Journal Description
Biologics
Biologics
is an international, peer-reviewed, open access journal on all areas of biologics derived from both novel and established biotechnologies, published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 27.7 days after submission; acceptance to publication is undertaken in 7.7 days (median values for papers published in this journal in the second half of 2023).
- Recognition of Reviewers: APC discount vouchers, optional signed peer review, and reviewer names published annually in the journal.
Latest Articles
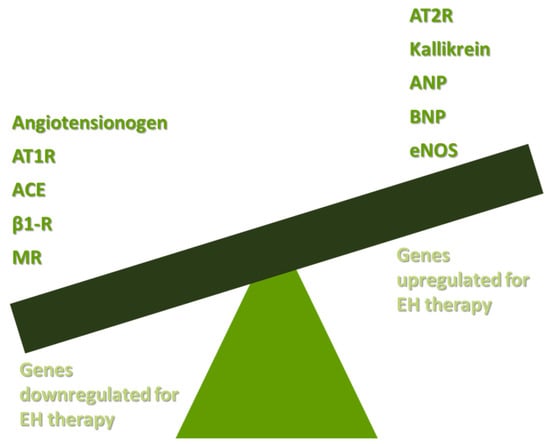
Gene Therapy for Hypertension, Atherosclerosis, and Familial Hypercholesterolemia: The Old Concepts and the New Era
Biologics 2024, 4(2), 143-160; https://doi.org/10.3390/biologics4020010 - 19 Apr 2024
Abstract
►
Show Figures
Cardiovascular disease remains the main cause of mortality in the 21st century. Hypertension, vessel atherosclerosis, and familial hypercholesterolemia (FH) are responsible for increased mortality and morbidity in patients. Therapies for cardiovascular disease are based on drug treatment options, but in the era of
[...] Read more.
Cardiovascular disease remains the main cause of mortality in the 21st century. Hypertension, vessel atherosclerosis, and familial hypercholesterolemia (FH) are responsible for increased mortality and morbidity in patients. Therapies for cardiovascular disease are based on drug treatment options, but in the era of precision medicine, personalized treatments are being developed. Studies have shown that these conditions have a strong genetic background, creating an opportunity for the implementation of gene therapy for these diseases. Currently, gene therapy is not widely used in clinical practice. Recent advances in this research field are making gene therapy a very promising preventive and therapeutic tool for cardiovascular disease. Essential hypertension’s (EH) pathophysiology is mostly based on the activation of both the sympathetic nervous system and the renin angiotensin aldosterone system (RAAS), natriuretic peptide production, and endothelial dysfunction. Plasmid DNA and viral vectors can be used, targeting the main mechanisms in the pathogenesis of EH. Many preclinical studies have been developed across the years, presenting a significant decrease in blood pressure. Nevertheless, no clinical studies have been developed studying the implementation of gene therapy in EH. Atherosclerotic damage is caused by monogenic diseases or is deteriorated by the activation of inflammation in the vessel wall. Gene therapy studies have been developed in the pre- and clinical phases targeting the lipoprotein and cholesterol metabolism and the inflammation of the vessels. FH is a common inherited metabolic disease associated with high levels of cholesterol in the blood. Clinical trials of gene therapy have been developed and presented optimistic results. In this review, the challenges of gene therapy for cardiovascular disease are outlined. Nevertheless, more clinical trials are needed to be performed for the development of convenient and safe drug schemes for our patients.
Full article
Open AccessReview
Tick-Borne Diseases—Still a Challenge: A Review
by
Radina Andonova, Dzhaner Bashchobanov, Veronika Gadzhovska and Georgi Popov
Biologics 2024, 4(2), 130-142; https://doi.org/10.3390/biologics4020009 - 15 Apr 2024
Abstract
Tick-borne diseases account for a large proportion of vector-borne illnesses. They include, for example, a variety of infections caused by bacteria, spirochetes, viruses, rickettsiae, and protozoa. We aim to present a review that demonstrates the connection between the diagnosis, treatment, prevention, and the
[...] Read more.
Tick-borne diseases account for a large proportion of vector-borne illnesses. They include, for example, a variety of infections caused by bacteria, spirochetes, viruses, rickettsiae, and protozoa. We aim to present a review that demonstrates the connection between the diagnosis, treatment, prevention, and the significance of certain emergency tick-borne diseases in humans and their clinical–epidemiological features. This review covers three diseases: anaplasmosis, ehrlichiosis, and babesiosis. The emergence of ehrlichiosis and anaplasmosis is become more frequently diagnosed as the cause of human infections, as animal reservoirs and tick vectors have increased in numbers and humans have inhabited areas where reservoir and tick populations are high. They belong to the order Rickettsiales and the family Anaplasmataceae, and the clinical manifestations typically coexist. Furthermore, prompt diagnosis and appropriate treatment are critical to the patient’s recovery. Similar to malaria, babesiosis causes hemolysis. It is spread by intraerythrocytic protozoa, and the parasitemia dictates how severe it can get. Left untreated, some patients might have a fatal outcome. The correct diagnosis can be difficult sometimes; that is why an in-depth knowledge of the diseases is required. Prevention, prompt diagnosis, and treatment of these tick-borne diseases depend on the understanding of their clinical, epidemiological, and laboratory features.
Full article
Open AccessReview
Classification and Molecular Functions of Heparan Sulfate Proteoglycans and Their Molecular Mechanisms with the Receptor
by
Yasunari Matsuzaka and Ryu Yashiro
Biologics 2024, 4(2), 105-129; https://doi.org/10.3390/biologics4020008 - 28 Mar 2024
Abstract
►▼
Show Figures
Heparan sulfate proteoglycans are highly glycosylated proteins in which heparan sulfate, a glycosaminoglycan sugar chain, is an acidic sugar chain consisting of a repeating disaccharide structure of glucuronic acid and N-acetylglucosamine is locally sulfated. Syndecan, one of the transmembrane HSPGs, functions as a
[...] Read more.
