Management of Invasive Infections in Diabetes Mellitus: A Comprehensive Review
Abstract
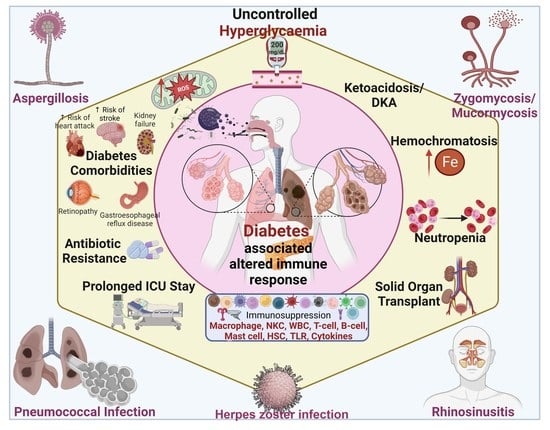
:1. Introduction
2. Altered Immune Response in Diabetes
2.1. Oxidative Stress in Diabetes
2.2. Cytokine Response in Diabetes
2.3. Neutrophil Function and Other Immune Abnormalities in Diabetes
2.4. Immune Abnormalities in T1 Diabetes
3. Invasive Infection Susceptibility and Management in Diabetes
3.1. Invasive Aspergillosis Infection
3.2. Invasive Zygomycosis
3.3. Invasive Pneumococcal Infection
3.4. Invasive Rhinosinusitis
- (a)
- CRS without nasal polyps (CRSsNP)
- (b)
- CRS with nasal polyps (CRSwNP)
- (i)
- Aspirin-exacerbated respiratory disease (AERD)
- (ii)
- Allergic fungal rhinosinusitis (AFRS)
- (a)
- Type 1 (IFN-g)
- (b)
- Type 2 (IL-5, and IL-13)
- (c)
- Type 3 (IL-17)
3.5. Invasive Mucormycosis
3.6. Herpes Zoster Infection
4. Treatment Possibilities for Invasive Infection in Diabetes with Impaired Immunity
4.1. Treatment Possibilities for Invasive Aspergillosis Infection
4.2. Treatment Possibilities for Invasive Zygomycosis
4.3. Treatment Possibilities for Invasive Pneumococcal Infection
4.4. Treatment Possibilities for Invasive Rhinosinusitis
4.5. Treatment Possibilities for Invasive Mucormycosis
4.6. Treatment Possibilities for Herpes Zoster
4.7. The Role of Beneficial Bacteria in Diabetic Immuno-Comptonization
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lao, M.; Li, C.; Li, J.; Chen, D.; Ding, M.; Gong, Y. Opportunistic Invasive Fungal Disease in Patients with Type 2 Diabetes Mellitus from Southern China: Clinical Features and Associated Factors. J. Diabetes Investig. 2020, 11, 731–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soysa, N.S.; Samaranayake, L.P.; Ellepola, A.N.B. Diabetes Mellitus as a Contributory Factor in Oral Candidosis. Diabet. Med. 2006, 23, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Srinivasan, K. Zinc Supplementation Alleviates Hyperglycemia and Associated Metabolic Abnormalities in Streptozotocin-Induced Diabetic Rats. Can. J. Physiol. Pharmacol. 2016, 94, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.R.; Srinivasan, K. Amelioration of Hyperglycemia and Associated Metabolic Abnormalities by a Combination of Fenugreek (Trigonella foenum-graecum) Seeds and Onion (Allium cepa) in Experimental Diabetes. J. Basic Clin. Physiol. Pharmacol. 2017, 28, 493–505. [Google Scholar] [CrossRef]
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 13 November 2022).
- Facts & Figures. Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 19 November 2022).
- Klekotka, R.B.; Mizgała, E.; Król, W. The Etiology of Lower Respiratory Tract Infections in People with Diabetes. Adv. Respir. Med. 2015, 83, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Janifer, J.; Geethalakshmi, S.; Satyavani, K.; Viswanathan, V. Prevalence of Lower Urinary Tract Infection in South Indian Type 2 Diabetic Subjects. Indian J. Nephrol. 2009, 19, 107. [Google Scholar] [CrossRef]
- Behzadi, P.; Carevic, B. Microbiology of Urinary Tract Infections: Microbial Agents and Predisposing Factors; BoD—Books on Demand: Norderstedt, Germany, 2019; ISBN 978-1-78984-955-4. [Google Scholar]
- Tognetti, L.; Martinelli, C.; Berti, S.; Hercogova, J.; Lotti, T.; Leoncini, F.; Moretti, S. Bacterial Skin and Soft Tissue Infections: Review of the Epidemiology, Microbiology, Aetiopathogenesis and Treatment. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 931–941. [Google Scholar] [CrossRef]
- Herold, K.C.; Rubenstein, A.H. Immunosuppression for Insulin-Dependent Diabetes. Available online: https://www.nejm.org/doi/pdf/10.1056/NEJM198803173181110 (accessed on 19 November 2022).
- Pickup, J.C.; Crook, M.A. Is Type II Diabetes Mellitus a Disease of the Innate Immune System? Diabetologia 1998, 41, 1241–1248. [Google Scholar] [CrossRef] [Green Version]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and Its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Donath, M.Y.; Halban, P.A. Decreased Beta-Cell Mass in Diabetes: Significance, Mechanisms and Therapeutic Implications. Diabetologia 2004, 47, 581–589. [Google Scholar] [CrossRef] [Green Version]
- Potenza, M.A.; Gagliardi, S.; Nacci, C.; Carratu, M.R.; Montagnani, M. Endothelial Dysfunction in Diabetes: From Mechanisms to Therapeutic Targets. Curr. Med. Chem. 2009, 16, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Meylan, E.; Tschopp, J.; Karin, M. Intracellular Pattern Recognition Receptors in the Host Response. Nature 2006, 442, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Srinivasan, K. Attenuation of Oxidative Stress and Cardioprotective Effects of Zinc Supplementation in Experimental Diabetic Rats. Br. J. Nutr. 2017, 117, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Pradeep, S.R.; Srinivasan, K. Amelioration of Oxidative Stress by Dietary Fenugreek (Trigonella foenum-graecum L.) Seeds Is Potentiated by Onion (Allium cepa L.) in Streptozotocin-Induced Diabetic Rats. Appl. Physiol. Nutr. Metab. 2017, 42, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Srinivasan, K. Zinc Supplementation Ameliorates Diabetic Cataract Through Modulation of Crystallin Proteins and Polyol Pathway in Experimental Rats. Biol. Trace Elem Res. 2019, 187, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, S.R.; Srinivasan, K. Ameliorative Influence of Dietary Fenugreek (Trigonella foenum-graecum) Seeds and Onion (Allium cepa) on Eye Lens Abnormalities via Modulation of Crystallin Proteins and Polyol Pathway in Experimental Diabetes. Curr. Eye Res. 2018, 43, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.S.M.; Ho, E.C.M.; Lam, K.S.L.; Chung, S.K. Contribution of Polyol Pathway to Diabetes-Induced Oxidative Stress. JASN 2003, 14, S233–S236. [Google Scholar] [CrossRef] [Green Version]
- Barman, S.; Srinivasan, K. Diabetes and Zinc Dyshomeostasis: Can Zinc Supplementation Mitigate Diabetic Complications? Crit. Rev. Food Sci. Nutr. 2022, 62, 1046–1061. [Google Scholar] [CrossRef]
- Urner, S.; Ho, F.; Jha, J.C.; Ziegler, D.; Jandeleit-Dahm, K. NADPH Oxidase Inhibition: Preclinical and Clinical Studies in Diabetic Complications. Antioxid. Redox Signal. 2020, 33, 415–434. [Google Scholar] [CrossRef]
- Wangoo, A.; Johnson, L.; Gough, J.; Ackbar, R.; Inglut, S.; Hicks, D.; Spencer, Y.; Hewinson, G.; Vordermeier, M. Advanced Granulomatous Lesions in Mycobacterium Bovis-Infected Cattle Are Associated with Increased Expression of Type I Procollagen, Γδ (WC1+) T Cells and CD 68+ Cells. J. Comp. Pathol. 2005, 133, 223–234. [Google Scholar] [CrossRef]
- Giacomini, E.; Iona, E.; Ferroni, L.; Miettinen, M.; Fattorini, L.; Orefici, G.; Julkunen, I.; Coccia, E.M. Infection of Human Macrophages and Dendritic Cells with Mycobacterium Tuberculosis Induces a Differential Cytokine Gene Expression That Modulates T Cell Response1. J. Immunol. 2001, 166, 7033–7041. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, P.A.; Alkanani, A.K.; Michels, A.W.; Lewis, E.C.; Shapiro, L.; Dinarello, C.A.; Zipris, D. A1-Antitrypsin Therapy Downregulates Toll-Like Receptor-Induced IL-1β Responses in Monocytes and Myeloid Dendritic Cells and May Improve Islet Function in Recently Diagnosed Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2014, 99, E1418–E1426. [Google Scholar] [CrossRef] [PubMed]
- Lachmandas, E.; Thiem, K.; van den Heuvel, C.; Hijmans, A.; de Galan, B.E.; Tack, C.J.; Netea, M.G.; van Crevel, R.; van Diepen, J.A. Patients with Type 1 Diabetes Mellitus Have Impaired IL-1β Production in Response to Mycobacterium Tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessaro, F.H.G.; Ayala, T.S.; Nolasco, E.L.; Bella, L.M.; Martins, J.O. Insulin Influences LPS-Induced TNF-α and IL-6 Release Through Distinct Pathways in Mouse Macrophages from Different Compartments. Cell. Physiol. Biochem. 2017, 42, 2093–2104. [Google Scholar] [CrossRef]
- Reinhold, D.; Ansorge, S. Elevated Glucose Levels Stimulate Transforming Growth Factor-Β1 (TGF-Β1), Suppress Interleukin IL-2, IL-6 and IL-10 Production and DNA Synthesis in Peripheral Blood Mononuclear Cells. Horm. Metab. Res. 1996, 28, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Spindler, M.P.; Ho, A.M.; Tridgell, D.; McCulloch-Olson, M.; Gersuk, V.; Ni, C.; Greenbaum, C.; Sanda, S. Acute Hyperglycemia Impairs IL-6 Expression in Humans. Immun. Inflamm. Dis. 2016, 4, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, Y.; Wang, L.; Fan, F.; Zhu, L.; Li, Z.; Ruan, X.; Huang, H.; Wang, Z.; Huang, Z.; et al. Amelioration of High Fat Diet Induced Liver Lipogenesis and Hepatic Steatosis by Interleukin-22. J. Hepatol. 2010, 53, 339–347. [Google Scholar] [CrossRef]
