Photochemistry of β-γ-Unsaturated Spirolactones
Abstract
:1. Introduction
2. Results and Discussion
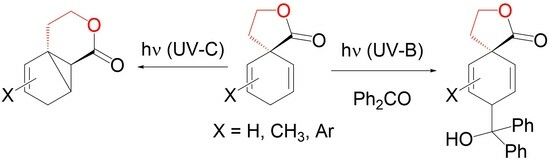
2.1. Photochemistry of Sole β-γ-Unsaturated Spirolactones 11
2.2. Photochemistry of β-γ-Unsaturated Spirolactones 11 in the Presence of Carbonyl Compounds
3. Materials and Methods
3.1. Chemicals and Instrumentation
3.2. Photochemistry of Sole Spirolactones 11
3.2.1. Kinetic Measurements
3.2.2. Analytical Data of Side Product 2-Oxaspiro[4.5]deca-6,9-diene-1,8-dione (12a)
3.2.3. Optimized Procedure for Oxa-Di-π-Methane Rearrangements of Spirolactones 11
3.2.4. Analytical Data of Oxa-Di-π-Methane Rearrangement Products 13
- 1,2,4b,5-tetrahydrocyclopenta[2,3]cyclopropa[1,2-c]pyran-4(4aH)-one (13a): Obtained as a colorless liquid. Rf = 0.21 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.33 (d, J = 2.9 Hz, 1H), 2.10 (ddd, J = 13.9, 3.5, 1.4 Hz, 1H), 2.44 (ddd, J = 13.9, 13.2, 5.8 Hz, 1H), 2.50 (dd, J = 6.9, 2.9 Hz, 1H), 2.55 (dt, J = 18.9, 2.2 Hz, 1H), 2.79 (ddt, J = 18.9, 6.9, 2.2 Hz, 1H), 4.10 (ddd, J = 13.2, 11.8, 3.5 Hz, 1H), 4.36 (ddd, J = 11.8, 5.8, 1.4 Hz, 1H), 5.61 (dt, J = 5.6, 2.2 Hz, 1H), 5.92 (dt, J = 5.6, 2.2 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ = 22.0 (t), 25.8 (d), 32.6 (d), 36.3 (t), 40.8 (s), 66.1 (t), 130.0 (d), 133.9 (d), 171.1 (s). IR (neat): ν = 2922, 1716, 1451, 1396, 1267, 1158, 1024, 912, 864 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C9H11O2 [M + H]+: 151.0759; Found: 151.0733.
- 4a,4b-dimethyl-1,2,4b,5-tetrahydrocyclopenta[2,3]cyclopropa[1,2-c]pyran-4(4aH)-one (13ba): Obtained as a colorless liquid. Rf = 0.29 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.27 (s, 1H), 1.57 (sext, J = 1.1 Hz, 3H), 1.67 (ddq, J = 2.6, 1.6, 1.1 Hz, 3H), 1.96 (ddd, J = 13.8, 3.5, 1.4 Hz, 1H), 2.33 (ddd, J = 13.8, 13.2, 5.8 Hz, 1H), 2.34 (ddqq, J = 18.0, 2.8, 1.6, 1.1 Hz, 1H), 2.40 (dd, J = 6.7, 2.8 Hz, 2H), 2.65 (ddqq, J = 18.0, 6.7, 2.6, 1.1 Hz, 1H), 4.07 (ddd, J = 13.2, 11.7, 3.5 Hz, 1H), 4.33 (ddd, J = 11.7, 5.8, 1.4 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ = 11.0 (q), 13.5 (q), 21.2 (t), 24.8 (d), 32.8 (d), 40.0 (t), 43.0 (s), 65.8 (t), 131.2 (s), 133.0 (s), 171.6 (s). IR (neat): ν = 2970, 2924, 1716, 1387, 1291, 1157, 1108, 1038, 779 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C11H15O2 [M + H]+: 179.1072; Found: 179.1053.
- 6,7-dimethyl-1,2,4b,5-tetrahydrocyclopenta[2,3]cyclopropa[1,2-c]pyran-4(4aH)-one (13bb): Obtained as a colorless liquid. Rf = 0.24 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.11 (s, 3H), 1.20 (s, 3H), 2.08 (ddd, J = 15.2, 11.5, 5.5 Hz, 1H), 2.17 (ddd, J = 15.2, 3.7, 2.7 Hz, 1H), 2.42 (t, J = 2.1 Hz, 2H), 4.21 (ddd, J = 11.5, 10.9, 3.7 Hz, 1H), 4.26 (ddd, J = 10.9, 5.5, 2.7 Hz, 1H), 5.63 (dt, J = 5.7, 2.1 Hz, 1H), 5.67 (dt, J = 5.7, 2.1 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ = 9.3 (q), 15.8 (q), 23.2 (t), 28.7 (s), 35.9 (s), 40.8 (t), 43.1 (s), 67.6 (t), 131.2 (d), 133.0 (d), 174.2 (s). IR (neat): ν = 2974, 2917, 1711, 1402, 1253, 1221, 1071, 937, 732 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C11H15O2 [M + H]+: 179.1072; Found: 179.1049.