Heparan sulfate proteoglycans are highly glycosylated proteins in which heparan sulfate, a glycosaminoglycan sugar chain, is an acidic sugar chain consisting of a repeating disaccharide structure of glucuronic acid and N-acetylglucosamine is locally sulfated. Syndecan, one of the transmembrane HSPGs, functions as a receptor that transmits signals from the extracellular microenvironment to the inside of the cell. In the vascular system, heparan sulfate proteoglycans, a major component of the glycocalyx, enable the binding of various plasma-derived molecules due to their diversity, epimerization of glycosaminoglycans chains, long chains, and sulfation. Heparan sulfate proteoglycans present in the extracellular matrix serve as a reservoir for bioactive molecules such as chemokines, cytokines, and growth factors. Aberrant expression of heparan sulfate proteoglycans, heparanase, and sulfatase is observed in many pathological conditions. Therefore, it can be applied to therapeutic strategies for a wide range of fields including Alzheimer’s disease, heart failure, cancer, organ transplants, diabetes, chronic inflammation, aging, and autoimmune diseases.
Full article
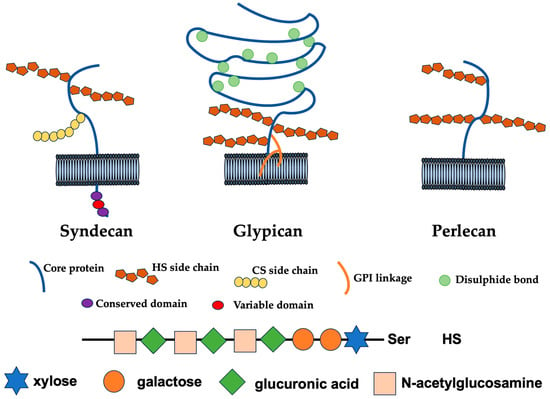
Figure 1
Open AccessReview
Extracellular Vesicles: Tiny Messengers for Mighty RNA Delivery
by
Alakesh Das
Biologics 2024, 4(1), 88-104; https://doi.org/10.3390/biologics4010007 - 06 Mar 2024
Abstract
Extracellular vesicles (EVs) encompass a diverse array of cell-derived vesicles, originating either from the endosomal compartment (exosomes) or generated through shedding from the cell membrane. These lipid bilayer nanovesicles carry a diverse cargo consisting of nucleic acids, various macromolecules, and growth factors, capable
[...] Read more.
Extracellular vesicles (EVs) encompass a diverse array of cell-derived vesicles, originating either from the endosomal compartment (exosomes) or generated through shedding from the cell membrane. These lipid bilayer nanovesicles carry a diverse cargo consisting of nucleic acids, various macromolecules, and growth factors, capable of being assimilated by nearby or distant cells through biofluids, thereby triggering a wide range of cellular responses. Given their distinctive biological characteristics and crucial roles in intercellular communication, EVs have garnered significant attention, especially concerning potential clinical applications. Inheriting cargo from their parent cells, EVs present promising resources for diverse disease biomarkers. Research elucidating the specific impacts of cargo on target cells has sparked enthusiasm for their therapeutic potential. Compelling evidence indicates that RNA cargo housed within EVs can modulate gene expression and influence cellular functions in recipient cells. However, despite significant progress, numerous aspects of EV biology remain obscure, encompassing selective cargo-loading mechanisms that yield distinct compositions from source cells, variability in size and content, and undisclosed pathways governing uptake and cargo fate in recipient cells. A thorough understanding of core EV mechanisms—such as generation, trafficking, and payload delivery—is essential for their effective clinical utilization. This review explores the current understanding of RNA loading and transportation within EVs, shedding light on the advancements made toward clinical applications.
Full article
(This article belongs to the Section Diagnostics)
►▼
Show Figures
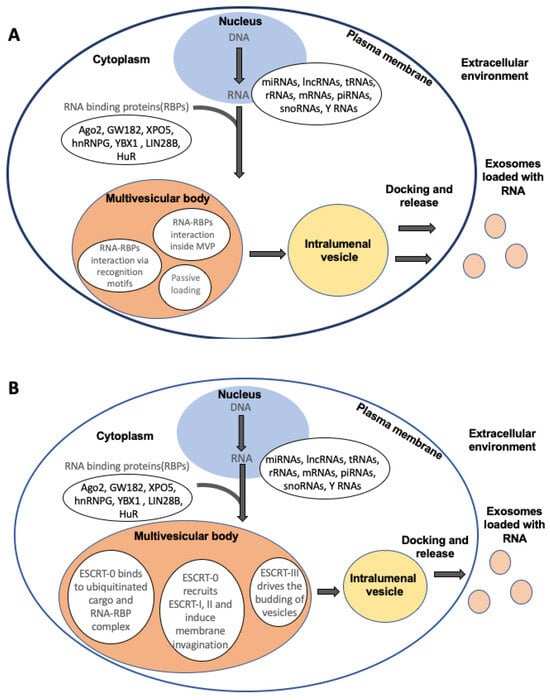
Figure 1
Open AccessArticle
A Molecular Modeling Study into Brønsted and Lewis Acid Catalyzed Conversion of CBD into Other Cannabinoids
by
Wim Buijs
Biologics 2024, 4(1), 75-87; https://doi.org/10.3390/biologics4010006 - 04 Mar 2024
Abstract
There is a continuous interest in cannabinoids like Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Previous experimental research has described the conversion of CBD to either Δ8-THC or Δ9-THC, depending on the acid catalyst applied. The use
[...] Read more.
There is a continuous interest in cannabinoids like Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD). Previous experimental research has described the conversion of CBD to either Δ8-THC or Δ9-THC, depending on the acid catalyst applied. The use of para-toluene sulfonic acid (pTSA) has led to the formation of Δ8-THC, while boron trifluoride etherate (BF3·Et2O) has mainly yielded Δ9-THC. The enormous difference in product selectivity between these two catalysts was investigated with Molecular Modeling, applying quantum chemical density functional theory. It was found that pTSA leads to fast isomerization of Δ9-CBD to Δ8-CBD and subsequent ring closure to Δ8-THC. BF3·Et2O catalysis leads to the formation of tertiary carbenium ions in the transition states, which yield Δ9-THC and some iso THC. Under dry conditions in refluxing toluene, it was found that pTSA is predominantly present as a dimer, and only a small fraction is available as monomeric catalyst. Applying the computationally derived activation barriers in transition state theory yielded reaction rates that predicted the amounts of cannabinoids that are in close agreement with the experimental findings from the previous literature.