- Tan, K.S.; Lee, K.O.; Low, K.C.; Gamage, A.M.; Liu, Y.; Tan, G.-Y.G.; Koh, H.Q.V.; Alonso, S.; Gan, Y.-H. Glutathione Deficiency in Type 2 Diabetes Impairs Cytokine Responses and Control of Intracellular Bacteria. J. Clin. Investig. 2012, 122, 2289–2300. [Google Scholar] [CrossRef]
- Restrepo, B.I.; Twahirwa, M.; Rahbar, M.H.; Schlesinger, L.S. Phagocytosis via Complement or Fc-Gamma Receptors Is Compromised in Monocytes from Type 2 Diabetes Patients with Chronic Hyperglycemia. PLoS ONE 2014, 9, e92977. [Google Scholar] [CrossRef] [Green Version]
- Pavlou, S.; Lindsay, J.; Ingram, R.; Xu, H.; Chen, M. Sustained High Glucose Exposure Sensitizes Macrophage Responses to Cytokine Stimuli but Reduces Their Phagocytic Activity. BMC Immunol. 2018, 19, 24. [Google Scholar] [CrossRef] [Green Version]
- Pamir, N.; McMillen, T.S.; Kaiyala, K.J.; Schwartz, M.W.; LeBoeuf, R.C. Receptors for Tumor Necrosis Factor-α Play a Protective Role against Obesity and Alter Adipose Tissue Macrophage Status. Endocrinology 2009, 150, 4124–4134. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Pradeep, S.R.; Srinivasan, K. Zinc Supplementation Alleviates the Progression of Diabetic Nephropathy by Inhibiting the Overexpression of Oxidative-Stress-Mediated Molecular Markers in Streptozotocin-Induced Experimental Rats. J. Nutr. Biochem. 2018, 54, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Ferracini, M.; Martins, J.O.; Campos, M.R.M.; Anger, D.B.C.; Jancar, S. Impaired Phagocytosis by Alveolar Macrophages from Diabetic Rats Is Related to the Deficient Coupling of LTs to the FcγR Signaling Cascade. Mol. Immunol. 2010, 47, 1974–1980. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Broedl, U.C.; Biller-Friedmann, I.M.; Vogeser, M.; Henschel, V.; Nassau, K.; Göke, B.; Kilger, E.; Parhofer, K.G. Serum Concentrations of Cortisol, Interleukin 6, Leptin and Adiponectin Predict Stress Induced Insulin Resistance in Acute Inflammatory Reactions. Crit. Care 2008, 12, R157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antoniades, C.; Tousoulis, D.; Marinou, K.; Papageorgiou, N.; Bosinakou, E.; Tsioufis, C.; Stefanadi, E.; Latsios, G.; Tentolouris, C.; Siasos, G.; et al. Effects of Insulin Dependence on Inflammatory Process, Thrombotic Mechanisms and Endothelial Function, in Patients with Type 2 Diabetes Mellitus and Coronary Atherosclerosis. Clin. Cardiol. 2007, 30, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Dinh, W.; Füth, R.; Nickl, W.; Krahn, T.; Ellinghaus, P.; Scheffold, T.; Bansemir, L.; Bufe, A.; Barroso, M.C.; Lankisch, M. Elevated Plasma Levels of TNF-Alpha and Interleukin-6 in Patients with Diastolic Dysfunction and Glucose Metabolism Disorders. Cardiovasc Diabetol 2009, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-F.; Zhang, H.-J.; Hu, Q.-X.; Liu, X.-Y.; Wang, Z.-Q.; Fan, J.-Y.; Zhan, M.; Chen, F.-L. Altered Polarization, Morphology, and Impaired Innate Immunity Germane to Resident Peritoneal Macrophages in Mice with Long-Term Type 2 Diabetes. BioMed Res. Int. 2012, 2012, e867023. [Google Scholar] [CrossRef] [Green Version]
- Stegenga, M.E.; van der Crabben, S.N.; Blümer, R.M.E.; Levi, M.; Meijers, J.C.M.; Serlie, M.J.; Tanck, M.W.T.; Sauerwein, H.P.; van der Poll, T. Hyperglycemia Enhances Coagulation and Reduces Neutrophil Degranulation, Whereas Hyperinsulinemia Inhibits Fibrinolysis during Human Endotoxemia. Blood 2008, 112, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.B.; Lad, A.; Bharath Prasad, A.S.; Balakrishnan, A.; Ramachandra, L.; Satyamoorthy, K. High Glucose Modulates IL-6 Mediated Immune Homeostasis through Impeding Neutrophil Extracellular Trap Formation. FEBS Lett. 2013, 587, 2241–2246. [Google Scholar] [CrossRef] [Green Version]
- Berrou, J.; Fougeray, S.; Venot, M.; Chardiny, V.; Gautier, J.-F.; Dulphy, N.; Toubert, A.; Peraldi, M.-N. Natural Killer Cell Function, an Important Target for Infection and Tumor Protection, Is Impaired in Type 2 Diabetes. PLoS ONE 2013, 8, e62418. [Google Scholar] [CrossRef] [Green Version]
- Kukreja, A.; Maclaren*, N.K. Current Cases in Which Epitope Mimicry Is Considered as a Component Cause of Autoimmune Disease: Immune-Mediated (Type 1) Diabetes. CMLS Cell. Mol. Life Sci. 2000, 57, 534–541. [Google Scholar] [CrossRef]
- van der Werf, N.; Kroese, F.G.M.; Rozing, J.; Hillebrands, J.-L. Viral Infections as Potential Triggers of Type 1 Diabetes. Diabetes/Metab. Res. Rev. 2007, 23, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Stabilini, A.; Migliavacca, B.; Horejs-Hoeck, J.; Kaupper, T.; Roncarolo, M.-G. Rapamycin Promotes Expansion of Functional CD4+CD25+FOXP3+ Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients1. J. Immunol. 2006, 177, 8338–8347. [Google Scholar] [CrossRef] [Green Version]
- Grazia Roncarolo, M.; Gregori, S.; Battaglia, M.; Bacchetta, R.; Fleischhauer, K.; Levings, M.K. Interleukin-10-Secreting Type 1 Regulatory T Cells in Rodents and Humans. Immunol. Rev. 2006, 212, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Marek-Trzonkowska, N.; Myśliwec, M.; Siebert, J.; Trzonkowski, P. Clinical Application of Regulatory T Cells in Type 1 Diabetes. Pediatr. Diabetes 2013, 14, 322–332. [Google Scholar] [CrossRef]
- Gong, Y.; Li, C.; Wang, C.; Li, J.; Ding, M.; Chen, D.; Lao, M. Epidemiology and Mortality-Associated Factors of Invasive Fungal Disease in Elderly Patients: A 20-Year Retrospective Study from Southern China. Infect. Drug Resist. 2020, 13, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassetti, M.; Garnacho-Montero, J.; Calandra, T.; Kullberg, B.; Dimopoulos, G.; Azoulay, E.; Chakrabarti, A.; Kett, D.; Leon, C.; Ostrosky-Zeichner, L.; et al. Intensive Care Medicine Research Agenda on Invasive Fungal Infection in Critically Ill Patients. Intensive Care Med. 2017, 43, 1225–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DM, S.; Govindakarnavar, A.; KE, V.; PS, R. Case Repot—Sino-Orbital Aspergillosis in a Diabetic Patient. Indian J. Med. Microbiol. 2006, 24, 138. [Google Scholar]
- Rankin, N.E. Disseminated Aspergillosis and Moniliasis Associated with Agranulocytosis and Antibiotic Therapy. Br. Med. J. 1953, 1, 918–919. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.E.; Chamilos, G.; Prince, R.A.; Kontoyiannis, D.P. Pretreatment with Empty Liposomes Attenuates the Immunopathology of Invasive Pulmonary Aspergillosis in Corticosteroid-Immunosuppressed Mice. Antimicrob. Agents Chemother. 2007, 51, 1078–1081. [Google Scholar] [CrossRef] [Green Version]
- McNeil, M.M.; Nash, S.L.; Hajjeh, R.A.; Phelan, M.A.; Conn, L.A.; Plikaytis, B.D.; Warnock, D.W. Trends in Mortality Due to Invasive Mycotic Diseases in the United States, 1980–1997. Clin. Infect. Dis. 2001, 33, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Kourkoumpetis, T.K.; Desalermos, A.; Muhammed, M.; Mylonakis, E. Central Nervous System Aspergillosis: A Series of 14 Cases from a General Hospital and Review of 123 Cases from the Literature. Medicine 2012, 91, 328–336. [Google Scholar] [CrossRef]
- Ghanaat, F.; Tayek, J.A. Weight Loss and Diabetes Are New Risk Factors for the Development of Invasive Aspergillosis Infection in Non-Immunocompromized Humans. Clin. Pract. 2017, 14, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, J.A.; Khan, M.A.; Afroze, N.; Haq, T. Localized Primary Renal Aspergillosis in a Diabetic Patient Following Lithotripsy—A Case Report. BMC Infect. Dis. 2007, 7, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Gui, L. Cavernous Sinus-Orbital Apex Aspergillus Infection in a Diabetic Patient. Medicine 2019, 98, e15041. [Google Scholar] [CrossRef]
- Remington, T.L.; Fuller, J.; Chiu, I. Chronic Necrotizing Pulmonary Aspergillosis in a Patient with Diabetes and Marijuana Use. CMAJ 2015, 187, 1305–1308. [Google Scholar] [CrossRef] [Green Version]
- Hartemink, K.J.; Paul, M.A.; Spijkstra, J.J.; Girbes, A.R.J.; Polderman, K.H. Immunoparalysis as a Cause for Invasive Aspergillosis? Intensive Care Med. 2003, 29, 2068–2071. [Google Scholar] [CrossRef]
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [Green Version]
- Rosenbloom, A.L. Hyperglycemic Crises and Their Complications in Children. J. Pediatr. Endocrinol. Metab. 2007, 20, 5–18. [Google Scholar] [CrossRef]
- Bitar, D.; Van Cauteren, D.; Lanternier, F.; Dannaoui, E.; Che, D.; Dromer, F.; Desenclos, J.-C.; Lortholary, O. Increasing Incidence of Zygomycosis (Mucormycosis), France, 1997–2006. Emerg. Infect. Dis. 2009, 15, 1395–1401. [Google Scholar] [CrossRef]
- Muszewska, A.; Pawłowska, J.; Krzyściak, P. Biology, Systematics, and Clinical Manifestations of Zygomycota Infections. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1273–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neonatal Skin Emergencies. Available online: https://journals.healio.com/doi/10.3928/19382359-20181210-03 (accessed on 19 November 2022).