- 1,2,4b,5-tetrahydroindeno[1′,2′:2,3]cyclopropa[1,2-c]pyran-4(4aH)-one (13c): Obtained as a white solid. Mp = 54–56 °C. Rf = 0.26 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.49 (d, J = 3.0 Hz, 1H), 2.26 (dd, J = 13.8, 3.4 Hz, 1H), 2.78 (ddd, J = 13.8, 13.3, 5.8 Hz, 1H), 2.80 (dd, J = 6.5, 3.0 Hz, 1H), 3.12 (d, J = 17.7 Hz, 1H), 3.31 (dd, J = 17.7, 6.5 Hz, 1H), 4.24 (ddd, J = 13.3, 11.8, 3.4 Hz, 1H), 4.47 (dd, J = 11.8, 5.8 Hz, 1H), 7.21–7.30 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 21.0 (t), 26.6 (d), 32.9 (d), 34.7 (t), 39.4 (s), 65.7 (t), 121.9 (d), 125.7 (d), 126.7 (d), 127.2 (d), 141.0 (s), 143.9 (s), 170.2 (s). IR (neat): ν = 2969, 2916, 1713, 1479, 1399, 1247, 1218, 1077, 1022, 937, 807, 754 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C13H13O2 [M + H]+: 201.0916; Found: 201.0904.
3.3. Photochemistry of Spirolactones 11 in the Presence of Carbonyl Compounds
3.3.1. Optimized Procedure for Reactions with Benzaldehyde (18) or Benzophenone (19)
3.3.2. Analytical Data of Products 20 and 26 from Reactions with Benzaldehyde (18)
- trans-8-[hydroxy(phenyl)methyl]-2-oxaspiro[4.5]deca-6,9-dien-1-one (trans-20a): Obtained as a colorless liquid. Rf = 0.10 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.98 (bs, 1H), 2.06 (t, J = 7.0 Hz, 2H), 3.37 (dtt, J = 5.2, 3.2, 2.0 Hz, 1H), 4.34 (t, J = 7.0 Hz, 2H), 4.79 (d, J = 5.2 Hz, 1H), 5.72 (dd, J = 10.3, 2.0 Hz, 1H), 5.74 (dd, J = 10.1, 2.0 Hz, 1H), 5.98 (ddd, J = 10.1, 3.2, 1.3 Hz, 1H), 5.99 (ddd, J = 10.3, 3.2, 1.3 Hz, 1H), 7.28–7.35 (m, 5H). 13C NMR (125 MHz, CDCl3): δ = 37.4 (t), 43.4 (d), 45.9 (s), 65.3 (t), 76.2 (d), 126.2 (d), 126.3 (d), 126.5 (d), 126.8 (d), 127.8 (d), 128.1 (d), 129.0 (d), 141.4 (s), 177.5 (s). IR (neat): ν = 3664, 3458, 2969, 2919, 1749, 1450, 1374, 1205, 1165, 1021, 914, 852, 766 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C16H17O3 [M + H]+: 257.1178; Found: 257.1186.
- cis-8-[hydroxy(phenyl)methyl]-2-oxaspiro[4.5]deca-6,9-dien-1-one (cis-20a): Obtained as a colorless liquid. Rf = 0.12 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.65 (bs, 1H), 2.39 (t, J = 7.0 Hz, 2H), 3.31 (dtt, J = 4.1, 3.8, 1.2 Hz, 1H), 4.48 (t, J = 7.0 Hz, 2H), 4.89 (d, J = 3.8 Hz, 1H), 5.73 (ddd, J = 9.9, 3.8, 1.5 Hz, 1H), 5.77 (ddd, J = 9.9, 2.0, 1.2 Hz, 1H), 5.80 (ddd, J = 9.8, 2.0, 1.2 Hz, 1H), 6.06 (ddd, J = 9.8, 4.1, 1.5 Hz, 1H), 7.27–7.39 (m, 5H). 13C NMR (125 MHz, CDCl3): δ = 36.6 (t), 44.8 (d), 46.6 (s), 65.7 (t), 74.4 (d), 126.0 (d), 127.1 (d), 127.2 (d), 127.3 (d), 127.6 (d), 128.2 (d), 130.3 (d), 142.7 (s), 178.8 (s). IR (neat): ν = 3454, 3029, 2915, 1751, 1373, 1205, 1166, 1021, 913, 851 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C16H17O3 [M + H]+: 257.1178; Found: 257.1182.