Full article
(This article belongs to the Section Natural Products)
►▼
Show Figures
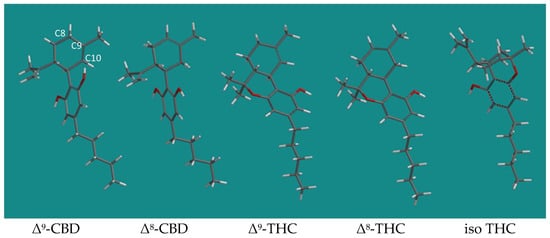
Figure 1
Open AccessReview
Monoclonal Antibody Development for Cancer Treatment Using the Phage Display Library Platform
by
Tiantian Zhang and Zhe Wang
Biologics 2024, 4(1), 55-74; https://doi.org/10.3390/biologics4010005 - 23 Feb 2024
Abstract
Thirty-four years ago, the groundbreaking work of John McCafferty and Sir Gregory Winter in developing phage display technology revolutionized the discovery of human antibodies, paving the way for diverse applications. Since then, numerous phage-derived antibodies have been successfully developed and advanced into clinical
[...] Read more.
Thirty-four years ago, the groundbreaking work of John McCafferty and Sir Gregory Winter in developing phage display technology revolutionized the discovery of human antibodies, paving the way for diverse applications. Since then, numerous phage-derived antibodies have been successfully developed and advanced into clinical studies, resulting in the approval of more than a dozen therapeutic antibodies. These antibodies have demonstrated efficacy across a spectrum of medical conditions, ranging from autoimmune diseases to various cancers. In this article, we provide an in-depth review of the development of phage display libraries as powerful platforms for therapeutic antibody discovery, elucidating the intricate procedures involved in antibody development. Additionally, we conduct a review of the current ntibody drugs for cancer treatment that have been developed using the phage display platform. Furthermore, we discuss the challenges inherent in this technology, offering insights into potential solutions to enhance crucial steps and facilitate more efficient drug discovery in the field of phage display technology.
Full article
(This article belongs to the Section Monoclonal Antibodies)
►▼
Show Figures
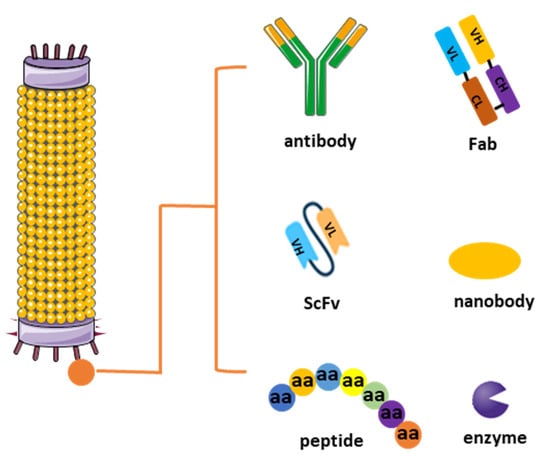
Figure 1
Open AccessArticle
Cytokines and Obstructive Sleep Apnea in Childhood: Study of a Group of Children
by
Luana Maria Nosetti, Claudio Tirelli, Franca Marino, Michela Gaiazzi, Lucia Sacchi, Mara De Amici, Fiorella Barocci, Ramona Maio, Marco Cosentino and Luigi Nespoli
Biologics 2024, 4(1), 44-54; https://doi.org/10.3390/biologics4010004 - 01 Feb 2024
Abstract
Introduction: Obstructive Sleep Apnea (OSA) in children is characterized by repeated episodes of partial or complete obstruction of the upper airways that impair normal ventilation and cause hypoxia and sleep disruption. These episodes activate innate and adaptive immunity resulting in the production of
[...] Read more.
Introduction: Obstructive Sleep Apnea (OSA) in children is characterized by repeated episodes of partial or complete obstruction of the upper airways that impair normal ventilation and cause hypoxia and sleep disruption. These episodes activate innate and adaptive immunity resulting in the production of proinflammatory cytokines: IL-1β, IL-6, TNF-α, and reactive oxygen species. The hypothalamic–pituitary–adrenal (HPT) axis is also activated with alteration of the circadian rhythm of cortisol synthesis. OSA in children, and even more in adults, induces a systemic inflammatory condition that contributes to the genesis of clinical complications: poor growth, learning disabilities, cardiovascular changes, insulin resistance, and metabolic syndrome. Methods: A total of 42 non-obese children (age 1–15 years) were enrolled among those sent to our sleep center to perform full polysomnography (PSG). After PSG, 6 children did not show OSA (controls), 20 had mild OSA (m OSA), and 16 had medium-severe OSA (MS OSA). In vitro IL-1β, TNF-α, and serum cortisol levels were measured at 2 and 8 am in the analyzed groups. Results: Cortisol levels did not differ between controls and OSA children. At 2 am, there were no differences between controls and OSA in TNF-α production, whereas at 8 am, TNF-α was reduced in MS-OSA. IL-1β production showed no differences between OSA and controls. Conclusions: In our population, only TNF-α production is suppressed in MS-OSA: this might indicate a role of OSA severity in inducing inflammation. In adults, the phenomenon is more pronounced due to the habitual greater severity/duration of OSA, presence of comorbidities (cardiovascular and metabolic), and different immune system function.