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The Rising Trend of Invasive Zygomycosis in Patients with Uncontrolled Diabetes Mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Das, A.; Sharma, A.; Panda, N.; Das, S.; Gupta, K.L.; Sakhuja, V. Ten Years’ Experience in Zygomycosis at a Tertiary Care Centre in India. J. Infect. 2001, 42, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Dekkers, R.; Verweij, P.E.; Weemaes, C.M.R.; Severijnen, R.S.V.M.; Van Krieken, J.H.J.M.; Warris, A. Gastrointestinal Zygomycosis Due to Rhizopus Microsporus Var. Rhizopodiformis as a Manifestation of Chronic Granulomatous Disease. Med. Mycol. 2008, 46, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Gugnani, H.C. A Review of Zygomycosis Due to Basidiobolus Ranarum. Eur. J. Epidemiol. 1999, 15, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.J.; Renshaw, G.; Andrews, J.; Kwon-Chung, J.; Cunnion, R.C.; Pass, H.I.; Taubenberger, J.; Wilson, W.; Pizzo, P.A. Invasive Zygomycosis Due to Conidiobolus Incongruus. Clin. Infect. Dis. 1994, 19, 423–430. [Google Scholar] [CrossRef]
- Yu, J.; Yu Li, R. Primary Renal Zygomycosis Due to Rhizopus Oryzae. Med. Mycol. 2006, 44, 461–466. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, A.; Marak, R.S.K.; Shivaprakash, M.R.; Gupta, S.; Garg, R.; Sakhuja, V.; Singhal, S.; Baghela, A.; Dixit, A.; Garg, M.K.; et al. Cavitary Pulmonary Zygomycosis Caused by Rhizopus homothallicus. J. Clin. Microbiol. 2010, 48, 1965–1969. [Google Scholar] [CrossRef] [Green Version]
- Kontoyiannis, D.P. Decrease in the Number of Reported Cases of Zygomycosis among Patients with Diabetes Mellitus: A Hypothesis. Clin. Infect. Dis. 2007, 44, 1089–1090. [Google Scholar] [CrossRef] [Green Version]
- Kwak, B.; Mulhaupt, F.; Myit, S.; Mach, F. Statins as a Newly Recognized Type of Immunomodulator. Nat. Med. 2000, 6, 1399–1402. [Google Scholar] [CrossRef]
- Lukács, G.; Papp, T.; Nyilasi, I.; Nagy, E.; Vágvölgyi, C. Differentiation of Rhizomucor Species on the Basis of Their Different Sensitivities to Lovastatin. J. Clin. Microbiol. 2004, 42, 5400–5402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamilos, G.; Lewis, R.E.; Kontoyiannis, D.P. Lovastatin Has Significant Activity against Zygomycetes and Interacts Synergistically with Voriconazole. Antimicrob. Agents Chemother. 2006, 50, 96–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kontoyiannis, D.P.; Lionakis, M.S.; Lewis, R.E.; Chamilos, G.; Healy, M.; Perego, C.; Safdar, A.; Kantarjian, H.; Champlin, R.; Walsh, T.J.; et al. Zygomycosis in a Tertiary-Care Cancer Center in the Era of Aspergillus-Active Antifungal Therapy: A Case-Control Observational Study of 27 Recent Cases. J. Infect. Dis. 2005, 191, 1350–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uçkay, I.; Chalandon, Y.; Sartoretti, P.; Rohner, P.; Berney, T.; Hadaya, K.; Van Delden, C. Invasive Zygomycosis in Transplant Recipients. Clin. Transplant. 2007, 21, 577–582. [Google Scholar] [CrossRef]
- Sundaram, C.; Mahadevan, A.; Laxmi, V.; Yasha, T.C.; Santosh, V.; Murthy, J.M.K.; Purohit, A.K.; Mohandas, S.; Shankar, S.K. Cerebral Zygomycosis. Mycoses 2005, 48, 396–407. [Google Scholar] [CrossRef]
- Giglio, M.; Caggiano, G.; De Blasi, R.; Brienza, N.; Bucaria, V.; Ladisa, P.; Ceci, G.; Dalfino, L.; Montagna, M.T.; Bruno, F.; et al. A Fatal Rhino-Cerebral Zygomycosis in a Young Woman with Latent Diabetes Mellitus and Cerebral Blood Vessel Agenesis. Med. Mycol. 2010, 48, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Reed, C.; Bryant, R.; Ibrahim, A.S.; Edwards, J., Jr.; Filler, S.G.; Goldberg, R.; Spellberg, B. Combination Polyene-Caspofungin Treatment of Rhino-Orbital-Cerebral Mucormycosis. Clin. Infect. Dis. 2008, 47, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial Fungal Infections: Epidemiology, Diagnosis, and Treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef]
- da Cunha, M.A.; Nery, A.F.; Lima, F.P.; Diniz Junior, J.; Maciel Neto, J.; Calado, N.B.; Luz, K.G.; Milan, E.P. Rhinocerebral Zygomycosis in a Diabetic Patient. Rev. Soc. Bras. Med. Trop. 2011, 44, 257–259. [Google Scholar] [CrossRef] [Green Version]
- Schoenmakers, M.C.J.; Hament, J.-M.; Fleer, A.; Aerts, P.C.; van Dijk, H.; Kimpen, J.L.L.; Wolfs, T.F.W. Risk Factors for Invasive Pneumococcal Disease. Rev. Res. Med. Microbiol. 2002, 13, 29–36. [Google Scholar] [CrossRef]
- Robinson, K.A.; Baughman, W.; Rothrock, G.; Barrett, N.L.; Pass, M.; Lexau, C.; Damaske, B.; Stefonek, K.; Barnes, B.; Patterson, J.; et al. Epidemiology of Invasive Streptococcus Pneumoniae Infections in the United States, 1995-1998Opportunities for Prevention in the Conjugate Vaccine Era. JAMA 2001, 285, 1729–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahl, M.S.; Trollfors, B.; Claesson, B.A.; Brandberg, L.L.; Rosengren, A. Invasive Pneumococcal Infections in Southwestern Sweden: A Second Follow-up Period of 15 Years. Scand. J. Infect. Dis. 2001, 33, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Akbar, D.H. Bacterial Pneumonia: Comparison between Diabetics and Non-Diabetics. Acta Diabetol. 2001, 38, 77–82. [Google Scholar] [CrossRef]
- Mohan, V.; Unnikrishnan, R.; Thomas, N.; Bhansali, A.; Wangnoo, S.K.; Thomas, K. Pneumococcal Infections and Immunization in Diabetic Patients. J. Postgrad. Med. 2011, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Saltzman, R.L.; Peterson, P.K. Immunodeficiency of the Elderly. Rev. Infect. Dis. 1987, 9, 1127–1139. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Weerarathna, T.; McCarthy, J.S.; Davis, T.M.E. Common Infections in Diabetes: Pathogenesis, Management and Relationship to Glycaemic Control. Diabetes/Metab. Res. Rev. 2007, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.C.W.; Raskin, P. Lung Function Changes Related to Diabetes Mellitus. Diabetes Technol. Ther. 2007, 9, S73–S82. [Google Scholar] [CrossRef] [PubMed]
- Davis, W.A.; Knuiman, M.; Kendall, P.; Grange, V.; Davis, T.M.E. Glycemic Exposure Is Associated with Reduced Pulmonary Function in Type 2 Diabetes: The Fremantle Diabetes Study. Diabetes Care 2004, 27, 752–757. [Google Scholar] [CrossRef] [Green Version]
- van den Borst, B.; Gosker, H.R.; Zeegers, M.P.; Schols, A.M.W.J. Pulmonary Function in Diabetes: A Metaanalysis. Chest 2010, 138, 393–406. [Google Scholar] [CrossRef]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.-C.; Allannic, H.; Genetet, B. Impaired Leucocyte Functions in Diabetic Patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]
- Moss, M.; Guidot, D.M.; Steinberg, K.P.; Duhon, G.F.; Treece, P.; Wolken, R.; Hudson, L.D.; Parsons, P.E. Diabetic Patients Have a Decreased Incidence of Acute Respiratory Distress Syndrome. Crit. Care Med. 2000, 28, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Liappis, A.P.; Kan, V.L.; Rochester, C.G.; Simon, G.L. The Effect of Statins on Mortality in Patients with Bacteremia. Clin. Infect. Dis. 2001, 33, 1352–1357. [Google Scholar] [CrossRef] [Green Version]
- Riahi, S.; Fonager, K.; Toft, E.; Hvilsted-Rasmussen, L.; Bendsen, J.; Paaske Johnsen, S.; Toft Sørensen, H. Use of Lipid-Lowering Drugs during 1991–98 in Northern Jutland, Denmark. Br. J. Clin. Pharmacol. 2001, 52, 307–311. [Google Scholar] [CrossRef] [Green Version]
- Bogaert, D.; Hermans, P.W.M.; Adrian, P.V.; Rümke, H.C.; de Groot, R. Pneumococcal Vaccines: An Update on Current Strategies. Vaccine 2004, 22, 2209–2220. [Google Scholar] [CrossRef] [PubMed]
- Cortese, M.M.; Wolff, M.; Almeido-Hill, J.; Reid, R.; Ketcham, J.; Santosham, M. High Incidence Rates of Invasive Pneumococcal Disease in the White Mountain Apache Population. Arch. Intern. Med. 1992, 152, 2277–2282. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Parkinson, A.J.; Bulkow, L.R.; Fitzgerald, M.A.; Peters, H.V.; Parks, D.J. The Epidemiology of Invasive Pneumococcal Disease in Alaska, 1986-1990 Ethnic Differences and Opportunities for Prevention. J. Infect. Dis. 1994, 170, 368–376. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Immunization and the Prevention of Influenza and Pneumococcal Disease in People with Diabetes. Diabetes Care 2003, 26, s126–s128. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, C. Rhinosinusitis Update. In Optimal Pain Management for the Dental Team; Renton, T., Ed.; BDJ Clinician’s Guides; Springer International Publishing: Cham, Switzerland, 2022; pp. 153–163. ISBN 978-3-030-86634-1. [Google Scholar]
- Stevens, W.W.; Peters, A.T.; Tan, B.K.; Klingler, A.I.; Poposki, J.A.; Hulse, K.E.; Grammer, L.C.; Welch, K.C.; Smith, S.S.; Conley, D.B.; et al. Associations Between Inflammatory Endotypes and Clinical Presentations in Chronic Rhinosinusitis. J. Allergy Clin. Immunol. Pract. 2019, 7, 2812–2820.e3. [Google Scholar] [CrossRef]