- trans-8-[hydroxy(phenyl)methyl]-6,7-dimethyl-2-oxaspiro[4.5]deca-6,9-dien-1-one (trans-20b): Obtained as a colorless liquid. Rf = 0.16 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.62 (bs, 1H), 1.78 (s, 3H), 1.99 (s, 3H), 2.19 (ddd, J = 13.4, 7.7, 4.3 Hz, 1H), 2.78 (ddd, J = 13.4, 9.0, 8.3 Hz, 1H), 3.14–3.16 (m, 1H), 4.47 (ddd, J = 9.3, 8.3, 7.7 Hz, 1H), 4.51 (ddd, J = 9.3, 9.0, 4.3 Hz, 1H), 5.12 (d, J = 2.7 Hz, 1H), 5.46 (dd, J = 9.9, 4.4 Hz, 1H), 5.74 (dd, J = 9.9, 1.2 Hz, 1H), 7.25–7.38 (m, 5H). 13C NMR (125 MHz, CDCl3): δ = 15.7 (q), 17.8 (q), 33.4 (t), 50.7 (s), 51.1 (d), 66.2 (t), 71.3 (d), 125.8 (d), 126.0 (d), 126.6 (s), 126.9 (s), 127.4 (d), 128.1 (d), 128.3 (d), 143.2 (s), 180.2 (s). IR (neat): ν = 3413, 3030, 2914, 1758, 1451, 1378, 1202, 1170, 1024, 847, 760 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C18H19O2 [M − H2O + H]+: 267.1385; Found: 267.1399.
- cis-8-[hydroxy(phenyl)methyl]-6,7-dimethyl-2-oxaspiro[4.5]deca-6,9-dien-1-one (cis-20b): Obtained as a colorless liquid. Rf = 0.08 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.75 (bs, 1H), 1.78 (s, 3H), 1.96 (s, 3H), 2.10 (ddd, J = 13.1, 7.0, 3.3 Hz, 1H), 2.54 (dt, J = 13.1, 9.3 Hz, 1H), 3.14–3.17 (m, 1H), 4.38 (td, J = 9.3, 7.0 Hz, 1H), 4.42 (td, J = 9.3, 3.3 Hz, 1H), 5.05 (d, J = 2.9 Hz, 1H), 5.54 (dd, J = 10.0, 4.2 Hz, 1H), 5.88 (dd, J = 10.0, 1.3 Hz, 1H), 7.30–7.40 (m, 5H). 13C NMR (125 MHz, CDCl3): δ = 15.5 (q), 17.9 (q), 36.1 (t), 50.2 (d), 50.6 (s), 65.5 (t), 72.7 (d), 125.7 (d), 126.0 (d), 127.3 (d), 127.4 (s), 127.8 (d), 128.2 (d), 130.0 (s), 141.8 (s), 178.0 (s). IR (neat): ν = 3434, 3031, 2919, 1757, 1450, 1376, 1206, 1172, 1025, 912, 729 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C18H19O2 [M − H2O + H]+: 267.1390; Found: 267.1399.
- 1,6-dimethyl-7-phenylspiro[8-oxabicyclo[4.2.0]oct-3-ene-2,3′-oxolan]-2′-one (26ba): Obtained as a colorless liquid. Rf = 0.30 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 0.87 (s, 3H), 1.54 (s, 3H), 2.23 (dd, J = 16.7, 7.4 Hz, 1H), 2.29 (ddd, J = 13.5, 6.7, 4.0 Hz, 1H), 2.68 (ddd, J = 16.7, 3.3, 2.2 Hz, 1H), 2.73 (dt, J = 13.5, 9.3 Hz, 1H), 4.43–4.47 (m, 2H), 5.28 (s, 1H), 5.99 (dd, J = 9.6, 3.3 Hz, 1H), 6.39 (ddd, J = 9.6, 7.4, 2.2 Hz, 1H), 7.26–7.40 (m, 5H). 13C NMR (125 MHz, CDCl3): bδ = 19.4 (q), 20.2 (q), 31.7 (t), 36.2 (t), 44.0 (s), 54.0 (s), 66.1 (t), 86.7 (d), 88.7 (s), 125.0 (d), 127.3 (d), 128.2 (d), 129.5 (d), 132.9 (d), 140.7 (s), 177.4 (s). IR (neat): ν = 2993, 2917, 1758, 1450, 1377, 1205, 1168, 1023, 848, 760 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C18H21O3 [M + H]+: 285.1491; Found: 285.1498.
