Microplastic Contamination in Cultured Mussels and Pearl Oysters in Greece
Abstract
:1. Introduction
2. Materials and Methods
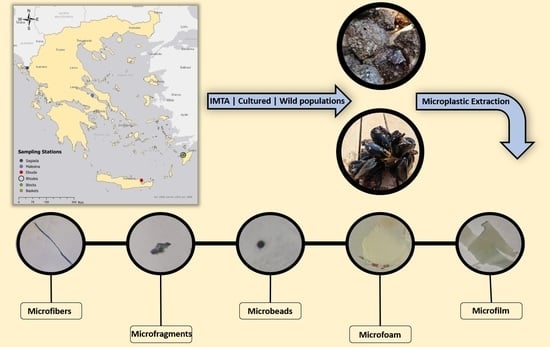
2.1. Study Sites and Sampling Stations
2.2. Sample Collection
2.3. Mussel and Oyster Sample Preparation and Digestion
2.4. Microscopic Inspection of Microplastics
2.5. Raman Spectroscopy
2.6. Data Analysis
2.7. Quality Control and Contamination Precautions
3. Results
3.1. Identification of MPs
3.2. Differences of MPs between the Two Bivalve Species
3.3. Differences of MPs between Stations
3.4. Differences of MPs in Every Station
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Thompson, R.C.; Galloway, T.S. The physical impacts of microplastics on marine organisms: A review. Environ. Pollut. 2013, 178, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, A.H.; Maldonado, E.A.L.; Alam, S.S.; López, J.R.L.; Herrera, P.F.M.; Mohamed, B.A.; Mahmoud, A.E.D.; Abutaleb, A.; Singh, L. Microplastics: Occurrences, treatment methods, regulations and foreseen environmental impacts. Environ. Res. 2022, 215, 114224. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, P.; Zhang, X.; Zhang, Y.; Xie, S.; Deng, J. Effect of microplastics exposure on the photosynthesis system of freshwater algae. J. Hazard. Mater. 2019, 374, 219–227. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; Van Loosdrecht, M.C.; Ni, B.-J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Setälä, O.; Norkko, J.; Lehtiniemi, M. Feeding type affects microplastic ingestion in a coastal invertebrate community. Mar. Pollut. Bull. 2016, 102, 95–101. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M.; Yakupitiyage, A.; Chavanich, S. Effects of microplastics on sessile invertebrates in the eastern coast of Thailand: An approach to coastal zone conservation. Mar. Pollut. Bull. 2017, 124, 349–355. [Google Scholar] [CrossRef]
- Ward, J.E.; Shumway, S.E. Separating the grain from the chaff: Particle selection in suspension-and deposit-feeding bivalves. J. Exp. Mar. Biol. Ecol. 2004, 300, 83–130. [Google Scholar] [CrossRef]
- Avio, C.G.; Gorbi, S.; Regoli, F. Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 2017, 128, 2–11. [Google Scholar] [CrossRef]
- Li, L.-L.; Amara, R.; Souissi, S.; Dehaut, A.; Duflos, G.; Monchy, S. Impacts of microplastics exposure on mussel (Mytilus edulis) gut microbiota. Sci. Total Environ. 2020, 745, 141018. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.S.; Gill, H.; Carrington, E. Microplastic changes the sinking and resuspension rates of marine mussel biodeposits. Mar. Pollut. Bull. 2021, 165, 112165. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.E.; Rosa, M.; Shumway, S.E. Capture, ingestion, and egestion of microplastics by suspension-feeding bivalves: A 40-year history. Anthr. Coasts 2019, 2, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Chatzivasileiou, D.; Dimitriou, P.D.; Theodorou, J.; Kalantzi, I.; Magiopoulos, I.; Papageorgiou, N.; Pitta, P.; Tsapakis, M.; Karakassis, I. An IMTA in Greece: Co-Culture of Fish, Bivalves, and Holothurians. J. Mar. Sci. Eng. 2022, 10, 776. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Ramfos, A.; Theodorou, J.A.; Katselis, G. Biological aspects, population and fishery dynamics of the non-indigenous pearl oyster Pinctada imbricata radiata (Leach, 1814) in the Eastern Mediterranean. Reg. Stud. Mar. Sci. 2021, 45, 101821. [Google Scholar] [CrossRef]