- Zhang, Z.; Adappa, N.D.; Lautenbach, E.; Chiu, A.G.; Doghramji, L.; Howland, T.J.; Cohen, N.A.; Palmer, J.N. The Effect of Diabetes Mellitus on Chronic Rhinosinusitis and Sinus Surgery Outcome. Int. Forum Allergy Rhinol. 2014, 4, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.; Peters, A.T. Pathophysiology of Chronic Rhinosinusitis with Nasal Polyp. Am. J. Rhinol. Allergy 2011, 25, 285–290. [Google Scholar] [CrossRef]
- Turner, J.H.; Soudry, E.; Nayak, J.V.; Hwang, P.H. Survival Outcomes in Acute Invasive Fungal Sinusitis: A Systematic Review and Quantitative Synthesis of Published Evidence. Laryngoscope 2013, 123, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Lanternier, F.; Poirée, S.; Kania, R.; Lortholary, O. Diabetes and Mucormycosis: A Complex Interplay. Diabetes Metab. 2012, 38, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Yohai, R.A.; Bullock, J.D.; Aziz, A.A.; Markert, R.J. Survival Factors in Rhino-Orbital-Cerebral Mucormycosis. Surv. Ophthalmol. 1994, 39, 3–22. [Google Scholar] [CrossRef]
- Barman, S.; Srinivasan, K. Ameliorative Effect of Zinc Supplementation on Compromised Small Intestinal Health in Streptozotocin-Induced Diabetic Rats. Chem.-Biol. Interact. 2019, 307, 37–50. [Google Scholar] [CrossRef]
- Barman, S.; Srinivasan, K. Enhanced Intestinal Absorption of Micronutrients in Streptozotocin-Induced Diabetic Rats Maintained on Zinc Supplementation. J. Trace Elem. Med. Biol. 2018, 50, 182–187. [Google Scholar] [CrossRef]
- Raizada, N.; Jyotsna, V.P.; Kandasamy, D.; Xess, I.; Thakar, A.; Tandon, N. Invasive Fungal Rhinosinusitis in Patients with Diabetes. J. Infect. Dev. Ctries. 2018, 12, 787–793. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Walsh, T.J.; Kontoyiannis, D.P. Pathogenesis of Mucormycosis. Clin. Infect. Dis. 2012, 54, S16–S22. [Google Scholar] [CrossRef] [Green Version]
- Petrikkos, G.; Skiada, A.; Lortholary, O.; Roilides, E.; Walsh, T.J.; Kontoyiannis, D.P. Epidemiology and Clinical Manifestations of Mucormycosis. Clin. Infect. Dis. 2012, 54, S23–S34. [Google Scholar] [CrossRef] [PubMed]
- Corzo-León, D.E.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes Mellitus as the Major Risk Factor for Mucormycosis in Mexico: Epidemiology, Diagnosis, and Outcomes of Reported Cases. Med. Mycol. 2018, 56, 29–43. [Google Scholar] [CrossRef] [Green Version]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 Cases Accrued by the Registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef] [Green Version]
- Lanternier, F.; Dannaoui, E.; Morizot, G.; Elie, C.; Garcia-Hermoso, D.; Huerre, M.; Bitar, D.; Dromer, F.; Lortholary, O.; French Mycosis Study Group. A Global Analysis of Mucormycosis in France: The RetroZygo Study (2005–2007). Clin. Infect. Dis. 2012, 54 (Suppl 1), S35–S43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, J.R.; Tullis, D.E.; Grossman, R.F.; Vellend, H.; Winton, T.L.; Patterson, G.A. Infectious Complications Following Isolated Lung Transplantation. Chest 1992, 101, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- Arce-Salinas, C.A.; Pérez-Silva, E. Mucormycosis Complications in Systemic Lupus Erythematosus. Lupus 2010, 19, 985–988. [Google Scholar] [CrossRef]
- Chegini, Z.; Didehdar, M.; Khoshbayan, A.; Rajaeih, S.; Salehi, M.; Shariati, A. Epidemiology, Clinical Features, Diagnosis and Treatment of Cerebral Mucormycosis in Diabetic Patients: A Systematic Review of Case Reports and Case Series. Mycoses 2020, 63, 1264–1282. [Google Scholar] [CrossRef]
- Vaughan, C.; Bartolo, A.; Vallabh, N.; Leong, S.C. A Meta-Analysis of Survival Factors in Rhino-Orbital-Cerebral Mucormycosis—Has Anything Changed in the Past 20 Years? Clin. Otolaryngol. 2018, 43, 1454–1464. [Google Scholar] [CrossRef]
- Chawla, U.; Chadha, G.; Anand, N.; Rani, A.; Bidhalan, T.; Chugh, J.P.; Chauhan, R.S.; Gupta, R.; Phogat, J.; Deswal, J.; et al. COVID 19 Associated Rhino-Orbital-Cerebral Mucormycosis- Clinicoetiological Profile and Management Outcome of Patients in Tertiary Eye Care Centre in Northern India. Saudi J. Med. Pharm. Sci. 2022, 8, 120–133. [Google Scholar] [CrossRef]
- Alqhamdi, S.; Idress, B.; Alharbi, A.; Aljurais, N. Case Report: Disseminated Pulmonary Mucormycosis Involving Spleen in Diabetic Patient with Aggressive Surgical Approach. Int. J. Surg. Case Rep. 2019, 54, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Rammaert, B.; Lanternier, F.; Zahar, J.-R.; Dannaoui, E.; Bougnoux, M.-E.; Lecuit, M.; Lortholary, O. Healthcare-Associated Mucormycosis. Clin. Infect. Dis. 2012, 54, S44–S54. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, S.; Hata, A.; Sadaoka, K.; Yamanishi, K.; Mori, Y. Comparison of Varicella-Zoster Virus-Specific Immunity of Patients with Diabetes Mellitus and Healthy Individuals. J. Infect. Dis. 2009, 200, 1606–1610. [Google Scholar] [CrossRef] [Green Version]
- Heymann, A.D.; Chodick, G.; Karpati, T.; Kamer, L.; Kremer, E.; Green, M.S.; Kokia, E.; Shalev, V. Diabetes as a Risk Factor for Herpes Zoster Infection: Results of a Population-Based Study in Israel. Infection 2008, 36, 226–230. [Google Scholar] [CrossRef]
- Hata, A.; Kuniyoshi, M.; Ohkusa, Y. Risk of Herpes Zoster in Patients with Underlying Diseases: A Retrospective Hospital-Based Cohort Study. Infection 2011, 39, 537–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weitzman, D.; Shavit, O.; Stein, M.; Cohen, R.; Chodick, G.; Shalev, V. A Population Based Study of the Epidemiology of Herpes Zoster and Its Complications. J. Infect. 2013, 67, 463–469. [Google Scholar] [CrossRef]
- Ke, C.-C.; Lai, H.-C.; Lin, C.-H.; Hung, C.-J.; Chen, D.-Y.; Sheu, W.H.-H.; Lui, P.-W. Increased Risk of Herpes Zoster in Diabetic Patients Comorbid with Coronary Artery Disease and Microvascular Disorders: A Population-Based Study in Taiwan. PLoS ONE 2016, 11, e0146750. [Google Scholar] [CrossRef] [Green Version]
- Kawai, K.; Yawn, B.P. Risk Factors for Herpes Zoster: A Systematic Review and Meta-Analysis. Mayo Clin. Proc. 2017, 92, 1806–1821. [Google Scholar] [CrossRef] [Green Version]
- Nassaji-Zavareh, M.; Taheri, R.; Ghorbani, R.; Aminian, M. Undiagnosed Diabetes Mellitus in Patients with Herpes Zoster. Indian J. Derm. 2008, 53, 119–121. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lin, I.-C.; Chen, H.-J.; Yeh, S.-Y.; Kao, C.-H. Association of Herpes Zoster and Type 1 Diabetes Mellitus. PLoS ONE 2016, 11, e0155175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-Quiles, C.; López-Lacort, M.; Ampudia-Blasco, F.J.; Díez-Domingo, J. Risk and Impact of Herpes Zoster on Patients with Diabetes: A Population-Based Study, 2009–2014. Hum. Vaccines Immunother. 2017, 13, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Guignard, A.P.; Greenberg, M.; Lu, C.; Rosillon, D.; Vannappagari, V. Risk of Herpes Zoster among Diabetics: A Matched Cohort Study in a US Insurance Claim Database before Introduction of Vaccination, 1997–2006. Infection 2014, 42, 729–735. [Google Scholar] [CrossRef] [Green Version]
- Aytac, U.; Dang, N.H. CD26 / Dipeptidyl Peptidase IV: A Regulator of Immune Function and a Potential Molecular Target for Therapy. Curr. Drug Targets-Immune Endocr. Metab. Disord. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, L.; Wang, X.; Zhou, Z. The New Insights from DPP-4 Inhibitors: Their Potential Immune Modulatory Function in Autoimmune Diabetes. Diabetes/Metab. Res. Rev. 2014, 30, 646–653. [Google Scholar] [CrossRef]
- Forbes, H.J.; Bhaskaran, K.; Thomas, S.L.; Smeeth, L.; Clayton, T.; Mansfield, K.; Minassian, C.; Langan, S.M. Quantification of Risk Factors for Postherpetic Neuralgia in Herpes Zoster Patients: A Cohort Study. Neurology 2016, 87, 94–102. [Google Scholar] [CrossRef] [Green Version]
- Suaya, J.A.; Chen, S.-Y.; Li, Q.; Burstin, S.J.; Levin, M.J. Incidence of Herpes Zoster and Persistent Post-Zoster Pain in Adults with or Without Diabetes in the United States. Open Forum Infect. Dis. 2014, 1, ofu049. [Google Scholar] [CrossRef] [PubMed]
- Bosco, D.; Plastino, M.; De Bartolo, M.; Cristiano, D.; Ettore, M.; Zurlo, G.; Bosco, F.; Colica, C.; Tallarigo, F.; Fava, A. Role of Impaired Glucose Metabolism in the Postherpetic Neuralgia. Clin. J. Pain 2013, 29, 733. [Google Scholar] [CrossRef]
- Torcel-Pagnon, L.; Bricout, H.; Bertrand, I.; Perinetti, E.; Franco, E.; Gabutti, G.; Volpi, A. Impact of Underlying Conditions on Zoster-Related Pain and on Quality of Life Following Zoster. J. Gerontol. Ser. A 2017, 72, 1091–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denning, D.W.; Stevens, D.A. Antifungal and Surgical Treatment of Invasive Aspergillosis: Review of 2,121 Published Cases. Rev. Infect. Dis. 1990, 12, 1147–1201. [Google Scholar] [CrossRef] [PubMed]
- Singer, C.; Armstrong, D.; Rosen, P.P.; Walzer, P.D.; Yu, B. Diffuse Pulmonary Infiltrates in Immunosuppressed Patients: Prospective Study of 80 Cases. Am. J. Med. 1979, 66, 110–120. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Marr, K.A.; Rex, J.H.; Cohen, S.H. Amphotericin B: Time for a New “Gold Standard”. Clin. Infect. Dis. 2003, 37, 415–425. [Google Scholar]