- 1,6-dimethyl-7-phenylspiro[8-oxabicyclo[4.2.0]oct-3-ene-2,3′-oxolan]-2′-one (26bb): Obtained as a colorless liquid. Rf = 0.18 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 0.88 (s, 3H), 1.48 (s, 3H), 2.16 (ddd, J = 13.3, 9.1, 7.1 Hz, 1H), 2.19 (ddd, J = 16.8, 3.3, 2.2 Hz, 1H), 2.49 (ddd, J = 13.3, 8.3, 5.1 Hz, 1H), 2.56 (dd, J = 16.8, 7.1 Hz, 1H), 4.21 (ddd, J = 9.1, 8.3, 7.1 Hz, 1H), 4.36 (td, J = 9.1, 5.1 Hz, 1H), 5.93 (s, 1H), 6.31 (ddd, J = 9.5, 7.1, 2.2 Hz, 1H), 6.40 (dd, J = 9.5, 3.3 Hz, 1H), 7.26 (tm, J = 7.3 Hz, 1H), 7.35 (tm, J = 7.3 Hz, 2H), 7.53 (dm, J = 7.3 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ = 14.4 (q), 25.4 (q), 31.8 (t), 37.9 (t), 49.5 (s), 50.5 (s), 66.1 (t), 83.7 (d), 85.0 (s), 126.3 (d), 127.1 (d), 128.0 (d), 129.4 (d), 132.9 (d), 140.8 (s), 179.8 (s). IR (neat): ν = 3028, 2918, 1750, 1377, 1173, 1024, 911, 837 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C18H21O3 [M + H]+: 285.1491; Found: 285.1484.
- 1,6-dimethyl-8-phenylspiro[7-oxabicyclo[4.2.0]oct-3-ene-2,3′-oxolan]-2′-one (26bc): Obtained as a colorless liquid. Rf = 0.40 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 0.80 (ddd, J = 13.2, 6.5, 1.1 Hz, 1H), 1.53 (s, 3H), 1.77 (s, 3H), 1.96 (ddd, J = 13.2, 11.1, 9.3 Hz, 1H), 2.46 (dd, J = 17.1, 7.2 Hz, 1H), 2.73 (ddd, J = 17.1, 3.3, 2.2 Hz, 1H), 3.98 (ddd, J = 11.1, 9.3, 6.5 Hz, 1H), 4.15 (td, J = 9.3, 1.1 Hz, 1H), 5.44 (dd, J = 9.6, 3.3 Hz, 1H), 5.50 (s, 1H), 6.20 (ddd, J = 9.6, 7.2, 2.2 Hz, 1H), 7.27–7.35 (m, 5H). 13C NMR (125 MHz, CDCl3): δ = 19.2 (q), 24.7 (q), 31.3 (t), 39.0 (t), 50.1 (s), 51.6 (s), 65.5 (t), 85.4 (s), 86.8 (d), 127.6 (d), 127.8 (d), 127.9 (d), 128.0 (d), 133.0 (d), 138.6 (s), 177.8 (s). IR (neat): ν = 2988, 2919, 1751, 1450, 1377, 1173, 1024, 842, 752 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C18H21O3 [M + H]+: 285.1491; Found: 285.1485.
- 4-[hydroxy(phenyl)methyl]-4H-spiro[naphthalene-1,3′-oxolan]-2′-one (20c) DS1: Obtained as a white solid. Mp = 65–66 °C. Rf = 0.24 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 2.15 (ddd, J = 13.2, 7.2, 5.7 Hz, 1H), 2.21 (ddd, J = 13.2, 7.8, 7.6 Hz, 1H), 2.80 (bs, 1H), 3.89 (ddd, J = 4.4, 3.7, 1.0 Hz, 1H), 4.40 (ddd, J = 9.2, 7.8, 5.7 Hz, 1H), 4.42 (ddd, J = 9.2, 7.6, 7.2 Hz, 1H), 5.11 (d, J = 3.7 Hz, 1H), 5.92 (dd, J = 10.0, 4.4 Hz, 1H), 5.97 (dd, J = 10.0, 1.0 Hz, 1H), 7.05–7.09 (m, 1H), 7.20–7.30 (m, 7H), 7.55–7.58 (m, 1H). 13C NMR (125 MHz, CDCl3): δ = 40.3 (t), 47.8 (d), 48.9 (s), 65.1 (t), 77.2 (d), 126.5 (d), 126.7 (d), 126.8 (d), 126.9 (d), 127.4 (d), 127.9 (d), 128.2 (d), 128.6 (d), 129.1 (d), 136.0 (s), 137.4 (s), 143.0 (s), 178.3 (s). IR (neat): ν = 3667, 3432, 2976, 2903, 1748, 1668, 1490, 1449, 1376, 1209, 1163, 1056, 1024 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C20H19O3 [M + H]+: 307.1334; Found: 307.1333.