- Theodorou, J.A.; Makri, M.; Douvi, X.; Ramfos, A.; Spinos, E. Seasonal variation in the biochemical composition, condition index, and meat yield of the non-indigenous pearl oyster Pinctada imbricata radiata (Leach, 1814) from the West of the Aegean Sea, Greece. Aquac. Fish. 2021, 8, 451–456. [Google Scholar] [CrossRef]
- Kalantzi, I.; Karakassis, I. Benthic impacts of fish farming: Meta-analysis of community and geochemical data. Mar. Pollut. Bull. 2006, 52, 484–493. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Kalantzi, I.; Karakassis, I. Effects of fish farming on the biological and geochemical properties of muddy and sandy sediments in the Mediterranean Sea. Mar. Environ. Res. 2010, 69, 326–336. [Google Scholar] [CrossRef]
- Pitta, P.; Tsapakis, M.; Apostolaki, E.T.; Tsagaraki, T.; Holmer, M.; Karakassis, I. ‘Ghost nutrients’ from fish farms are transferred up the food web by phytoplankton grazers. Mar. Ecol. Prog. Ser. 2009, 374, 1–6. [Google Scholar] [CrossRef]
- Alexander, K.; Angel, D.; Freeman, S.; Israel, D.; Johansen, J.; Kletou, D.; Meland, M.; Pecorino, D.; Rebours, C.; Rousou, M.; et al. Improving sustainability of aquaculture in Europe: Stakeholder dialogues on integrated multi-trophic aquaculture (IMTA). Environ. Sci. Policy 2016, 55, 96–106. [Google Scholar] [CrossRef]
- Chopin, T.; Cooper, J.A.; Reid, G.; Cross, S.; Moore, C. Open-water integrated multi-trophic aquaculture: Environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquac. 2012, 4, 209–220. [Google Scholar] [CrossRef]
- Freeman, S.; Angel, D.; Freeman, S. Integrated aquaculture (INTAQ) as a tool for an ecosystem approach to the marine farming sector in the Mediterranean Sea. Integr. Maric. 2019, 529, 133–183. [Google Scholar]
- Chen, G.; Li, Y.; Wang, J. Occurrence and ecological impact of microplastics in aquaculture ecosystems. Chemosphere 2021, 274, 129989. [Google Scholar] [CrossRef] [PubMed]
- Piperagkas, O.; Papageorgiou, N.; Karakassis, I. Qualitative and quantitative assessment of microplastics in three sandy Mediterranean beaches, including different methodological approaches. Estuar. Coast. Shelf Sci. 2019, 219, 169–175. [Google Scholar] [CrossRef]
- Munno, K.; Helm, P.A.; Jackson, D.A.; Rochman, C.; Sims, A. Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem. 2018, 37, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.; Jeyasanta, K.I.; Laju, R.; Edward, J.P. Microplastic contamination in Indian edible mussels (Perna perna and Perna viridis) and their environs. Mar. Pollut. Bull. 2021, 171, 112678. [Google Scholar] [CrossRef]
- Wakkaf, T.; El Zrelli, R.; Kedzierski, M.; Balti, R.; Shaiek, M.; Mansour, L.; Tlig-Zouari, S.; Bruzaud, S.; Rabaoui, L. Microplastics in edible mussels from a southern Mediterranean lagoon: Preliminary results on seawater-mussel transfer and implications for environmental protection and seafood safety. Mar. Pollut. Bull. 2020, 158, 111355. [Google Scholar] [CrossRef]
- Yozukmaz, A. Investigation of microplastics in edible wild mussels from Izmir Bay (Aegean Sea, Western Turkey): A risk assessment for the consumers. Mar. Pollut. Bull. 2021, 171, 112733. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the marine environment: A review of the methods used for identification and quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- De Witte, B.; Devriese, L.; Bekaert, K.; Hoffman, S.; Vandermeersch, G.; Cooreman, K.; Robbens, J. Quality assessment of the blue mussel (Mytilus edulis): Comparison between commercial and wild types. Mar. Pollut. Bull. 2014, 85, 146–155. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B.; Crawford, C.; Quinn, B. Microplastic identification techniques. Microplastic Pollut. 2017, 10, 219–267. [Google Scholar]