- Gonçalves, S.S.; Souza, A.C.R.; Chowdhary, A.; Meis, J.F.; Colombo, A.L. Epidemiology and Molecular Mechanisms of Antifungal Resistance in Candida and Aspergillus. Mycoses 2016, 59, 198–219. [Google Scholar] [CrossRef]
- Azzola, A.; Passweg, J.R.; Habicht, J.M.; Bubendorf, L.; Tamm, M.; Gratwohl, A.; Eich, G. Use of Lung Resection and Voriconazole for Successful Treatment of Invasive Pulmonary Aspergillus ustus Infection. J. Clin. Microbiol. 2004, 42, 4805–4808. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, S.S.; Stchigel, A.M.; Cano, J.; Guarro, J.; Colombo, A.L. In Vitro Antifungal Susceptibility of Clinically Relevant Species Belonging to Aspergillus Section Flavi. Antimicrob. Agents Chemother. 2013, 57, 1944–1947. [Google Scholar] [CrossRef] [Green Version]
- Shivaprakash, M.R.; Geertsen, E.; Chakrabarti, A.; Mouton, J.W.; Meis, J.F. In Vitro Susceptibility of 188 Clinical and Environmental Isolates of Aspergillus flavus for the New Triazole Isavuconazole and Seven Other Antifungal Drugs. Mycoses 2011, 54, e583–e589. [Google Scholar] [CrossRef] [PubMed]
- Safdar, A.; Ma, J.; Saliba, F.; Dupont, B.; Wingard, J.R.; Hachem, R.Y.; Mattiuzzi, G.N.; Chandrasekar, P.H.; Kontoyiannis, D.P.; Rolston, K.V.; et al. Drug-Induced Nephrotoxicity Caused by Amphotericin B Lipid Complex and Liposomal Amphotericin B: A Review and Meta-Analysis. Medicine 2010, 89, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Crippa, F.; Leisenring, W.; Hoyle, M.; Boeckh, M.; Balajee, S.A.; Nichols, W.G.; Musher, B.; Corey, L. Itraconazole versus Fluconazole for Prevention of Fungal Infections in Patients Receiving Allogeneic Stem Cell Transplants. Blood 2004, 103, 1527–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.-W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus Amphotericin B for Primary Therapy of Invasive Aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theuretzbacher, U.; Ihle, F.; Derendorf, H. Pharmacokinetic/Pharmacodynamic Profile of Voriconazole. Clin. Pharm. 2006, 45, 649–663. [Google Scholar] [CrossRef]
- von Mach, M.A.; Burhenne, J.; Weilemann, L.S. Accumulation of the Solvent Vehicle Sulphobutylether Beta Cyclodextrin Sodium in Critically Ill Patients Treated with Intravenous Voriconazole under Renal Replacement Therapy. BMC Clin. Pharm. 2006, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Hamada, Y.; Tokimatsu, I.; Mikamo, H.; Kimura, M.; Seki, M.; Takakura, S.; Ohmagari, N.; Takahashi, Y.; Kasahara, K.; Matsumoto, K.; et al. Practice Guidelines for Therapeutic Drug Monitoring of Voriconazole: A Consensus Review of the Japanese Society of Chemotherapy and the Japanese Society of Therapeutic Drug Monitoring. J. Infect. Chemother. 2013, 19, 381–392. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.; Andes, D. Therapeutic Drug Monitoring of Antifungals: Pharmacokinetic and Pharmacodynamic Considerations. Ther. Drug Monit. 2008, 30, 167–172. [Google Scholar] [CrossRef]
- Imhof, A.; Balajee, S.A.; Fredricks, D.N.; Englund, J.A.; Marr, K.A. Breakthrough Fungal Infections in Stem Cell Transplant Recipients Receiving Voriconazole. Clin. Infect. Dis. 2004, 39, 743–746. [Google Scholar] [CrossRef] [Green Version]
- Walsh, T.J.; Finberg, R.W.; Arndt, C.; Hiemenz, J.; Schwartz, C.; Bodensteiner, D.; Pappas, P.; Seibel, N.; Greenberg, R.N.; Dummer, S.; et al. Liposomal Amphotericin B for Empirical Therapy in Patients with Persistent Fever and Neutropenia. N. Engl. J. Med. 1999, 340, 764–771. [Google Scholar] [CrossRef]
- Denning, D.W. Echinocandin Antifungal Drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Maertens, J.; Raad, I.; Petrikkos, G.; Boogaerts, M.; Selleslag, D.; Petersen, F.B.; Sable, C.A.; Kartsonis, N.A.; Ngai, A.; Taylor, A.; et al. Efficacy and Safety of Caspofungin for Treatment of Invasive Aspergillosis in Patients Refractory to or Intolerant of Conventional Antifungal Therapy. Clin. Infect. Dis. 2004, 39, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Anahita, S.; Nahal, M.; Azadeh, M.; Zahra, S.; Seyed Davood, M. Is Pulmonary Aspergillosis Common in Diabetes Mellitus Patients? TANAFFOS (Respir.) 2010, 9, 69–74. [Google Scholar]
- Johnson, A.K.; Ghazarian, Z.; Cendrowski, K.D.; Persichino, J.G. Pulmonary Aspergillosis and Mucormycosis in a Patient with COVID-19. Med. Mycol. Case Rep. 2021, 32, 64–67. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, D.; Pandhi, N. Pulmonary Fungal Infections in Diabetic Postcoronavirus Disease 2019 Patients—A Report of Three Cases. Indian J. Respir. Care 2022, 11, 60. [Google Scholar]
- PII: 0926-6542(63)90061-1|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/0926654263900611?token=BFB298646EA60BAE5049302CD7B0303F81D1D52FBEA1C967D7D087B5D1B86F28F6BE221D2CCFD084A166C8DF89B22FBF&originRegion=us-east-1&originCreation=20221114193639 (accessed on 14 November 2022).
- Kumagai, R.; Ohara, G.; Sato, S.; Miyazaki, K.; Kagohashi, K.; Kurishima, K.; Satoh, H. Successfully Treated Invasive Pulmonary Aspergillosis in a Patient with Diabetic Ketoacidosis. Cent. Eur. J. Med. 2013, 8, 685–688. [Google Scholar] [CrossRef] [Green Version]
- Nugroho, G.M.S.; Wulandari, L. Hemoptysis in a Patient with Pulmonary Aspergilloma and Type 2 Diabetes Mellitus: A Rare Case in an Indonesian Adult. Int. J. Surg. Case Rep. 2021, 84, 106125. [Google Scholar] [CrossRef] [PubMed]
- Rit, K.; Saha, R.; Dey, R.; Barik, G. Rhino-Oculo-Cerebral Aspergillus and Mucor Co-Infections in an Immunocompromised Patient with Type 2 Diabetes Mellitus. Med. J. Dr Patil Univ. 2014, 7, 486. [Google Scholar] [CrossRef]
- Gargouri, M.; Marrakchi, C.; Feki, W.; Charfi, S.; Maaloul, I.; Lahiani, D.; Elleuch, E.; Koubaa, M.; Mnif, Z.; Ayadi, A.; et al. Combination of Amphotericin B and Caspofungin in the Treatment of Mucormycosis. Med. Mycol. Case Rep. 2019, 26, 32–37. [Google Scholar] [CrossRef]
- Paydar, S.; Baezzat, S.R.; Fazelzadeh, A.; Geramizadeh, B. A Case of Gastric Zygomycosis in a Diabetic Patient Successfully Treated with Total Gastrectomy. Middle East J. Dig. Dis. MEJDD 2010, 2, 46–48. [Google Scholar] [CrossRef]
- Garg, J.; Sujatha, S.; Garg, A.; Parija, S.C. Nosocomial Cutaneous Zygomycosis in a Patient with Diabetic Ketoacidosis. Int. J. Infect. Dis. 2009, 13, e508–e510. [Google Scholar] [CrossRef] [Green Version]
- Liang, K.P.; Tleyjeh, I.M.; Wilson, W.R.; Roberts, G.D.; Temesgen, Z. Rhino-Orbitocerebral Mucormycosis Caused by Apophysomyces elegans. J. Clin. Microbiol. 2006, 44, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, M.; Kaundal, P.; Sharma, S.; Rana, K. Mucormycosis in the Urinary Bladder—The Devil Is in the Details. Indian J. Surg. 2022, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Diwakar, A.; Dewan, R.K.; Chowdhary, A.; Randhawa, H.S.; Khanna, G.; Gaur, S.N. Zygomycosis—A Case Report and Overview of the Disease in India. Mycoses 2007, 50, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Caputo, G.M.; Weitekamp, M.R.; Karchmer, A.W. Infections in Patients with Diabetes Mellitus. N. Engl. J. Med. 1999, 341, 1906–1912. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Yang, G.-Y.; Loh, C.-H.; Liou, S.-H.; Su, W.-L.; Lin, S.-H.; Chou, C.-C. Cost Benefits of Targeting the Pneumococcal Vaccination Program to the Elderly Population in Taiwan. Am. J. Infect. Control 2006, 34, 597–599. [Google Scholar] [CrossRef]
- American Diabetes Association. Influenza and Pneumococcal Immunization in Diabetes. Diabetes Care 2004, 27, s111–s113. [Google Scholar] [CrossRef] [Green Version]
- Davis, T.M.E.; Kauhanen, J.; Davis, W.A. Pneumococcal Vaccination and Incident Hospitalisation for Pneumonia in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. Intern. Med. J. 2017, 47, 1206–1210. [Google Scholar] [CrossRef]
- Liu, M.; Spellberg, B.; Phan, Q.T.; Fu, Y.; Fu, Y.; Lee, A.S.; Edwards, J.E.; Filler, S.G.; Ibrahim, A.S. The Endothelial Cell Receptor GRP78 Is Required for Mucormycosis Pathogenesis in Diabetic Mice. J. Clin. Investig. 2010, 120, 1914–1924. [Google Scholar] [CrossRef] [Green Version]
- Rathod, M.; Patel, J.; Prajapati, M.; Oza, M. A Comprehensive Review on Mucormycosis. Int. j. pharm. res. appl. 2022, 7, 883–890. [Google Scholar] [CrossRef]
- Natesan, S.K.; Chandrasekar, P.H. Isavuconazole for the Treatment of Invasive Aspergillosis and Mucormycosis: Current Evidence, Safety, Efficacy, and Clinical Recommendations. Infect. Drug Resist. 2016, 9, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Brunet, K. Optimisation du Traitement Préventif et Curatif de la Mucormycose Pulmonaire. Ph.D. Thesis, Université de Poitiers, Poitiers, France, 2020. [Google Scholar]