Full article
(This article belongs to the Section Cytokines and Allied Mediators)
►▼
Show Figures
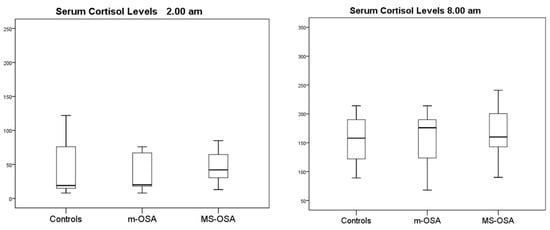
Figure 1
Open AccessArticle
Phytotherapy Used in Ailments of the Digestive System by Andean Inhabitants of Pampas, Huancavelica, Peru
by
Charles Frank Saldaña-Chafloque, Mercedes Acosta-Román, José Torres-Huamaní and José Luis Castillo-Zavala
Biologics 2024, 4(1), 30-43; https://doi.org/10.3390/biologics4010003 - 26 Jan 2024
Abstract
The use of medicinal plants for the therapy of diseases of the digestive system, where the Andean peoples developed various forms of administration. The objective is to identify medicinal plants used in the therapy of ailments of the digestive system by the Andean
[...] Read more.
The use of medicinal plants for the therapy of diseases of the digestive system, where the Andean peoples developed various forms of administration. The objective is to identify medicinal plants used in the therapy of ailments of the digestive system by the Andean inhabitants of Pampas, Tayacaja, Huancavelica, Peru. Methods: Non-probabilistic sampling, using the “snowball” technique, carrying out semi-structured surveys, allowing information to be collected on the prevalence of ailments or diseases of the digestive system treated with medicinal plants, with inhabitants over 20 years of age participating and using the medicinal plants in the therapy of your digestive system ailments, and exclude those inhabitants who do not comply with it. Results: A total of 16 families, 33 genera, and 34 species are reported, where the families that present the greatest abundance of species are Asteraceae and Lamiaceae. The widely used species are Minthostachys mollis (11.9%), Aloe vera (10.4%), Clinopodium bolivianum (9%), Artemisia absinthium (9%), and Matricaria chamomilla (8.2%). Concluding with the identification of a diversity of medicinal flora, used in the therapy of diseases of the digestive system, such as stomach pain, constipation, gallbladder ailments, gastritis, and gastrointestinal, and liver diseases.
Full article
(This article belongs to the Section Natural Products)
►▼
Show Figures
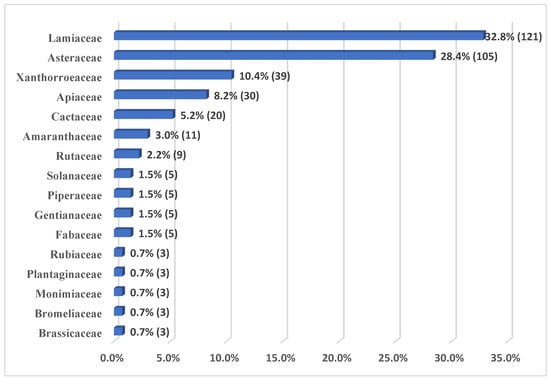
Figure 1
Open AccessReview
Respiratory Syncytial Virus Vaccines for the Prevention of Lower Respiratory Tract Infections in Patients Living with Chronic Obstructive Pulmonary Disease: A Rapid Review
by
Paul M. Boylan, Megan E. Fleischman, Nathan Pinner, Joseph Andrew Woods and Adam Welch
Biologics 2024, 4(1), 17-29; https://doi.org/10.3390/biologics4010002 - 25 Jan 2024
Abstract
Background: Patients living with chronic obstructive pulmonary disease (COPD) are at risk for lower respiratory tract infections caused by respiratory syncytial virus (RSV). The first RSV vaccines were approved in 2023 for adults ages 60 years and older. The safety and efficacy of
[...] Read more.
Background: Patients living with chronic obstructive pulmonary disease (COPD) are at risk for lower respiratory tract infections caused by respiratory syncytial virus (RSV). The first RSV vaccines were approved in 2023 for adults ages 60 years and older. The safety and efficacy of the RSV vaccines and their clinical implications in patients living with COPD, apart from composite comorbidity results, are under-reported. Methods: This rapid review aimed to collect and report data pertaining to RSV vaccine safety and efficacy in patients living with COPD. Resources searched included Ovid MEDLINE, EMBASE, International Pharmaceutical Abstracts, published peer-reviewed abstracts, ClinicalTrials.gov, and the United States Food and Drug Administration (FDA) website. Results: Seven records were included: five research manuscripts and two ongoing clinical trials. Patients living with COPD were included in RSV vaccine clinical trials, but outcomes of RSV vaccine safety and efficacy in patients living with COPD were grossly unreported. Conclusions: Future clinical trials of patients living with COPD and subgroup analyses of patients living with COPD within existing studies evaluating RSV vaccine safety and efficacy are necessary to substantiate outcomes in this population.
Full article
(This article belongs to the Section Vaccines)
►▼
Show Figures
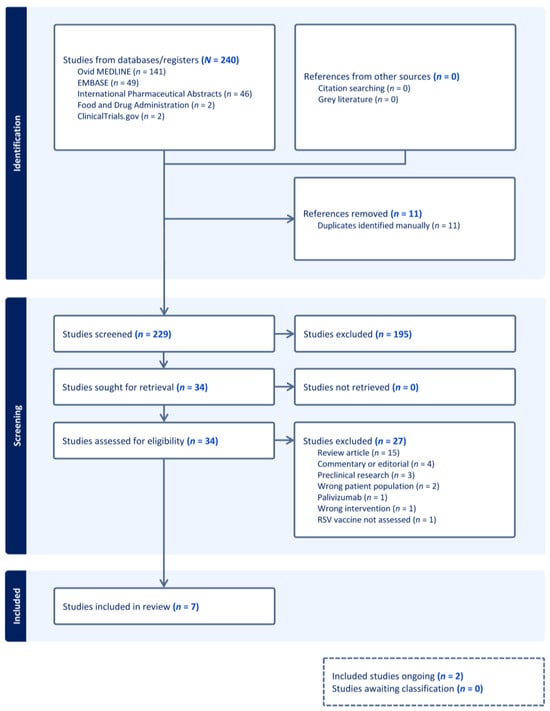
Figure 1
Open AccessReview
Recent Advances in Arboviral Vaccines: Emerging Platforms and Promising Innovations
by
Sujit Pujhari
Biologics 2024, 4(1), 1-16; https://doi.org/10.3390/biologics4010001 - 22 Dec 2023
Abstract
Arboviruses are a group of viruses that are transmitted by arthropods, such as mosquitoes, and cause significant morbidity and mortality worldwide. Currently, there are only a few options, with restricted use, for effective vaccines against these viruses. However, recent advances in arboviral vaccine
[...] Read more.