- Philippidis, A.; Mikallou, A.; Anglos, D. Determining optimum irradiation conditions for the analysis of vermilion by Raman spectroscopy. Eur. Phys. J. Plus 2021, 136, 1194. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker version 3.0, a handy set for conservation scientists: A free online Raman spectra database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- da Silva, D.J.; Parra, D.F.; Wiebeck, H. Applying confocal Raman spectroscopy and different linear multivariate analyses to sort polyethylene residues. Chem. Eng. J. 2021, 426, 131344. [Google Scholar] [CrossRef]
- Li, J.; Lusher, A.L.; Rotchell, J.M.; Deudero, S.; Turra, A.; Bråte, I.L.N.; Sun, C.; Hossain, M.S.; Li, Q.; Kolandhasamy, P.; et al. Using mussel as a global bioindicator of coastal microplastic pollution. Environ. Pollut. 2019, 244, 522–533. [Google Scholar] [CrossRef]
- Beyer, J.; Green, N.W.; Brooks, S.; Allan, I.J.; Ruus, A.; Gomes, T.; Bråte, I.L.N.; Schøyen, M. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: A review. Mar. Environ. Res. 2017, 130, 338–365. [Google Scholar] [CrossRef]
- Strokov, K.; Schäfer, A.H.; Dobrindt, U.; Galstyan, A. Facile fabrication of silicon (IV) phthalocyanine-embedded poly (vinyl alcohol)-based antibacterial and antifouling interfaces. ACS Appl. Bio Mater. 2020, 3, 3751–3760. [Google Scholar] [CrossRef]
- Turner, S.; Horton, A.A.; Rose, N.L.; Hall, C. A temporal sediment record of microplastics in an urban lake, London, UK. J. Paleolimnol. 2019, 61, 449–462. [Google Scholar] [CrossRef]
- Digka, N.; Tsangaris, C.; Torre, M.; Anastasopoulou, A.; Zeri, C. Microplastics in mussels and fish from the Northern Ionian Sea. Mar. Pollut. Bull. 2018, 135, 30–40. [Google Scholar] [CrossRef]
- Naji, A.; Nuri, M.; Vethaak, A.D. Microplastics contamination in molluscs from the northern part of the Persian Gulf. Environ. Pollut. 2018, 235, 113–120. [Google Scholar] [CrossRef]
- Gedik, K.; Eryaşar, A.R.; Gözler, A.M. The microplastic pattern of wild-caught Mediterranean mussels from the Marmara Sea. Mar. Pollut. Bull. 2022, 175, 113331. [Google Scholar] [CrossRef]
- Leslie, H.; Brandsma, S.; Van Velzen, M.; Vethaak, A. Microplastics en route: Field measurements in the Dutch river delta and Amsterdam canals, wastewater treatment plants, North Sea sediments and biota. Environ. Int. 2017, 101, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Green, C.; Reynolds, A.; Shi, H.; Rotchell, J.M. Microplastics in mussels sampled from coastal waters and supermarkets in the United Kingdom. Environ. Pollut. 2018, 241, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef]
- Li, H.-X.; Ma, L.-S.; Lin, L.; Ni, Z.-X.; Xu, X.-R.; Shi, H.-H.; Yan, Y.; Zheng, G.-M.; Rittschof, D. Microplastics in oysters Saccostrea cucullata along the Pearl River estuary, China. Environ. Pollut. 2018, 236, 619–625. [Google Scholar] [CrossRef]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef]
- Saldaña-Serrano, M.; Bastolla, C.L.; Mattos, J.J.; Lima, D.; Freire, T.B.; Nogueira, D.J.; De-la-Torre, G.E.; Righetti, B.P.; Zacchi, F.L.; Gomes, C.H.; et al. Microplastics and linear alkylbenzene levels in oysters Crassostrea gigas driven by sewage contamination at an important aquaculture area of Brazil. Chemosphere 2022, 307, 136039. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Le, X.T.T.; Nguyen, D.T.; Phung, T.V.; Vu, D.N.; Pham, H.V. Abundance of microplastics in cultured oysters (Crassostrea gigas) from Danang Bay of Vietnam. Mar. Pollut. Bull. 2022, 180, 113800. [Google Scholar]