- Daniels, C.C.; Rogers, P.D.; Shelton, C.M. A Review of Pneumococcal Vaccines: Current Polysaccharide Vaccine Recommendations and Future Protein Antigens. J. Pediatr. Pharmacol. Ther. 2016, 21, 27–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.-H.; Chen, C.-C.; Ho, C.-W.; Hsieh, M.-C.; Hsu, S.-P.; Lin, C.-L.; Kao, C.-H. Dipeptidyl Peptidase-4 Inhibitor Treatment Could Decrease Klebsiella pneumoniae Pneumonia in Patients with Type 2 Diabetes. Postgrad. Med. 2020, 132, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Chen, H.-H.; Lai, C.-C.; Lin, C.-L.; Kao, C.-H. Dipeptidyl Peptidase-4 Inhibitor Treatment Could Decrease Chronic Rhinosinusitis in Diabetic Patients. QJM Int. J. Med. 2020, 113, 181–185. [Google Scholar] [CrossRef]
- Chamilos, G.; Marom, E.M.; Lewis, R.E.; Lionakis, M.S.; Kontoyiannis, D.P. Predictors of Pulmonary Zygomycosis versus Invasive Pulmonary Aspergillosis in Patients with Cancer. Clin. Infect. Dis. 2005, 41, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skiada, A.; Lass-Floerl, C.; Klimko, N.; Ibrahim, A.; Roilides, E.; Petrikkos, G. Challenges in the Diagnosis and Treatment of Mucormycosis. Med. Mycol. 2018, 56, S93–S101. [Google Scholar] [CrossRef] [Green Version]
- Galletti, B.; Freni, F.; Meduri, A.; Oliverio, G.W.; Signorino, G.A.; Perroni, P.; Galletti, C.; Aragona, P.; Galletti, F. Rhino-Orbito-Cerebral Mucormycosis in Diabetic Disease Mucormycosis in Diabetic Disease. J. Craniofacial Surg. 2020, 31, e321. [Google Scholar] [CrossRef]
- Simmons, J.H.; Zeitler, P.S.; Fenton, L.Z.; Abzug, M.J.; Fiallo-Scharer, R.V.; Klingensmith, G.J. Rhinocerebral Mucormycosis Complicated by Internal Carotid Artery Thrombosis in a Pediatric Patient with Type 1 Diabetes Mellitus: A Case Report and Review of the Literature. Pediatr. Diabetes 2005, 6, 234–238. [Google Scholar] [CrossRef]
- Artis, W.M.; Fountain, J.A.; Delcher, H.K.; Jones, H.E. A Mechanism of Susceptibility to Mucormycosis in Diabetic Ketoacidosis: Transferrin and Iron Availability. Diabetes 1982, 31, 1109–1114. [Google Scholar] [CrossRef]
- Ibrahim, A.S.; Spellberg, B.; Edwards, J. Iron Acquisition: A Novel Perspective on Mucormycosis Pathogenesis and Treatment. Curr. Opin. Infect. Dis. 2008, 21, 620–625. [Google Scholar] [CrossRef] [Green Version]
- Skiada, A.; Lanternier, F.; Groll, A.H.; Pagano, L.; Zimmerli, S.; Herbrecht, R.; Lortholary, O.; Petrikkos, G.L. Diagnosis and Treatment of Mucormycosis in Patients with Hematological Malignancies: Guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica 2013, 98, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.J.; Schell, W.A.; Covel, J.; Duboc, G.; Giamberardino, C.; Kapoor, M.; Moloney, M.; Soltow, Q.A.; Tenor, J.L.; Toffaletti, D.L.; et al. In Vitro and In Vivo Evaluation of APX001A/APX001 and Other Gwt1 Inhibitors against Cryptococcus. Antimicrob. Agents Chemother. 2018, 62, e00523-18. [Google Scholar] [CrossRef] [Green Version]
- Alkhazraji, S.; Gebremariam, T.; Alqarihi, A.; Gu, Y.; Mamouei, Z.; Singh, S.; Wiederhold, N.P.; Shaw, K.J.; Ibrahim, A.S. Fosmanogepix (APX001) Is Effective in the Treatment of Immunocompromised Mice Infected with Invasive Pulmonary Scedosporiosis or Disseminated Fusariosis. Antimicrob. Agents Chemother. 2020, 64, e01735-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colley, T.; Sehra, G.; Chowdhary, A.; Alanio, A.; Kelly, S.L.; Kizawa, Y.; Armstrong-James, D.; Fisher, M.C.; Warrilow, A.G.S.; Parker, J.E.; et al. In Vitro and In Vivo Efficacy of a Novel and Long-Acting Fungicidal Azole, PC1244, on Aspergillus fumigatus Infection. Antimicrob. Agents Chemother. 2018, 62, e01941-17. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.S.; Edwards, J.E., Jr.; Fu, Y.; Spellberg, B. Deferiprone Iron Chelation as a Novel Therapy for Experimental Mucormycosis. J. Antimicrob. Chemother. 2006, 58, 1070–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, A.S.; Gebermariam, T.; Fu, Y.; Lin, L.; Husseiny, M.I.; French, S.W.; Schwartz, J.; Skory, C.D.; Edwards, J.E.; Spellberg, B.J. The Iron Chelator Deferasirox Protects Mice from Mucormycosis through Iron Starvation. J. Clin. Investig. 2007, 117, 2649–2657. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Zarazúa, O.; Vélez-Ramírez, L.N.; Alcocer-León, M.; Utrilla-Álvarez, J.D.; Martínez-Rivera, M.A.; Flores-Saldaña, G.A.; Mondragón, J.D. A Case of Concomitant Pulmonary Tuberculosis and Mucormycosis in an Insulin-Dependent Diabetic Patient. J. Clin. Tuberc. Mycobact. Dis. 2019, 16, 100105. [Google Scholar] [CrossRef]
- Chaulk, A.L.; Do, T.H.; Supsupin, E.P.; Bhattacharjee, M.B.; Richani, K.; Adesina, O.-O.O. A Unique Radiologic Case of Optic Nerve Infarction in a Patient with Mucormycosis. J. Neuroophthalmol. 2021, 41, e354. [Google Scholar] [CrossRef]
- Lau, C.-I.; Wang, H.-C.; Yeh, H.-L.; Li, C.-H. Isolated Orbito-Cerebral Mucormycosis. Neurologist 2011, 17, 151. [Google Scholar] [CrossRef]
- Zafar, S.; Prabhu, A. Rhino-Orbito-Cerebral Mucormycosis: Recovery against the Odds. Pract. Neurol. 2017, 17, 485–488. [Google Scholar] [CrossRef]
- Rangel-Guerra, R.A.; Martínez, H.R.; Sáenz, C.; Bosques-Padilla, F.; Estrada-Bellmann, I. Rhinocerebral and Systemic Mucormycosis. Clinical Experience with 36 Cases. J. Neurol. Sci. 1996, 143, 19–30. [Google Scholar] [CrossRef]
- De, L.; Feitoza, M.; Altemani, A.; da Silva Junior, N.; Reis, F. Teaching NeuroImages: Mucormycosis-Associated Vasculitis A New Sequence to Show an Old Invasive Infection. Neurology 2019, 92, e1796–e1797. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.T.; Leavitt, J.A.; Moore, E.J.; Noseworthy, J.H. MRI Findings of Rapidly Progressive Ophthalmoplegia and Blindness in Mucormycosis. Neurology 2006, 66, E40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karakurum, B.; Karatas, M.; Cagici, A.C.; Uncu, H.; Yildirim, T.; Hurcan, C.; Karaca, S.; Kizilkilic, E.; Tan, M. Mucormycosis Presenting with Painful Ophthalmoplegia. Acta Neurol. Belg. 2005, 105, 201–205. [Google Scholar]
- Salinas-Lara, C.; Rembao-Bojórquez, D.; de la Cruz, E.; Márquez, C.; Portocarrero, L.; Tena-Suck, M.L. Pituitary Apoplexy Due to Mucormycosis Infection in a Patient with an ACTH Producing Pulmonary Tumor. J. Clin. Neurosci. 2008, 15, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Stemler, J.; Hamed, K.; Salmanton-García, J.; Rezaei-Matehkolaei, A.; Gräfe, S.K.; Sal, E.; Zarrouk, M.; Seidel, D.; Abdelaziz Khedr, R.; Ben-Ami, R.; et al. Mucormycosis in the Middle East and North Africa: Analysis of the FungiScope® Registry and Cases from the Literature. Mycoses 2020, 63, 1060–1068. [Google Scholar] [CrossRef]
- Macdonell, R.A.; Donnan, G.A.; Kalnins, R.M.; Richards, M.J.; Bladin, P.F. Otocerebral Mucormycosis—A Case Report. Clin. Exp. Neurol. 1987, 23, 225–232. [Google Scholar] [PubMed]
- Yang, H.; Wang, C. Looks like Tuberculous Meningitis, But Not: A Case of Rhinocerebral Mucormycosis with Garcin Syndrome. Front. Neurol. 2016, 7, 181. [Google Scholar] [CrossRef] [Green Version]
- Sarvestani, A.S.; Pishdad, G.; Bolandparvaz, S. Predisposing Factors for Mucormycosis in Patients with Diabetes Mellitus; An Experience of 21 Years in Southern Iran. Bull. Emerg. Trauma 2013, 1, 164–170. [Google Scholar]
- Sasannejad, P.; Ghabeli-Juibary, A.; Aminzadeh, S.; Olfati, N. Cerebellar Infarction and Aneurysmal Subarachnoid Hemorrhage: An Unusual Presentation and Rare Complications of Rhinocerebral Mucormycosis. Iran. J. Neurol. 2015, 14, 222–224. [Google Scholar]
- Dolatabadi, S.; Ahmadi, B.; Rezaei-Matehkolaei, A.; Zarrinfar, H.; Skiada, A.; Mirhendi, H.; Nashibi, R.; Niknejad, F.; Nazeri, M.; Rafiei, A.; et al. Mucormycosis in Iran: A Six-Year Retrospective Experience. J. Mycol. Médicale 2018, 28, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Abdolalizadeh, P.; Kashkouli, M.B.; Khademi, B.; Karimi, N.; Hamami, P.; Es’haghi, A. Diabetic versus Non-Diabetic Rhino-Orbito-Cerebral Mucormycosis. Mycoses 2020, 63, 573–578. [Google Scholar] [CrossRef]
- Patel, A.; Kaur, H.; Xess, I.; Michael, J.S.; Savio, J.; Rudramurthy, S.; Singh, R.; Shastri, P.; Umabala, P.; Sardana, R.; et al. A Multicentre Observational Study on the Epidemiology, Risk Factors, Management and Outcomes of Mucormycosis in India. Clin. Microbiol. Infect. 2020, 26, 944.e9–944.e15. [Google Scholar] [CrossRef] [PubMed]
- Mahalaxmi, I.; Jayaramayya, K.; Venkatesan, D.; Subramaniam, M.D.; Renu, K.; Vijayakumar, P.; Narayanasamy, A.; Gopalakrishnan, A.V.; Kumar, N.S.; Sivaprakash, P.; et al. Mucormycosis: An Opportunistic Pathogen during COVID-19. Environ. Res. 2021, 201, 111643. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R.; Lewis, P.; Shimabukuro, T.T.; Su, J.; Moro, P.; Woo, E.J.; Jankosky, C.; Cano, M. Post-Licensure Safety Surveillance of Zoster Vaccine Live (Zostavax®) in the United States, Vaccine Adverse Event Reporting System (VAERS), 2006–2015. Hum. Vaccines Immunother. 2018, 14, 1963–1969. [Google Scholar] [CrossRef] [Green Version]