Arboviruses are a group of viruses that are transmitted by arthropods, such as mosquitoes, and cause significant morbidity and mortality worldwide. Currently, there are only a few options, with restricted use, for effective vaccines against these viruses. However, recent advances in arboviral vaccine development have shown promising innovations that have potential in preclinical and clinical studies. Insect-specific viruses have been explored as a novel vaccine platform that can induce cross-protective immunity against related arboviruses. Nanoparticle-based vaccines have also been developed to enhance the immunogenicity and stability of viral antigens. Additionally, vaccines against mosquito salivary proteins that can modulate the host immune response and interfere with arboviral transmission are being explored. Synonymous recoding, such as random codon shuffling, codon deoptimization, and codon-pair deoptimization, is being investigated as a strategy to attenuate the replication of arboviruses in vertebrate cells, reducing the risk of reverting to wild-type virulence. Finally, mRNA vaccines have been developed to rapidly generate and express viral antigens in the host cells, eliciting robust and durable immune responses. The challenges and opportunities for arboviral vaccine development are outlined, and future directions for research and innovation are discussed.
Full article
(This article belongs to the Special Issue Novel Vaccine Technologies and Platforms to Protect from Infectious Diseases)
►▼
Show Figures
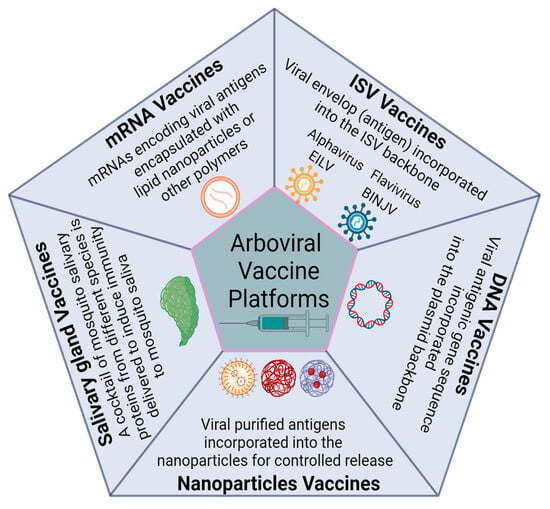
Figure 1
Open AccessReview
Targeting Hyaluronan Synthesis in Cancer: A Road Less Travelled
by
Theodoros Karalis
Biologics 2023, 3(4), 402-414; https://doi.org/10.3390/biologics3040022 - 12 Dec 2023
Abstract
►▼
Show Figures
Hyaluronan is one of the major components of the extracellular matrix and is involved in the regulation of multiple processes in both human physiology and disease. In human cancers, hyaluronan metabolism displays remarkable alterations, leading to the accumulation of large amounts of hyaluronan
[...] Read more.
Hyaluronan is one of the major components of the extracellular matrix and is involved in the regulation of multiple processes in both human physiology and disease. In human cancers, hyaluronan metabolism displays remarkable alterations, leading to the accumulation of large amounts of hyaluronan matrices in the tumoural tissues. The altered levels of hyaluronan in the tumours stem from the enhanced expression and activity of hyaluronan synthases in both tumour and stromal cells. Moreover, hyaluronidase activity is also upregulated in cancer, leading to the generation of lower molecular weight hyaluronan fragments that in turn assist tumour growth, neo-angiogenesis and the metastatic cascade. Hyaluronan accumulation in malignant tissues not only assists tumour growth and metastases but is also associated with worse outcomes in cancer patients. Therefore, targeting hyaluronan synthesis emerges as an interesting strategy that might be employed for cancer treatment. This review article summarises current evidence and discusses ways to move forward in the field of targeting hyaluronan synthesis for cancer therapy.
Full article
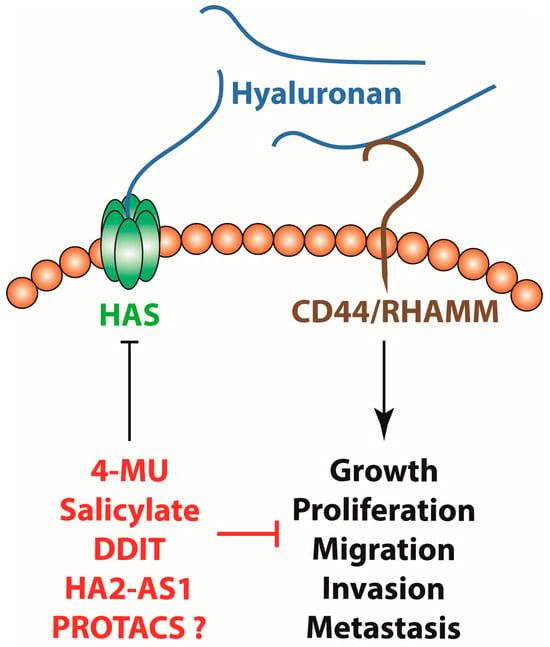
Figure 1
Open AccessReview
Advances in Escherichia coli-Based Therapeutic Protein Expression: Mammalian Conversion, Continuous Manufacturing, and Cell-Free Production
by
Sarfaraz K. Niazi and Matthias Magoola
Biologics 2023, 3(4), 380-401; https://doi.org/10.3390/biologics3040021 - 29 Nov 2023
Cited by 3
Abstract
Therapeutic proteins treat many acute and chronic diseases that were until recently considered untreatable. However, their high development cost keeps them out of reach of most patients around the world. One plausible solution to lower-cost manufacturing is to adopt newer technologies like using
[...] Read more.
Therapeutic proteins treat many acute and chronic diseases that were until recently considered untreatable. However, their high development cost keeps them out of reach of most patients around the world. One plausible solution to lower-cost manufacturing is to adopt newer technologies like using Escherichia coli to express larger molecules, including full-length antibodies, generally relegated to Chinese Hamster Ovary (CHO) cells, adopt continuous manufacturing, and convert the manufacturing to cell-free synthesis. The advantages of using E. coli include a shorter production cycle, little risk of viral contamination, cell host stability, and a highly reproducible post-translational modification.