- 4-[hydroxy(phenyl)methyl]-4H-spiro[naphthalene-1,3′-oxolan]-2′-one (20c) DS2: Obtained as a white solid. Mp = 66–67 °C. Rf = 0.20 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.28 (bs, 1H), 2.64 (ddd, J = 13.7, 8.3, 5.0 Hz, 1H), 3.04 (ddd, J = 13.7, 9.0, 7.3 Hz, 1H), 3.97 (ddd, J = 4.6, 3.2, 1.4 Hz, 1H), 4.66 (ddd, J = 9.5, 8.3, 7.3 Hz, 1H), 4.74 (ddd, J = 9.5, 9.0, 5.0 Hz, 1H), 5.24 (d, J = 3.2 Hz, 1H), 5.80 (dd, J = 10.0, 4.6 Hz, 1H), 5.95 (dd, J = 10.0, 1.4 Hz, 1H), 7.31–7.44 (m, 9H). 13C NMR (125 MHz, CDCl3): δ = 38.0 (t), 48.4 (d), 48.5 (s), 66.5 (t), 77.7 (d), 125.7 (d), 125.9 (d), 126.3 (d), 127.1 (d), 127.5 (d), 127.8 (d), 128.2 (d), 128.6 (d), 128.7 (d), 135.5 (s), 135.9 (s), 143.0 (s), 179.8 (s). IR (neat): ν = 3439, 3030, 2922, 1761, 1510, 1450, 1374, 1268, 1165, 1024, 961, 759 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C20H19O3 [M + H]+: 307.1334; Found: 307.1333.
- 4-[hydroxy(phenyl)methyl]-4H-spiro[naphthalene-1,3′-oxolan]-2′-one (20c) DS3: Obtained as a colorless liquid Rf = 0.18 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.70 (bs, 1H), 1.59 (ddd, J = 13.2, 7.3, 5.7 Hz, 1H), 2.21 (dt, J = 13.2, 7.7 Hz, 1H), 4.04 (dd, J = 5.6, 4.8 Hz, 1H), 4.30 (ddd, J = 9.3, 7.7, 7.3 Hz, 1H), 4.32 (ddd, J = 9.3, 7.7, 5.7 Hz, 1H), 5.02 (d, J = 5.6 Hz, 1H), 5.88 (d, J = 10.1 Hz, 1H), 6.23 (dd, J = 10.1, 4.8 Hz, 1H), 7.00–7.03 (m, 1H), 7.20–7.24 (m, 3H), 7.30–7.36 (m, 3H). 13C NMR (125 MHz, CDCl3): δ = 39.8 (t), 47.5 (d), 48.6 (s), 65.2 (t), 79.1 (d), 126.7 (d), 126.9 (d), 127.2 (d), 127.4 (d), 127.5 (d), 127.6 (d), 127.7 (d), 128.0 (d), 129.4 (d), 134.7 (s), 136.3 (s), 140.9 (s), 178.7 (s). IR (neat): ν = 3450, 3061, 3029, 2922, 1760, 1677, 1489, 1449, 1372, 1161, 1024, 909, 761 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C20H19O3 [M + H]+: 307.1334; Found: 307.1346.
- 4-[hydroxy(phenyl)methyl]-4H-spiro[naphthalene-1,3′-oxolan]-2′-one (20c) DS4: Obtained as a white solid. Mp = 64–65 °C. Rf = 0.15 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.60 (bs, 1H), 2.61 (ddd, J = 13.6, 8.3, 5.1 Hz, 1H), 3.02 (ddd, J = 13.6, 8.7, 7.3 Hz, 1H), 3.87 (dd, J = 5.8, 5.0 Hz, 1H), 4.63 (ddd, J = 9.5, 8.3, 7.3 Hz, 1H), 4.71 (ddd, J = 9.5, 8.7, 5.01Hz, 1H), 4.95 (d, J = 5.8 Hz, 1H), 5.88 (d, J = 9.8 Hz, 1H), 5.97 (dd, J = 9.8, 5.0 Hz, 1H), 6.93 (dm, J = 7.8 Hz, 1H), 7.19 (tm, J = 7.8 Hz, 1H), 7.30–7.44 (m, 7H). 13C NMR (125 MHz, CDCl3): δ = 38.3 (t), 48.5 (d), 48.6 (s), 66.2 (t), 77.6 (d), 125.6 (d), 126.8 (d), 127.1 (d), 127.4 (d), 127.6 (d), 128.2 (d), 128.5 (d), 130.4 (d), 130.4 (d), 134.3 (s), 136.1 (s), 142.7 (s), 178.8 (s). IR (neat): ν = 3666, 3434, 2971, 2920, 1759, 1599, 1445, 1374, 1259, 1160, 1025, 909, 759 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C20H19O3 [M + H]+: 307.1334; Found: 307.1334.
