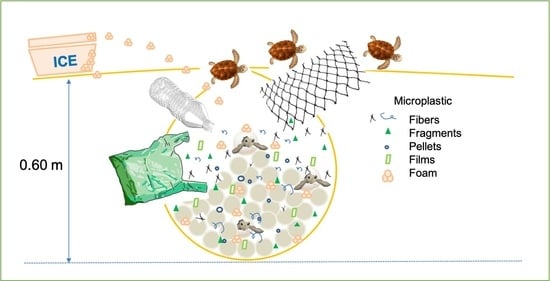
Microplastic Pollution in Sea Turtle Nests on the Beaches of Nautla and Vega de Alatorre, Veracruz
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
2.3. Sample Drying
2.4. Extraction of Microplastics
2.5. Classification of Microplastics
2.6. Statistic Analysis
3. Results
3.1. Abundance of Microplastics in Nests
3.2. Concentration of Microplastics in Nests
3.3. Classification of Microplastics Found in Nests
3.4. Implications of This Work
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, M.; Li, J.; Wang, P.; Hozzein, W.; Li, W. Environmental perspectives of microplastic pollution in the aquatic environment: A review. Mar. Life Sci. Technol. 2020, 2, 414–430. [Google Scholar] [CrossRef]
- Greenpeace. Plásticos | Greenpeace España—ES | Greenpeace España. Available online: https://es.greenpeace.org/es/trabajamos-en/consumismo/plasticos/ (accessed on 22 November 2022).
- Shah, A.; Kato, S.; Shintani, N.; Ramudu, N.; Nakajima, T. Microbial degradation of aliphatic and aliphatic-aromatic co-polyesters. Appl. Microbiol. Biotechnol. 2014, 98, 3437–3447. [Google Scholar] [CrossRef] [PubMed]
- Derraik, J. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Giraldez, L.; Braz, F.; Lacerda, A.; Ferraz, L.; Moura, D.; Gonçalves, D. Efeitos dos microplásticos no meio ambiente: Um macroproblema emergente. RECyT 2020, 33, 100–107. [Google Scholar] [CrossRef]
- Herrera, A.; Martínez, I.; Gómez, M. Bases para la planificación sostenible de áreas marinas en la macaronesia. Revista de la Sociedad Atlántica de Oceanógrafos. 2020. Available online: https://gmrcanarias.com/wp-content/uploads/2020/11/AF-ES-Flyer-PLASMAR-1.pdf (accessed on 22 November 2022).
- Botello, A. La Contaminación Marina y la Urgencia de su Legislación. Omnia; Instituto de Ciencias del Mar y Limnología, UNAM: Mexico City, Mexico, 1991; Volume 7, pp. 59–63. [Google Scholar]
- Beg, M.; Al-Muzaini, S.; Saeed, T.; Jacob, P.; Beg, K.; Al-Bahloul, M.; Kurian, A. Chemical contamination and toxicity of sediment from a coastal area receiving industrial effluents in Kuwait. Arch. Environ. Contam. Toxicol. 2001, 41, 289–297. [Google Scholar] [PubMed]
- Bollaín, C.; Agulló, D. Presencia de Microplásticos en Aguas y su Potencial Impacto en la Salud Pública. Rev. Esp. Salud Pública 2019, 93, 1–10. [Google Scholar]
- Cabrera, M. Evaluación de la Estación Depuradora de Agua Residual (Edar) el Trocadero del Municipio de Puerto Real Como Ruta de Entrada de Microplástico al Medio Acuático. Master’s Thesis, Universidad de Cádiz, Cádiz, España, 2018; 65p. [Google Scholar]
- Costa, L.; Vasco, O.; Galindo, A. Una mirada hacia los contaminantes emergentes “microplasticos” en Colombia. In Proceedings of the Encuentro Internacional de Educacion en Ingenieria, Cartagena de Indias, Colombia, 13–16 September 2022. [Google Scholar]
- Zhang, H. Transport of microplastics in coastal seas. Estuar. Coast. Shelf Sci. 2017, 199, 74–86. [Google Scholar] [CrossRef]
- Cruz, A.; Álvarez, J.; Martínez, C.; Enríquez, M.; Gutiérrez, M.; Vázquez, A.; Ojeda, S. Cuantificación y caracterización de microplásticos y residuos sólidos urbanos en playa Zipolite, Oaxaca. Cienc. Y Mar XXIV 2020, 71, 3–21. [Google Scholar]
- Laglbauer, B.; Franco, R.; Andreu, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in Slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef]
- Yang, C.Z.; Yaniger, S.I.; Jordan, V.C.; Klein, D.J.; Bittner, G.D. Most plastic products release estrogenic chemicals: A potential health problem that can be solved. Environ. Health Perspect 2011, 119, 989–996. [Google Scholar] [CrossRef]
