar] [CrossRef]
- Usui, Y.; Sato, K.; Tanaka, M. Catalytic dihydroxylation of olefins with hydrogen peroxide: An organic-solvent- and metal-free system. Angew. Chem. Int. Ed. 2003, 42, 5623–5625. [Google Scholar] [CrossRef] [PubMed]
- Maras, A.; Erden, M.; Secen, H.; Sutbeyaz, P. One-pot synthesis from 1,4-cyclohexadiene of (+/−)-1,4/2,5-cyclohexanetetrol, a naturally occurring cyclitol derivative. Carbohydr. Res. 1998, 308, 435–437. [Google Scholar] [CrossRef]
- Geary, L.M.; Chen, T.-Y.; Montgomery, T.P.; Krische, M.J. Benzannulation via Ruthenium-Catalyzed Diol–Diene [4+2] Cycloaddition: One- and Two-Directional Syntheses of Fluoranthenes and Acenes. J. Am. Chem. Soc. 2014, 136, 5920–5922. [Google Scholar] [CrossRef] [PubMed]
- Kasun, Z.A.; Geary, L.M.; Krische, M.J. Ring expansion of cyclic 1,2-diols to form medium sized rings via ruthenium catalyzed transfer hydrogenative [4+2] cycloaddition. Chem. Commun. 2014, 50, 7545–7547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suemune, H.; Hasegawa, A.; Sakai, K. Highly enantio- and diastereo-selective synthesis of C2-symmetric 3,5-cyclohexadiene-1,2-diol and D2-symmetric cyclohexane-1,2,4,5-tetrol. Tetrahedron Asymmetry 1995, 6, 55–58. [Google Scholar] [CrossRef]
- McCasland, G.E.; Furuta, S.; Johnson, L.F.; Shoolery, J.N. Synthesis of the Five Diastereomeric 1,2,4,5-Cyclohexanetetrols. Nuclear Magnetic Resonance Configurational Proofs1,2. J. Org. Chem. 1963, 28, 894–900. [Google Scholar] [CrossRef]
- Cavdar, H.; Saracoglu, N. Ring opening of epoxides with NaHSO4: Isolation of beta-hydroxy sulfate esters and an effective synthesis for trans-diols. Tetrahedron 2009, 65, 985–989. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Afonso, C.A.M.; Branco, L.C. Oxidation of Cyclohexene to trans-1,2-Cyclohexanediol Promoted by p-Toluenesulfonic Acid without Organic Solvents. J. Chem. Educ. 2011, 88, 1002–1003. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Afonso, C.A.M. Brønsted Acid-Catalyzed Dihydroxylation of Olefins in Aqueous Medium. Adv. Synth. Catal. 2011, 353, 2920–2926. [Google Scholar] [CrossRef]
- Tschamber, T.; Backenstrass, F.; Fritz, H.; Streith, J. Catalytic One-Pot Osmylation of Cyclohexadienes: Stereochemical and conformational studies of the resulting polyols. Helv. Chim. Acta 1992, 75, 1052–1060. [Google Scholar] [CrossRef]


 |  |
| Melting point: 203–204 °C (Lit. 208 °C [9,10,11]) 1H NMR (300 MHz, D2O) δ 3.62 (m, 4H), 1.69 (m, 4H). 13C NMR (75 MHz, D2O) δ 69.4, 33.61 (Figure A1 and Figure A2). Literature [9]: 1HNMR (400 MHz, D2O): δ 3.76 (m, 4H), 1.84 (m, 4H) ppm. 13C NMR (100 MHz, D2O): δ 74.4, 38.3 ppm. | Melting point: 148 °C (Lit. 148 °C [11], 147–148 °C [12]) 1H NMR (300 MHz, CDCl3) δ 5.03 (m, 4H), 2.02 (m, 16H). 13C NMR (75 MHz, CDCl3) δ 169.88, 77.51, 77.09, 76.66, 70.10, 69.08, 30.13, 20.96, 20.81 (Figure A3 and Figure A4). Literature [7,11]: 1HNMR (200 MHz, CDCl3) d 5.08–5.04 (m, OCH, 4H), 2.11–2.01 (m, CH2, 4H), 2.06 (s, OAc, 12H); 13C NMR (50 MHz, CDCl3) d 171.6, 71.2, 32.2, 22.9. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosatella, A.A.; Afonso, C.A.M. Stereoselective Synthesis and Isolation of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol. AppliedChem 2022, 2, 142-148. https://doi.org/10.3390/appliedchem2030010
Rosatella AA, Afonso CAM. Stereoselective Synthesis and Isolation of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol. AppliedChem. 2022; 2(3):142-148. https://doi.org/10.3390/appliedchem2030010
Chicago/Turabian StyleRosatella, Andreia A., and Carlos A. M. Afonso. 2022. "Stereoselective Synthesis and Isolation of (±)-trans,trans-Cyclohexane-1,2,4,5-tetraol" AppliedChem 2, no. 3: 142-148. https://doi.org/10.3390/appliedchem2030010