3.3.3. Analytical Data of Products 27 from Reactions with Benzophenone (19)
- trans-8-[hydroxy(diphenyl)methyl]-2-oxaspiro[4.5]deca-6,9-dien-1-one (trans-27a): Obtained as a white solid. Mp = 225–226 °C. Rf = 0.28 (PE/MTBE 2:1). 1H NMR (600 MHz, CDCl3): δ = 1.58 (bs, 1H), 2.28 (t, J = 7.0 Hz, 2H), 4.24 (tt, J = 3.0, 2.1 Hz, 1H), 4.40 (t, J = 7.0 Hz, 2H), 5.81 (dd, J = 10.3, 2.1 Hz, 2H), 5.88 (dd, J = 10.3, 3.0 Hz, 2H), 7.25 (tm, J = 7.4 Hz, 2H), 7.35 (ddm, J = 8.3, 7.4 Hz, 4H), 7.57 (dm, J = 8.3 Hz, 4H). 13C NMR (150 MHz, CDCl3): δ = 37.3 (t), 44.2 (d), 46.0 (s), 65.3 (t), 79.3 (s), 125.6 (d), 127.0 (d), 127.9 (d), 128.2 (d), 128.5 (d), 144.9 (s), 177.1 (s). IR (neat): ν = 3446, 3026, 2919, 1754, 1489, 1446, 1167, 1021, 913, 846, 753 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C22H19O2 [M − H2O + H]+: 315.1385; Found: 315.1374.
- cis-8-[hydroxy(diphenyl)methyl]-2-oxaspiro[4.5]deca-6,9-dien-1-one (cis-27a): Obtained as a white solid. Mp = 189–190 °C. Rf = 0.16 (PE/MTBE 2:1). 1H NMR (600 MHz, CDCl3): δ = 1.59 (bs, 1H), 2.38 (t, J = 7.0 Hz, 2H), 4.27 (tt, J = 3.8, 1.3 Hz, 1H), 4.46 (t, J = 7.0 Hz, 2H), 5.75 (dd, J = 10.3, 1.3 Hz, 2H), 5.80 (dd, J = 10.3, 3.8 Hz, 2H), 7.21 (tm, J = 7.4 Hz, 2H), 7.34 (ddm, J = 8.3, 7.4 Hz, 4H), 7.67 (dm, J = 8.3 Hz, 4H). 13C NMR (150 MHz, CDCl3): δ = 36.6 (t), 45.0 (d), 46.5 (s), 65.8 (t), 77.8 (s), 125.7 (d), 126.6 (d), 128.1 (d), 128.3 (d), 128.5 (d), 145.9 (s), 178.7 (s). IR (neat): ν = 3446, 3027, 2919, 1753, 1489, 1446, 1167, 1020, 913, 846, 753 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C22H19O2 [M − H2O + H]+: 315.1385; Found: 315.1372.
- trans-8-[hydroxy(diphenyl)methyl]-6,7-dimethyl-2-oxaspiro[4.5]deca-6,9-dien-1-one (trans-27b): Obtained as a white solid. Mp = 189–190 °C. Rf = 0.28 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.18 (s, 3H), 1.57 (s, 3H), 2.11 (ddd, J = 13.2, 7.4, 3.5 Hz, 1H), 2.30 (bs, 1H), 2.51 (dt, J = 13.2, 9.2 Hz, 1H), 4.12 (dd, J = 4.5, 0.8 Hz, 1H), 4.35 (td, J = 9.2, 7.4 Hz, 1H), 4.41 (td, J = 9.2, 3.5 Hz, 1H), 5.83 (dd, J = 10.0, 0.8 Hz, 1H), 5.96 (dd, J = 10.0, 4.5 Hz, 1H), 7.27–7.37 (m, 8H), 7.52 (dm, J = 8.5 Hz, 1H), 7.60 (dm, J = 8.5 Hz, 1H). 13C NMR (125 MHz, CDCl3): δ = 16.0 (q), 20.6 (q), 35.6 (t), 50.9 (s), 51.6 (d), 65.6 (t), 79.6 (s), 125.3 (d), 127.0 (s), 128.0 (d), 128.1 (d), 128.2 (d), 129.5 (s), 145.4 (s), 177.9 (s). IR (neat): ν = 3523, 2920, 1749, 1447,m1375, 1170, 1026, 949, 910 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C24H23O2 [M − H2O + H]+: 343.1698; Found: 343.1657.