- Castro, S.; Barrera, A.; González, A.; Pinot, A.; Vargas, J.; Sierra, I.; Huchin, J. Contaminación por microplásticos en ecosistemas acuáticos. XXVI Verano Cienc. 2021, 10, 1–9. [Google Scholar]
- Beckwith, V.; Fuentes, M. Microplastic at nesting grounds used by the northern Gulf of Mexico loggerhead recovery unit. Mar. Pollut. Bull. 2018, 131, 32–37. [Google Scholar] [CrossRef] [PubMed]
- São Miguel, R.; Anastácio, R.; Pereira, M. Sea Turtle Nesting: What Is Known and What Are the Challenges under a Changing Climate Scenario. Open J. Ecol. 2022, 12, 1–35. [Google Scholar] [CrossRef]
- Zavaleta, L. Factores que Influyen en la Anidación de Tortuga Verde (Chelonia mydas) en Veracruz. Ph.D. Thesis, Universidad Veracruzana, Veracruz, Mexico, 2013; 122p. [Google Scholar]
- Andrady, A. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Wen, J. Heat capacities of polymers. In Physical Properties of Polymers Handbook; Mark, J.E., Ed.; Springer: New York, NY, USA, 2007; pp. 145–154. [Google Scholar]
- Lithner, D.; Larsson, A.; Dave, G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011, 409, 3309–3324. [Google Scholar] [CrossRef]
- Khuyen, V.T.K.; Le, D.V.; Le, H.A.; Fischer, A.R.; Dornack, C. Assessing Microplastic Prevalence and Dispersion from Saigon Urban Canals via Can Gio Mangrove Reserve to East Sea by Raman Scattering Microscopy. Microplastics 2022, 1, 536–553. [Google Scholar] [CrossRef]
- Mohamed Nor, N.; Obbard, J. Microplastics in Singapore’s coastal mangrove ecosystems. Mar. Pollut. Bull. 2014, 79, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, J.; Cruz, A.; Vázquez, A.; Ojeda, S. Method for quantifying and characterization of microplastics in sand beaches. Rev. Int. Contam. Ambie 2020, 36, 151–164. [Google Scholar] [CrossRef]
- Zarfl, C. Promising techniques and open challenges for microplastic identification and quantification in environmental matrices. Anal. Bioanal. Chem. 2019, 411, 3743–3756. [Google Scholar] [CrossRef]
- Norén, F. Small Plastic Particles in Coastal Swedish Waters. N. Research Reportcommissioned by KIMO Sweeden 2007. Available online: researchgate.net (accessed on 10 September 2022).
- Cruz, A. Evaluación de la Calidad Ambiental y su Relación Con la Presencia de Microplásticos en Cinco Playas Mexicanas. Master’s Thesis, Universidad Autónoma Metropolitana Azcapotzalco, Mexico City, Mexico, 2020; 224p. [Google Scholar]
- Herrera, A.; Garrido, P.; Martínez, I.; Samper, M.; López, J.; Gómez, M.; Packard, T. Novel methodology to isolate microplastics from vegetal-rich samples. Mar. Pollut. Bull. 2018, 129, 61–69. [Google Scholar] [CrossRef]
- Boerger, C.; Lattin, G.; Moore, S.; Moore, C. Plastic ingestion by planktivorous fishes in the North Pacific Central Gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef]
- Rosado, V.; Mendoza, N.; Vázquez, A.; Álvarez-Zeferino, J.; Beltrán, M.; Ojeda, S. Caracterización de Microplásticos y Muestreo de Residuos Sólidos Urbanos de la Playa de Tuxpan, Veracruz. In Proceedings of the 90 Encuentro Nacional de Expertos en Residuos Sólidos. Guadalajara, Jalisco, 20 June 2018; pp. 64–72. [Google Scholar]
- Piñon, T.; Rodriguez, R.; Pastrana, M.; Rogel, E.; Wakida, F. Microplastics on sandy beaches of the Baja California Peninsula, Mexico. Mar. Pollut. Bull. 2018, 131, 63–71. [Google Scholar] [CrossRef]