- Waite, H.R.; Donnelly, M.J.; Walters, L.J. Quantity and types of microplastics in the organic tissues of the eastern oyster Crassostrea virginica and Atlantic mud crab Panopeus herbstii from a Florida estuary. Mar. Pollut. Bull. 2018, 129, 179–185. [Google Scholar] [CrossRef]
- Aslam, S.; Tzoraki, O.; Krasakopoulou, E. Anthropogenic litter in freshwater bodies and their estuaries: An empirical analysis in Lesvos, Greece. Environ. Sci. Pollut. Res. 2022, 29, 16563–16575. [Google Scholar] [CrossRef]
- Conti, I.; Simioni, C.; Varano, G.; Brenna, C.; Costanzi, E.; Neri, L.M. Legislation to limit the environmental plastic and microplastic pollution and their influence on human exposure. Environ. Pollut. 2021, 288, 117708. [Google Scholar] [CrossRef] [PubMed]






| Study Site | MP Type | Mytilus galloprovincialis (%) | Pinctada imbricata radiata (%) |
|---|---|---|---|
| Malesina | Microfibers | 47.74 | 34.98 |
| Microfragments | 42.96 | 49.89 | |
| Microbeads | 9.29 | 14.44 | |
| Microfoam | - | 0.34 | |
| Microfilm | - | 0.34 |
| Study Site | Microfibers (%) | Microfragments (%) | Microbeads (%) | Microfoam (%) | Microfilm (%) |
|---|---|---|---|---|---|
| Sagiada | 15.82 | 79.06 | 4.05 | 1.07 | - |
| Malesina | 34.98 | 49.89 | 14.44 | 0.34 | 0.34 |
| Elounda | 11.57 | 85.69 | 1.56 | - | 1.18 |
| Rhodes-blocks | 6.46 | 91.25 | 1.47 | 0.42 | 0.40 |
| Rhodes-baskets | 13.01 | 80.05 | 6.94 | - | - |
| Species | MP Concentration | Filter Pore Size (μm) | Study Site | Reference |
|---|---|---|---|---|
| Mytilus galloprovincialis | 5.3 ± 0.5 | 1.2 | Ionian Sea (wild) | [39] |
| 5.0 ± 0.96 | 0.8 | Evoikos Gulf, Malesina (farmed) | This study | |
| 2.81 to 4.98 | 0.7 | Izmir Bay (wild) | [28] | |
| 2.5 ± 0.3 | 1.2 | Ionian Sea (farmed) | [39] | |
| 1.12 | 1.2 | Marmara Sea (wild) | [41] | |
| Mytilus edulis | 13.2 | 0.7 | Dutch North Sea Coast (wild) | [42] |
| 0.7 to 2.9 | 5 | U.K. (wild) | [43] | |
| 2.7 | 5 | China (wild) | [33,44,45] | |
| 1.6 | 5 | China (farmed) | [44] | |
| 0.36 ± 0.07 | 0.8 | North Sea, Germany (farmed) | [46] | |
| Pinctada imbricata radiata | 1.54 to 3.56 | 0.8 | Sagiada, Malesina, Rhodes, Greece (farmed) | This study |
| 2.06 to 3.03 | 0.8 | Elounda bay, Rhodes, Greece (wild) | This study | |
| 0.2 to 2.2 | 0.45 | Persian Gulf, Iran (wild) | [40] | |
| Saccostrea cucullata | 1.5 to 7.2 | 20 | Pearl River, Estuary, China (wild) | [45] |
| Crassostrea gigas | 0.27–0.64 | 0.6 | Santa Caterina Island, Brazil | [47] |
| 0.47 ± 0.16 | 0.8 | Brittany, France, Atlantic Ocean (commercial) | [46] | |
| 1.88 ± 1.58 | 0.6 | Danang Bay, Vietnam | [48] | |
| Crassostrea virginica | 3.84 ± 3.39 | 0.45 | Mosquito Lagoon, Indian River, Florida | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manolaki, S.M.; Chatzivasileiou, D.; Lampa, M.; Dimitriou, P.D.; Philippidis, A.; Karakassis, I.; Papageorgiou, N. Microplastic Contamination in Cultured Mussels and Pearl Oysters in Greece. Microplastics 2023, 2, 168-181. https://doi.org/10.3390/microplastics2020013
Manolaki SM, Chatzivasileiou D, Lampa M, Dimitriou PD, Philippidis A, Karakassis I, Papageorgiou N. Microplastic Contamination in Cultured Mussels and Pearl Oysters in Greece. Microplastics. 2023; 2(2):168-181. https://doi.org/10.3390/microplastics2020013
Chicago/Turabian StyleManolaki, Stefania M., Dimitra Chatzivasileiou, Maria Lampa, Panagiotis D. Dimitriou, Aggelos Philippidis, Ioannis Karakassis, and Nafsika Papageorgiou. 2023. "Microplastic Contamination in Cultured Mussels and Pearl Oysters in Greece" Microplastics 2, no. 2: 168-181. https://doi.org/10.3390/microplastics2020013