- cis-8-[hydroxy(diphenyl)methyl]-6,7-dimethyl-2-oxaspiro[4.5]deca-6,9-dien-1-one (cis-27b): Obtained as a white solid. Mp = 176–177 °C. Rf = 0.26 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 1.10 (d, J = 0.5 Hz, 3H), 1.61 (bs, 1H), 1.68 (d, J = 1.0 Hz, 3H), 2.16 (ddd, J = 13.4, 7.7, 4.2 Hz, 1H), 2.77 (ddd, J = 13.4, 9.1, 8.4 Hz, 1H), 4.25 (dquint.q, J = 4.6, 1.0, 0.5 Hz, 1H), 4.44 (ddd, J = 9.3, 8.4, 7.7 Hz, 1H), 4.50 (ddd, J = 9.3, 9.1, 4.2 Hz, 1H), 5465 (dd, J = 9.9, 1.0 Hz, 1H), 5.80 (dd, J = 9.9, 4.6 Hz, 1H), 7.16–7.20 (m, 2H), 7.27–7.35 (m, 4H), 7.68–7.75 (m, 4H). 13C NMR (125 MHz, CDCl3): δ = 16.4 (q), 20.5 (q), 33.8 (t), 50.8 (s), 51.5 (d), 66.3 (t), 77.9 (s), 125.2 (d), 127.2 (s), 128.1 (d), 128.2 (d), 128.4 (d), 131.1 (s), 147.2 (s), 180.1 (s). IR (neat): ν = 3524, 2973, 2912, 1748, 1491, 1446, 1375, 1170, 1028, 948, 819, 746 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C24H23O2 [M − H2O + H]+: 343.1698; Found: 343.1702.
- trans-4-[hydroxy(diphenyl)methyl]-4H-spiro[naphthalene-1,3′-oxolan]-2′-one (trans-27c): Obtained as a white solid. Mp = 213–214 °C. Rf = 0.24 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 2.23 (bs, 1H), 2.47 (ddd, J = 13.1, 8.0, 6.4 Hz, 1H), 2.63 (ddd, J = 13.1, 7.9, 6.3 Hz, 1H), 4.51 (ddd, J = 9.4, 8.0, 6.3 Hz, 1H), 4.55 (ddd, J = 9.4, 7.9, 6.4 Hz, 1H), 4.74 (dd, J = 4.9, 0.8 Hz, 1H), 6.03 (dd, J = 10.1, 0.8 Hz, 1H), 6.26 (dd, J = 10.1, 4.9 Hz, 1H), 6.38 (dd, J = 7.9, 1.4 Hz, 1H), 6.86 (ddd, J = 8.3, 7.9, 1.4 Hz, 1H), 7.07 (dd, J = 7.9, 1.4 Hz, 1H), 7.19–7.42 (m, 9H), 7.64 (dm, J = 8.5 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ = 40.0 (t), 49.0 (d), 49.2 (s), 65.6 (t), 81.0 (s), 126.0 (d), 126.1 (d), 126.6 (d), 126.7 (d), 127.0 (d), 127.3 (d), 127.4 (d), 127.9 (d), 128.4 (d), 129.2 (d), 129.8 (d), 130.8 (d), 133.5 (s), 137.4 (s), 144.5 (s), 145.3 (s), 179.0 (s). IR (neat): ν = 3520, 2975, 2913, 1751, 1490, 1446, 1373, 1163, 1024, 950, 828, 766, 744 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C26H21O2 [M − H2O + H]+: 365.1542; Found: 365.1533.
- cis-4-[hydroxy(diphenyl)methyl]-4H-spiro[naphthalene-1,3′-oxolan]-2′-one (cis-27c): Obtained as a white solid. Mp = 225–226 °C. Rf = 0.20 (PE/MTBE 2:1). 1H NMR (500 MHz, CDCl3): δ = 2.63 (ddd, J = 13.8, 8.8, 5.8 Hz, 1H), 2.94 (ddd, J = 13.8, 8.8, 6.5 Hz, 1H), 4.64 (ddd, J = 9.3, 8.8, 6.5 Hz, 1H), 4.73 (ddd, J = 9.3, 8.8, 5.8 Hz, 1H), 4.81 (bs, 1H), 4.88 (d, J = 4.9 Hz, 1H), 5.82 (d, J = 10.1 Hz, 1H), 6.18 (dd, J = 10.1, 4.9 Hz, 1H), 6.20 (d, J = 7.8 Hz, 1H), 6.83 (t, J = 7.8 Hz, 1H), 7.18–7.30 (m, 6H), 7.40 (t, J = 7.8 Hz, 2H), 7.54 (d, J = 7.8 Hz, 2H), 7.82 (d, J = 7.8 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ = 40.6 (t), 50.0 (d), 50.2 (s), 68.2 (t), 80.8 (s), 126.08 (d), 127.5 (d), 127.6 (d), 127.9 (d), 128.1 (d), 128.3 (d), 128.8 (d), 129.2 (d), 129.7(d), 130.2 (d), 130.4 (d), 131.1 (d), 134.3 (s), 138.3 (s), 147.4 (s), 148.1 (s), 181.4 (s). IR (neat): ν = 3665, 2980, 2905, 1763, 1488, 1448, 1375, 1160, 1028, 758 cm−1. HRMS (ESI-Q-TOF): m/z Calcd for C26H21O2 [M − H2O + H]+: 365.1542; Found: 365.1560.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srinivasan, R.; White, L.S.; Rossi, A.R.; Epling, G.A. Organic photochemistry with 6.7-eV photons: 1,4-cyclohexadiene and 1,4-cyclohexadiene-3,3,6,6-d4. J. Am. Chem. Soc. 1981, 103, 7299–7304. [Google Scholar] [CrossRef]