Full article
(This article belongs to the Section Protein Therapeutics)
►▼
Show Figures
Graphical abstract
Open AccessReview
mRNA and Synthesis-Based Therapeutic Proteins: A Non-Recombinant Affordable Option
by
Sarfaraz K. Niazi and Matthias Magoola
Biologics 2023, 3(4), 355-379; https://doi.org/10.3390/biologics3040020 - 15 Nov 2023
Cited by 4
Abstract
Recombinant technology has been around for nearly three quarters of a century and has revolutionized protein therapy. However, the cost of developing recombinant therapeutic proteins and the manufacturing infrastructure keeps their cost unaffordable for most patients. Proteins are produced in the body via
[...] Read more.
Recombinant technology has been around for nearly three quarters of a century and has revolutionized protein therapy. However, the cost of developing recombinant therapeutic proteins and the manufacturing infrastructure keeps their cost unaffordable for most patients. Proteins are produced in the body via messenger RNA (mRNA) translation. This process can be readily replicated through administering a chemical nucleic acid product to manufacture the same protein recombinantly. The progress made in creating these proteins ex vivo in a cell-free system also offers a lower-cost option to produce therapeutic proteins. This article compares these alternative methods for recombinant protein production, assessing their respective advantages and limitations. While developers and regulatory agencies may encounter significant challenges in navigating product approval, including many unresolved intellectual property issues, these technologies are now proven and offer the most logical solution to making therapeutic proteins accessible to most patients.
Full article
(This article belongs to the Section Protein Therapeutics)
►▼
Show Figures

Figure 1
Open AccessReview
The Role of Anti-DFS70 in the Diagnosis of Systemic Autoimmune Rheumatic Diseases
by
Liudmila Zotova, Victoria Kotova and Zakhar Kuznetsov
Biologics 2023, 3(4), 342-354; https://doi.org/10.3390/biologics3040019 - 14 Nov 2023
Abstract
The diagnosis of systemic autoimmune rheumatic disease (SARD) or its exclusion is carried out taking into account the results of immunological studies, primarily antinuclear antibodies (ANA) and specific autoantibodies. Often, during ANA analysis via indirect immunofluorescence reaction on cellular and tissue substrates, a
[...] Read more.
The diagnosis of systemic autoimmune rheumatic disease (SARD) or its exclusion is carried out taking into account the results of immunological studies, primarily antinuclear antibodies (ANA) and specific autoantibodies. Often, during ANA analysis via indirect immunofluorescence reaction on cellular and tissue substrates, a dense fine speckled 70 (DFS70) fluorescence pattern is observed. Studies on the diagnostic significance of antibodies to anti-DFS70 allow for optimizing the stepwise diagnosis of SARD. Currently, a two-step strategy for laboratory diagnostic investigation is recommended: in the first step, ANA screening is performed, and in the second step, patients with positive results undergo confirmatory tests to detect specific antibodies against individual nuclear antigens. The detection of anti-DFS70 in ANA-seropositive patients without clinical and/or other specific serological markers characteristic of a particular disease within the SARD group may be considered a negative prognostic marker. Also, in the process of decision making in clinical practice, we should remember that anti-DFS70 can be found in the blood of patients with a different, non-SARD pathology and that most people showing anti-DFS70 are healthy individuals.
Full article
(This article belongs to the Section Diagnostics)
Open AccessCase Report
Is Metagenomics the Future Routine Diagnosis Tool for Brain Abscesses? About a Case
by
William Lars, Claudie Lamoureux, Jérémy Picard, Christophe Rodriguez, Clémence Beauruelle, Luc Quaesaet, Geneviève Héry-Arnaud, Séverine Ansart and Anne Coste
Biologics 2023, 3(4), 335-341; https://doi.org/10.3390/biologics3040018 - 27 Oct 2023
Abstract
Shotgun metagenomics (SMg) usefulness for brain abscess diagnosis is not known. We describe a case of brain abscess diagnosed with SMg and provide a review of the literature. A 70-year-old woman was diagnosed with multiple brain abscesses. Standard culture techniques and 16S rRNA
[...] Read more.
Shotgun metagenomics (SMg) usefulness for brain abscess diagnosis is not known. We describe a case of brain abscess diagnosed with SMg and provide a review of the literature. A 70-year-old woman was diagnosed with multiple brain abscesses. Standard culture techniques and 16S rRNA gene sequencing of abscess samples remained negative. SMg finally revealed the presence of sequences from Streptococcus anginosus and Fusobacterium nucleatum, leading to antimicrobial treatment adaptation and corticosteroids initiation. The patient finally recovered. A literature review retrieved fifteen other cases of brain abscesses diagnosed with SMg. SMg results led to changes in patient management in most cases. The existing literature about the performances of SMg, its advantages, future evolutions, and limitations is then discussed. SMg place in routine should be evaluated and defined through prospective studies.
Full article
(This article belongs to the Section Diagnostics)
►▼
Show Figures
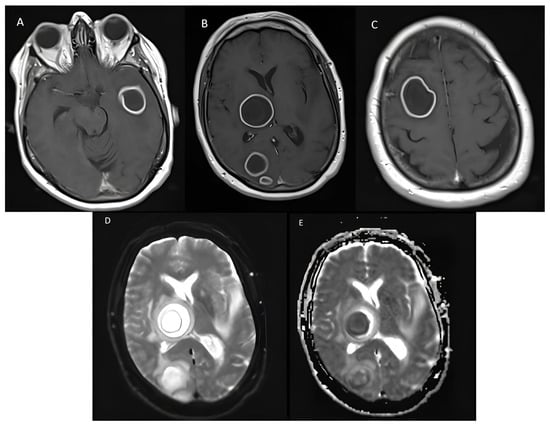
Figure 1
Open AccessReview
Neoantigens: The Novel Precision Cancer Immunotherapy
by
Tiantian Zhang, Esaw Kurban and Zhe Wang
Biologics 2023, 3(4), 321-334; https://doi.org/10.3390/biologics3040017 - 18 Oct 2023
Abstract
►▼
Show Figures
The past few decades have witnessed the remarkable progress of cancer immunotherapy. Neoantigens, also known as tumor-specific antigens, are novel antigens originating from tumor-specific alterations such as genomic mutations, dysregulated RNA splicing, and post-translational modifications. Neoantigens, recognized as non-self entities, trigger immune responses
[...] Read more.