- Hata, A.; Inoue, F.; Yamasaki, M.; Fujikawa, J.; Kawasaki, Y.; Hamamoto, Y.; Honjo, S.; Moriishi, E.; Mori, Y.; Koshiyama, H. Safety, Humoral and Cell-Mediated Immune Responses to Herpes Zoster Vaccine in Subjects with Diabetes Mellitus. J. Infect. 2013, 67, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of Gut Microbiota in Type 2 Diabetes Pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, A.; Roy, V.; Priyadarshini, I. A Mini Review: Mucormycosis in Coronavirus Disease-19, Host-Iron Assimilation, and Probiotics as Novel Therapy. J. Pharmacol. Pharmacother. 2021, 12, 120–124. [Google Scholar] [CrossRef]
- Samson, R.; Dharne, M. COVID-19 Associated Mucormycosis: Evolving Technologies for Early and Rapid Diagnosis. 3 Biotech 2021, 12, 6. [Google Scholar] [CrossRef]

| Condition | Causative Organism | Patient/Number of Patients | Symptoms | Country | Diagnosis | Treatment | References |
|---|---|---|---|---|---|---|---|
| Renal Aspergillosis | Aspergillus fumigatus | Forty-five-year-old diabetic male | Mild pain in the left lumbar region; irregular, low-grade fever; and occasional dysuria for 3 months. | Malaysia | Ultrasound scan and intravenous urogram (IVU) revealed a cystic lesion (4 × 3.9 cm) at the lower pole cortical region of the left kidney. | Amphotericin B for 2 weeks at 1.25 mg/kg/day, which was replaced by oral itraconazole (200 mg twice daily for two months and 100 mg twice daily for one month) as the person could not tolerate Amphotericin B. | [58] |
| Pulmonary aspergillosis | Aspergillus | (a) Forty-six-year-old diabetic female with hypertension (b) Forty-five-year-old diabetic male smoker | wheezing, chronic productive cough, and dyspnoea. Intermittent fever for a month, dyspnoea, dry cough, and weight loss. | Iran | Elevated ESR [erythrocyte sedimentation rate] (126 mm/1st h) and fasting blood sugar: 229 mg/dl; the galactomannan level in serum was 1.7. Chest X-ray showed cavitary formation in the right upper lobe, spiral CT-scan of thorax with revealed large cavitation in anterior segment of right upper lobe, trans-bronchial lung biopsy. Galactomannan serum level was 1.8. Elevated ESR (66 mm/1st h), white blood cells:18,700/mm3, Neut.: 88%, and blood sugar: 380 mg/dl. Cavitation in upper lobe nodules was revealed on chest X-ray and CT-scan of thorax. | Itraconazole Itraconazole | [159] |
| Pulmonary aspergillosis | Aspergillus versicolor and Aspergillus ochraceus | Twenty-nine-year-old Type 1 diabetic male with marijuana use. | Chest pain for a week, weight loss, dyspnoea, fever, and night sweats for a year, | Canada | A radiograph of the chest showed pneumothorax and air space disease in the left lower lobe. Computed tomography of the chest showed consolidation and cavitation in the left lower lobe, annexing the pleura, pneumothorax, a chest tube and subcutaneous emphysema. | Surgery and six-months course of voriconazole. | [60] |
| Pulmonary aspergillosis and mucormycosis | Aspergillus fumigatus, Rhizopus arrhizus | Seventy-nine-year-old diabetic, Latino male with hypertension and COVID-19 | Fever, rigors, dry cough, and dyspnoea for 10 days. | USA | Nasopharyngeal swab PCR (Polymerase Chain Reaction) test was positive for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Chest radiograph (CXR) revealed patchy bibasilar infiltrates. Computed tomography (CT) revealed moderate, bilateral, ground-glass opacities and infiltrates. On day 13, a broncho-alveolar lavage showed thick respiratory secretions. On day 19, the chest CT was repeated. | Five days of treatment with ceftriaxone, azithromycin, and remdesivir. A 7 day course of IV (intravenous) vancomycin 1250 mg every 8 h and IV ceftriaxone 1 g daily for treating ventilator-associated pneumonia. On day 14, 200 mg of IV voriconazole was administered twice daily for treating aspergillosis. From the 19th, day liposomal Amphotericin B was started for treating mucormycosis. On day 23, a tracheostomy was performed, and on day 25 was a percutaneous endoscopic gastrostomy. | [160] |
| Pulmonary aspergillosis | Aspergillus fumigatus | Forty-four-year-old diabetic male | Fever, haemoptysis, cough, and dyspnoea for 3 weeks. | India | Sputum analysis for fungus, chest X-ray, and contrast-enhanced computed tomography were performed, which showed multifocal areas of cavitation involving bilateral upper and lower lobes of the lung. Patient underwent flexible bronchoscopy and subsequent biopsy. | IV voriconazole, 6 mg/kg b.d., followed by 4 mg/kg b.d. for 2 weeks. | [161] |
| Pulmonary aspergillosis | Aspergillus fumigatus | Forty-five-year-old diabetic male with ketoacidosis and hypertension. | Flu-like illness with pyrexia. | UK | Chest radiography showed patchy consolidation and volume loss of the left lower zone. Bronchoscopy showed thick, white plaques over the left main bronchus, extending into left upper and lower lobe bronchi. | Initially started with intravenous cefotaxime, flucloxacillin, and metronidazole. From day, 5 piperacillin, gentamicin and metronidazole were administered. Intravenous fluconazole was given for candida albicans infection. From day 7, oral itraconazole, 200 ug b.d., and an intravenous amphotericin B colloidal dispersion (2 mg/kg bw increased to 4 mg/kg bw) were treated. Itraconazole was given for six months. | [162] |
| Pulmonary aspergillosis | Aspergillus | Fifty-three-year-old diabetic female with keto-acidosis. | Fever, vomiting, and confusion. | Japan | A chest radiograph demonstrated infiltrates in the middle and lower zones of both lungs. A chest CT scan showed nodules with the halo sign and focal ground glass opacity and cavitation. Bronchoscopy showed thick, white plaques. | Intravenous ampicillin sodium/sulbactam and peramivir were initiated for her pneumonia. This was shifted to L-amphotericin B (100 mg/body per day for a month). | [163] |
| Pulmonary aspergillosis/aspergilloma | Aspergillus sp. | Forty-five-year-old diabetic female | Chest pain, cough, decreased appetite, weight loss, and chronic haemoptysis. | Indonesia | X-ray and CT scan showed a mass in the upper lobe of left lung. Fine needle aspiration biopsy showed Aspergillus sp. surgery. | Initial treatment included the administration of codeine (3 × 10 mg), tranexamic acid (3 × 500 mg iv), novorapid (3 × 10 units), evemir (12 units, night), and Fluconazole (1 × 400 mg for 1 day, followed by 200 mg). Surgery was performed using pulmonary wedge resection | [164] |
| Rhino-oculo-cerebral aspergillus and mucor co-infection | Rhizopus & Aspergillus flavus | Forty-six-year-old diabetic male with hypertension and epistaxis in his past | proptosis of the left eye, ptosis, and diminution of vision for a month. Confused state | India | The radiological examination revealed opacification. Squamous cell carcinoma revealed on histopathology. X-ray and CT scan were performed for diagnosis. Sinoscopy confirmed a severely inflamed maxillary sinus. Fifteen days after the diagnosis of aspergillosis, histopathological studies revealed septate hyphae showing mucor infection. | Voriconazole treatment for seven days. After 15 days, Amphotericin B was given, but the patient expired due to intolerance. | [165] |
| Condition | Causative Organism | Patient/Number of Patients | Symptoms | Country | Diagnosis | Treatment | References |
|---|---|---|---|---|---|---|---|
| Gastrointestinal | Zygomycosis | Thirty-six-year-old diabetic female | Epigastric-pain, bilious vomiting, weight loss, fever, and constipation. | Iran | Endoscopy disclosed an extensive sub-mucosal haemorrhage. A biopsy demonstrated broad, aseptate fungal elements, a laparotomy showed rubbery, grey/brown necrotic tissue | Total gastrectomy with Roux-en-Y esophago-jejunostomy and surgical debridement, Amphotericin B lipid complex (1 mg/kg/dose). | [167] |
| Cutaneous | Rhizopus arrhizus | Twenty-six-year-old diabetic female with ketoacidosis. | Severe lower abdominal pain and intense thirst. | India | Biopsy specimen showed non-septate hyphae with right angle branching. | Local surgical debridement and Amphotericin B (4 mg/kg/day). | [168] |
| Cutaneous and rhino-orbitocerebral | Apophysomyces elegans | Fifty-year-old diabetic male | Facial pain and diplopia, right eye proptosis. | USA | Physical examination, CT, and MRI of the head showed right eye proptosis with inflammatory changes. Histopathology showed aseptate hyphae. | Liposomal Amphotericin B and multiple debridements. | [169] |
| Urinary bladder | Mucorales | Fifty-five-year-old diabetic male | Fever, dysuria, obstructive urinary symptoms, deranged creatinine, and flank pain. | India | Non-contrast CT scan showed right mild hydronephrosis. Cystoscopy showed yellowish-white material in urinary bladder. Histopathology studies revealed the fungal attack. | Posaconazole | [170] |