- Retama, I.; Jonathan, M.; Shruti, V.; Velumani, S.; Sarkar, S.; Roy, P.; Rodríguez, P. Microplastics in tourist beaches of Huatulco Bay, Pacific coast of southern Mexico. Mar. Pollut. Bull. 2016, 113, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Duncan, E.; Arrowsmith, J.; Bain, C.; Broderick, A.; Lee, J.; Metcalfe, K.; Pikesley, S.K.; Snape, R.T.; van Sebille, E.; Godley, B. The true depth of the Mediterranean plastic problem: Extreme microplastic pollution on marine turtle nesting beaches in Cyprus. Mar. Pollut. Bull. 2018, 136, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Lin, L.; Li, D.; Wang, J.; Liu, Y.; Li, R.; Wu, S.; Shi, H. Microplastic pollution at Qilianyu, the largest green sea turtle nesting grounds in the northern South China Sea. PeerJ 2022, 10, e13536. [Google Scholar] [CrossRef]
- Álvarez, J.; Ojeda, S.; Cruz, A.; Martínez, C.; Vázquez, A. Microplastics in Mexican beaches. Resour. Conserv. Recycl. 2020, 155, 104633. [Google Scholar] [CrossRef]
- Beltrán, M.; Mendoza, N.; Vázquez, A.; Álvarez, J. Presencia de hidrocarburos en microplásticos en una playa mexicana. In Memorias de Congreso Nacional AMICA, del 28 al 30 de octubre; Instituto de ingeniería de la UNAM: Ciudad de México, Mexico, 2019; pp. 83–87. [Google Scholar]
- Álvarez, J.; Vázquez, A.; Cruz, A.; Ojeda, S.; Beltrán, M.; Sotelo, S.; Martínez, C. Microplásticos en ambientes marinos, obtención de datos iniciales para entender la problemática nacional. In Memorias de Congreso Nacional AMICA, del 28 al 30 de octubre; Instituto de ingeniería de la UNAM: Ciudad de México, Mexico, 2019; pp. 139–144. [Google Scholar]
- Ríos, L.; Ontiveros, J.; León, D.; Ruiz, A.; Rangel, M.; Pérez, L.; Sánchez, J. Microplastic contamination and fluxes in a touristic area at the SE Gulf of California. Mar. Pollut. Bull. 2021, 170, 112638. [Google Scholar] [CrossRef] [PubMed]
- Escobar, J. La Contaminación de Los Ríos y sus Efectos en las Áreas Costeras y el Mar. CEPAL, Santiago de chile 2002. Available online: https://repositorio.cepal.org/bitstream/handle/11362/6411/S0210820_es.pdf?sequence=1&isAllowed=y (accessed on 20 November 2022).
- Rochman, C.; Brookson, C.; Bikker, J.; Djuric, N.; Earn, A.; Bucci, K.; Athey, S.; Huntington, A.; McIlwraith, H.; Munno, K.; et al. Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 2019, 38, 703–711. [Google Scholar] [CrossRef]



| Sampling Site | Average Abundance of Microplastics (#MP/Kg SS) |
|---|---|
| Beacon 1 Raudal Beach | 1 ± 0.84 |
| Beacon 4 Raudal Beach | 1.61 ± 1.24 |
| Beacon 13 Laurel Beach | 3.33 ± 3.10 |
| Beacon 31 Navarro Beach | 3.78 ± 3.50 |
| Pen 1 Raudal Beach | 1.88 ± 2.37 |
| Pen 2 Laurel Beach | 1.81 ± 1.21 |
| Sampling Site | Average Concentration of Microplastics (mgMP/Kg SS) |
|---|---|
| Beacon 1 Raudal Beach | 0.0030 ± 0.0069 |
| Beacon 4 Raudal Beach | 0.0038 ± 0.0037 |
| Beacon 13 Laurel Beach | 0.0062 ± 0.0078 |
| Beacon 31 Navarro Beach | 0.0139 ± 0.0445 |
| Pen 1 Raudal Beach | 0.0095 ± 0.0244 |
| Pen 2 Laurel Beach | 0.0040 ± 0.0083 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estrella-Jordan, B.A.; Lango-Reynoso, F.; Castañeda-Chávez, M.d.R.; Montoya-Mendoza, J.; Reynier-Valdes, D. Microplastic Pollution in Sea Turtle Nests on the Beaches of Nautla and Vega de Alatorre, Veracruz. Microplastics 2023, 2, 182-191. https://doi.org/10.3390/microplastics2020014
Estrella-Jordan BA, Lango-Reynoso F, Castañeda-Chávez MdR, Montoya-Mendoza J, Reynier-Valdes D. Microplastic Pollution in Sea Turtle Nests on the Beaches of Nautla and Vega de Alatorre, Veracruz. Microplastics. 2023; 2(2):182-191. https://doi.org/10.3390/microplastics2020014
Chicago/Turabian StyleEstrella-Jordan, Belem Anahy, Fabiola Lango-Reynoso, María del Refugio Castañeda-Chávez, Jesús Montoya-Mendoza, and David Reynier-Valdes. 2023. "Microplastic Pollution in Sea Turtle Nests on the Beaches of Nautla and Vega de Alatorre, Veracruz" Microplastics 2, no. 2: 182-191. https://doi.org/10.3390/microplastics2020014