The past few decades have witnessed the remarkable progress of cancer immunotherapy. Neoantigens, also known as tumor-specific antigens, are novel antigens originating from tumor-specific alterations such as genomic mutations, dysregulated RNA splicing, and post-translational modifications. Neoantigens, recognized as non-self entities, trigger immune responses that evade central and peripheral tolerance mechanisms. With the notable strides in cancer genomics facilitated by next-generation sequencing technologies, neoantigens have emerged as a promising avenue for tumor-specific immunotherapy grounded in genomic profiling-based precision medicine. Furthermore, a growing number of preclinical and clinical investigations are harnessing the potential synergies between neoantigens and other immunotherapies such as adoptive cell therapy and immune checkpoint inhibitors. In this review, we will provide a comprehensive perspective encompassing the trajectory of neoantigens, neoantigen design strategies, and the diverse array of clinical applications inherent in immunotherapy strategies centered around neoantigens. Moreover, we delve into the inherent prospects and challenges that accompany the clinical adoption of neoantigen-based immunotherapies while also putting forth potential solutions to address these challenges.
Full article
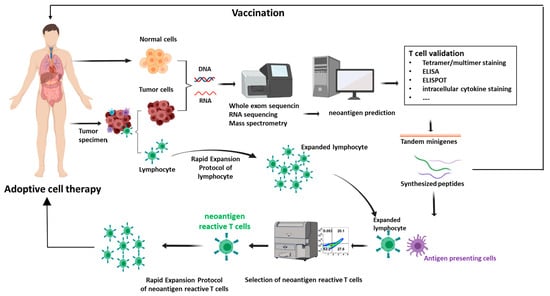
Figure 1
Open AccessArticle
Dose-Dependency of the Glycemic Response to Polyphenol-Rich Sugarcane Extract (PRSE)
by
Matthew Flavel, Julian Neoh and Kosta Fremielle Lim
Biologics 2023, 3(4), 308-320; https://doi.org/10.3390/biologics3040016 - 10 Oct 2023
Abstract
Foods high in available carbohydrates, such as plain white sugar or sucrose, increase the postprandial blood glucose levels that may aggravate the risk of developing Type 2 Diabetes. One class of compounds that is gaining popularity due to its potential application in reducing
[...] Read more.
Foods high in available carbohydrates, such as plain white sugar or sucrose, increase the postprandial blood glucose levels that may aggravate the risk of developing Type 2 Diabetes. One class of compounds that is gaining popularity due to its potential application in reducing the release of sugars for absorption into the body is polyphenols. The study aimed to investigate the effect of adding different doses of polyphenol-rich sugarcane extract (PRSE) to sucrose to lower the postprandial glycemia of the participants in a non-randomized study. The four test samples’ Glycemic Index (GI) values were calculated based on the standardized recommended methodology by comparing the area under the curve (AUC) of the test samples against the glucose standard. The glucose concentration curves were similar for the four test foods. The glucose response curves, and GI values were decreased in a dose-dependent manner. The results of this study indicate that PRSE-coated sugar can lower postprandial glycemia in normal individuals. Additionally, decreasing GI values with an increasing concentration of polyphenols suggests a dose-dependent effect between polyphenol levels and GI.
Full article
(This article belongs to the Section Natural Products)
►▼
Show Figures
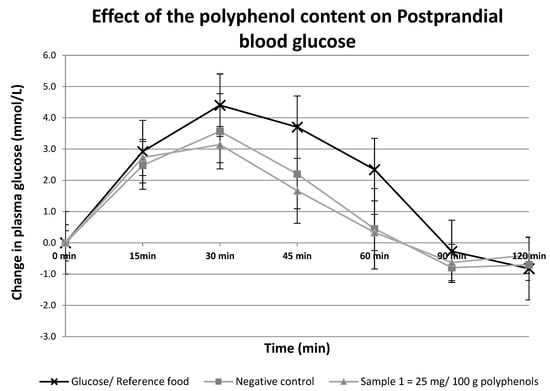
Figure 1
Open AccessArticle
Robust Porcine GFR Measurement with Radiotracers and Only Late Blood Samples
by
Lars Jødal, Juan Ignacio Brignone, Pui-Ki Chan Ladefoged, Lars Lund and Trine Borup Andersen
Biologics 2023, 3(4), 296-307; https://doi.org/10.3390/biologics3040015 - 29 Sep 2023
Abstract
(1) Pigs are physiologically very relevant as animal models of human physiology. Radiotracer methods for porcine GFR (glomerular filtration rate) determination exist but require full-curve blood sampling or the application of correction formulas, which vary among studies. (2) We used porcine GFR data
[...] Read more.
(1) Pigs are physiologically very relevant as animal models of human physiology. Radiotracer methods for porcine GFR (glomerular filtration rate) determination exist but require full-curve blood sampling or the application of correction formulas, which vary among studies. (2) We used porcine GFR data (40 datapoints from 20 juvenile pigs) for which the GFR was measured as the plasma clearance of [99mTc]Tc-DTPA. The reference clearance (Cl, GFR; range 41–85 mL/min) was measured from the full curve under the data. For simpler determination, an approximate clearance, Cl1, was based on the last five blood samples (acquired 120–240 min post injection). (3) The following formula for the GFR was developed: Cl = 1.27 · (Cl1)0.92. The spread (SD) was within 4% of the reference GFR. A comparison with the literature data showed that our correction formula was robust in pigs of various breeds, sizes up to approximately 200 kg, and GFRs up to approximately 400 mL/min, with a spread of up to 8%. The formula was also applicable for iohexol as the tracer. (4) A formula was developed that allows porcine GFR to be measured using only 4–5 late blood samples. This new formula can be applied across a wide range of swine breeds, animal sizes, and GFR ranges, allowing for robust determination of the GFR in pigs without full-curve blood sampling and without urine collection.