| Pulmonary | Rhizopus oryzae | Sixty-one-year-old diabetic male | Productive cough, fever, anorexia, and weakness. | India | A thick-walled cavity in the hilar region was seen from the chest X-ray. CECT (contrast-enhanced computed tomography) of the chest revealed a cavitating lesion. Histopathological studies showed fungal hyphae. | Amphotericin B for a cumulative dose of 3 g. | [171] |
| Age at First Dose (Months) | Primary Dose | Booster Dose | Reference | |
|---|---|---|---|---|
| Pn7 | Pn7 | PPV23 | ||
| 2–6 months | 3 doses (2/4/6 months) | 12–15 months of age | After 2 years of age. | [176,177,178,179] |
| 7–11 months | 2 doses (0/2 months) | 12–15 months of age | First dose: At least 6–8 weeks after the last dose of pnc7. | |
| 12–23 months | 2 doses (0/2 months) | None | Second dose: 5 years after the pn23 dose. | |
| ≥24 months | 2 doses (0/2 months) | None | - | [89,176,177,178,179] |
| 5–64 years | None | None | None | [89,180] |
| >64 years | None | None | Second dose if the vaccine was administered >5 years ago. | |
| Underlying Diseases | Organ/Region Infected with Fungus | Objective | Patient/Number of Patients | Country | Methodology | Results and Conclusion | Reference | |
|---|---|---|---|---|---|---|---|---|
| Diabetes | Lungs | Pulmonary mucormycosis and tuberculosis | A diabetic case with fungal co-infection. | A fifty-six-year-old female | Netherlands | X-ray, CT scan, RTPCR, and lobectomy. | Treatment with TB and mycosis medicines resulted in little side effects. Patients with diabetes should undergo testing for certain co-infections | [195] |
| Diabetes mellitus | Eye | Mucormycosis | Infarction of the optic nerve caused by mucormycosis in a diabetic patient. | A fifty-one-year-old male | USA | MRI, exenteration and sinus debridement. | Extensive infarction of the left optic nerve with inflammation of the ipsilateral and periorbital adnexa. Histopathology demonstrated the presence of mucormycosis. | [196] |
| Renal failure and diabetes mellitus | Eye | Mucormycosis | ROCM detected in an ocular nerve infection case. | A thirty-four-year-old man. | Taiwan | Ophthalmic and neurological examination, CSF (cerebrospinal fluid) examination, and MRI. | There were black eschars going from the bilateral canthi to the vascular area. It extended to cerebral and bilateral ophthalmic nerves. People with immunocompromised patients might consider ROCM if they have neuro-ophthalmological symptoms. | [197] |
| Diabetic ketoacidosis with ophthalmoplegia | Nostril region | Mucormycosis | An instance of recovery from mucormycosis infection. | A twenty-two-year-old women | USA | CT scan, nasoendoscopy, and biopsy. | Surgical excision of the right eye, paranasal sinuses, maxilla, and palate, suboccipital craniectomy, and shunt for hydrocephalus, followed by an 18 month course of antifungal medication. The chance of infection was increased with several surgical procedures. | [198] |
| Diabetes, kidney failure, myelodysplastic syndrome, and acute leukaemia | Cerebral region | Mucormycosis | Retrospective study of 36 cases with Mucormycosis | Thirty-six cases | Mexico | Surgical debridement, CT scan, and MRI. | Systemic and rhino-cerebral mucormycosis. The report suggested medicinal and surgical treatment. | [199] |
| HIV infection and diabetes | Cerebral region | Mucormycosis | Mucomycorsis with vasculitis in a diabetic case. | A fifty-four-year-old woman | Brazil | CSF analysis, CT scan, histopathologic analysis, and angiography with HR-VWI (high-resolution vessel wall imaging). | Vasculitis accompanied with inflammation. More research is necessary to evaluate the accuracy of mucormycosis tests. | [200] |
| Diabetes mellitus | Cerebral region | Mucormycorsis | Progressive ophthalmoplegia and blindness in infection. | Eighteen-year-old woman | USA | Surgical debridements, MRI, lumbar puncture, funduscopic examination, and surgical debridement. | Observation of fungal hyphae in the ophthalmic artery and optic nerve perineurals in the absence of substantial optic nerve inflammation. Diabetic individuals with ophthalmoplegia and blindness should be evaluated for infection. | [201] |
| Diabetes mellitus (three patients) and chronic leukaemia (one patient) | Cerebral region | mucormycosis | Examining fungal infections in four patients with underlying illnesses. | Four cases | Turkey | CT scan Otorhinolaryngologic examination | There were neurological abnormalities detected. Two patients had passed away. Investigating mucormycosis in ophthalmoplegia and ensuring quick diagnosis should be considered. | [202] |
| Diabetes mellitus with Cushing’s syndrome | Cerebral region | Mucormycosis | Cushing’s syndrome and solid tumours are associated with infection. | Forty-two-year-old woman | Mexico | CT scan Autopsy | Infarction of the left temporal lobe. The cause of the patient’s death was determined to be a multihormonal pituitary adenoma with expansion to the sphenoid bone and sellar erosion. ACTH (ectopic adrenocorticotropic hormone) was detected in the left lung. The research established a correlation between ACTH and ectopic pulmonary tumours, pituitary apoplexy, and mucormycosis. | [203] |
| Diabetes mellitus and immunosuppression conditions | Cerebral region | Mucormycosis | Reginal differences in the infection and its causes. | - | Middle East and North Africa | Data collection | A total of 310 instances of infection. Most cases were associated with diabetes and immunosuppression. It is necessary to put into practice efficient treatment and preventive measures. | [204] |
| Diabetes | Cerebral region | Mucormycosis | A case of diabetes infected with mucormycosis. | Elder man | Canada | CT scan Autopsy | Thrombosis with infection in cerebral region. Early diagnosis is the key to effective therapy. | [205] |
| Diabetes mellitus with Garcin syndrome | Cerebral region | Mucormycosis | Analysis of infection and tuberculosis meningitis in a case with underlying disease. | - | China | CT scan with X-ray | Tuberculosis meningitis developed to mucormycosis. Diagnostics should be first in identifying the infection. | [206] |
| Diabetes mellitus | Cerebral region | Mucormycosis | To determine the prevalence and risk factors of mucormycosis in individuals with diabetes mellitus. | Total of 162 patients | Iran | Detailed history, otorhinolaryngologic, ophthalmic, and neurologic examinations | A total of 30 individuals had diabetes (19 were women and 11 were men). Diabetes might be a risk factor for fungal infections. | [207] |
| Diabetes mellitus | Cerebral region | Mucormycosis | Identification of infection in diabetes patient with complication to acute infarction | Fifty-seven-year-old man | Iran | CT scan Biopsy | Subarachnoid haemorrhage associated with a stroke. The biopsy revealed a mucormycosis infection. Early actions are required to prevent severe consequences. | [208] |
| Diabetes mellitus | Sinus region | Rhizopusaarhisus | To estimate the distribution of infection and its associated factors. | A total of 208 cases | Iran | Sequencing and data collection. | From 2008 to 2014, there was an increase in infections. It is crucial to monitor and identify this infection. | [209] |
| Diabetes and non-diabetic patients | Rhino-orbito- cerebral | Mucorales | To compare fungal infections in people with and without diabetes. | Total of 63 patients | Iran | Ophthalmic investigation, imaging studies, and biopsy. | Survival was recorded in 51% of diabetic patients and 70% of non-diabetic patients. Neither group’s rate of vision survival differed from the other. | [210] |
| Diabetes mellitus, Malignancy, transplant | Rhino-orbital | Rhizopus | Mucormycosis was the subject of a prospective observational research that was carried out across 12 locations in India. | Total of 465 patients | India | Questionnaire analysis | Symptoms with a shorter duration. The shorter duration of antifungal medication and the use of Amphotericin B were independent risk factors for death. Diabetes was the primary risk factor. | [211] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, A.; Hithamani, G.; Naveen, J.; Pradeep, S.R.; Barman, S.; Srinivasan, K. Management of Invasive Infections in Diabetes Mellitus: A Comprehensive Review. Biologics 2023, 3, 40-71. https://doi.org/10.3390/biologics3010004
Khanam A, Hithamani G, Naveen J, Pradeep SR, Barman S, Srinivasan K. Management of Invasive Infections in Diabetes Mellitus: A Comprehensive Review. Biologics. 2023; 3(1):40-71. https://doi.org/10.3390/biologics3010004
Chicago/Turabian StyleKhanam, Anjum, Gavirangappa Hithamani, Jayapala Naveen, Seetur R. Pradeep, Susmita Barman, and Krishnapura Srinivasan. 2023. "Management of Invasive Infections in Diabetes Mellitus: A Comprehensive Review" Biologics 3, no. 1: 40-71. https://doi.org/10.3390/biologics3010004