Full article
(This article belongs to the Section Diagnostics)
►▼
Show Figures
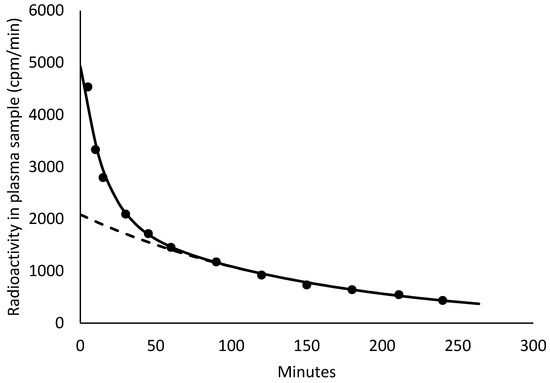
Figure 1
Open AccessReview
The Dawn of In Vivo Gene Editing Era: A Revolution in the Making
by
Sarfaraz K. Niazi
Biologics 2023, 3(4), 253-295; https://doi.org/10.3390/biologics3040014 - 25 Sep 2023
Cited by 1
Abstract
►▼
Show Figures
Gene or genome editing (GE) revises, removes, or replaces a mutated gene at the DNA level; it is a tool. Gene therapy (GT) offsets mutations by introducing a “normal” version of the gene into the body while the diseased gene remains in the
[...] Read more.
Gene or genome editing (GE) revises, removes, or replaces a mutated gene at the DNA level; it is a tool. Gene therapy (GT) offsets mutations by introducing a “normal” version of the gene into the body while the diseased gene remains in the genome; it is a medicine. So far, no in vivo GE product has been approved, as opposed to 22 GT products approved by the FDA, and many more are under development. No GE product has been approved globally; however, critical regulatory agencies are encouraging their entry, as evidenced by the FDA issuing a guideline specific to GE products. The potential of GE in treating diseases far supersedes any other modality conceived in history. Still, it also presents unparalleled risks—from off-target impact, delivery consistency and long-term effects of gene-fixing leading to designer babies and species transformation that will keep the bar high for the approval of these products. These challenges will come to the light of resolution only after the FDA begins approving them and opening the door to a revolution in treating hundreds of untreatable diseases that will be tantamount to a revolution in the making. This article brings a perspective and a future analysis of GE to educate and motivate developers to expand GE products to fulfill the needs of patients.
Full article

Graphical abstract
Open AccessReview
Unraveling the Immunopathogenesis of Multiple Sclerosis: The Dynamic Dance of Plasmablasts and Pathogenic T Cells
by
Yasunari Matsuzaka and Ryu Yashiro
Biologics 2023, 3(3), 232-252; https://doi.org/10.3390/biologics3030013 - 14 Sep 2023
Abstract
►▼
Show Figures
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system, characterized by multiple lesions occurring temporally and spatially. Additionally, MS is a disease that predominates in the white population. In recent years, there has been a rapid increase in
[...] Read more.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system, characterized by multiple lesions occurring temporally and spatially. Additionally, MS is a disease that predominates in the white population. In recent years, there has been a rapid increase in the number of patients, and it often occurs in young people, with an average age of onset of around 30 years old, but it can also occur in children and the elderly. It is more common in women than men, with a male-to-female ratio of approximately 1:3. As the immunopathogenesis of MS, a group of B cells called plasmablasts controls encephalomyelitis via IL-10 production. These IL-10-producing B cells, called regulatory B cells, suppress inflammatory responses in experimental mouse models of autoimmune diseases including MS. Since it has been clarified that these regulatory B cells are plasmablasts, it is expected that the artificial control of plasmablast differentiation will lead to the development of new treatments for MS. Among CD8-positive T cells in the peripheral blood, the proportion of PD-1-positive cells is decreased in MS patients compared with healthy controls. The dysfunction of inhibitory receptors expressed on T cells is known to be the core of MS immunopathology and may be the cause of chronic persistent inflammation. The PD-1+ CD8+ T cells may also serve as indicators that reflect the condition of each patient in other immunological neurological diseases such as MS. Th17 cells also regulate the development of various autoimmune diseases, including MS. Thus, the restoration of weakened immune regulatory functions may be a true disease-modifying treatment. So far, steroids and immunosuppressants have been the mainstream for autoimmune diseases, but the problem is that this kills not only pathogenic T cells, but also lymphocytes, which are necessary for the body. From this understanding of the immune regulation of MS, we can expect the development of therapeutic strategies that target only pathogenic immune cells.
Full article
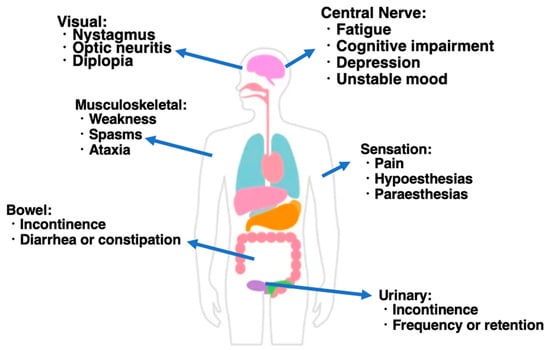
Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Biologics, Molecules, Pharmaceuticals, Pharmaceutics
Biosimilars and Interchangeability
Topic Editors: Alexis Oliva, Shein-Chung Chow, Joao GoncalvesDeadline: 31 December 2024

Conferences
Special Issues
Special Issue in
Biologics
Novel Vaccine Technologies and Platforms to Protect from Infectious Diseases
Guest Editors: Alexander Zakhartchouk, Sams SadatDeadline: 1 May 2024
Special Issue in
Biologics
Anti-SARS-CoV-2/COVID-19 Drugs and Vaccines: Part II
Guest Editors: Vasso Apostolopoulos, Majid DavidsonDeadline: 30 June 2024
Special Issue in
Biologics
The Role of Extracellular Vesicles in Cancer
Guest Editor: Yasunari MatsuzakaDeadline: 25 November 2024