- Kumar, A.; Chowdhury, P.K.; Rama Rao, K.V.S.; Mittal, J.P. Real-time observation of concerted benzene formation in the infrared multiphoton dissociation of 1,4-cyclohexadiene. Chem. Phys. Lett. 1991, 182, 165–170. [Google Scholar] [CrossRef]
- Kumar, A.; Naik, P.D.; Saini, R.D.; Mittal, J.P. Direct evidence of a radical channel in photodissociation of 1,4-cyclohexadiene with ArF laser at 193 nm. Chem. Phys. Lett. 1999, 309, 191–197. [Google Scholar] [CrossRef]
- Zilberg, S.; Haas, Y. The photochemistry of 1,4-cyclohexadiene in solution and in the gas phase: Conical intersections and the origin of the “helicopter-type” motion of H2 photo-generated in the isolated molecule. Phys. Chem. Chem. Phys. 2002, 4, 34–42. [Google Scholar] [CrossRef]
- Hixson, S.S.; Mariano, P.S.; Zimmerman, H.E. Di-π-methane and oxa-di-π-methane rearrangements. Chem. Rev. 1973, 73, 531–551. [Google Scholar] [CrossRef]
- Zimmerman, H.E.; Armesto, D. Synthetic Aspects of the Di-π-methane Rearrangement. Chem. Rev. 1996, 96, 3065–3112. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.E. Di-π-Methane Rearrangement. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; Two Volume Set; CRC Press: Boca Raton, FL, USA, 2019; pp. 511–526. ISBN 9780429100253. [Google Scholar]
- Tröster, A.; Bach, T. Triplet-sensitised di-π-methane rearrangement of N-substituted 2-azabarrelenones. Chem. Commun. 2019, 55, 302–305. [Google Scholar] [CrossRef]
- Poplata, S.; Tröster, A.; Zou, Y.-Q.; Bach, T. Recent Advances in the Synthesis of Cyclobutanes by Olefin [2+2] Photocycloaddition Reactions. Chem. Rev. 2016, 116, 9748–9815. [Google Scholar] [CrossRef]
- Jennings, W.; Hill, B. Photodimerization of norbornadiene using chromium hexacarbonyl. J. Am. Chem. Soc. 1970, 92, 3199–3200. [Google Scholar] [CrossRef]
- Huang, D.-J.; Cheng, C.-H. [2 + 2] Dimerization of norbornadiene and its derivatives in the presence of nickel complexes and zinc metal. J. Organomet. Chem. 1995, 490, C1–C7. [Google Scholar] [CrossRef]
- Malik, C.K.; Vaultier, M.; Ghosh, S. Copper(I)-catalyzed [2 + 2] photocycloaddition of nonconjugated alkenes in room-temperature ionic liquids. Synthesis 2007, 1247–1250. [Google Scholar] [CrossRef]
- Linker, T.; Fröhlich, L. Regio- and Diastereoselective Photooxygenation of Chiral 2,5-Cyclohexadiene-l-carboxylic Acids. Angew. Chem. Int. Ed. Engl. 1994, 33, 1971–1972. [Google Scholar] [CrossRef]
- Vorndran, K.; Linker, T. Simple two-step ipso substitution of aromatic carboxylic acids by alkyl halides. Angew. Chem. Int. Ed. Engl. 2003, 42, 2489–2491. [Google Scholar] [CrossRef] [PubMed]
- Krüger, T.; Vorndran, K.; Linker, T. Regioselective arene functionalization: Simple substitution of carboxylate by alkyl groups. Chem.-A Eur. J. 2009, 15, 12082–12091. [Google Scholar] [CrossRef]
- Krüger, T.; Linker, T. Synthesis of γ-Spirolactams by Birch Reduction of Arenes. Eur. J. Org. Chem. 2021, 1585–1591. [Google Scholar] [CrossRef]
- Krüger, T.; Bramborg, A.; Kelling, A.; Sperlich, E.; Linker, T. Birch Reduction of Arenes as an Easy Entry to γ-Spirolactones. Eur. J. Org. Chem. 2021, 6383–6391. [Google Scholar] [CrossRef]
- Martin, H.-D.; Mayer, B. Proximity Effects in Organic Chemistry—The Photoelectron Spectroscopic Investigation of Non-Bonding and Transannular Interactions. Angew. Chem. Int. Ed. Engl. 1983, 22, 283–314. [Google Scholar] [CrossRef]
- Czarnecki, M.; Wessig, P. Scaling Up UV-Mediated Intramolecular Photodehydro-Diels–Alder Reactions Using a Homemade High-Performance Annular Continuous-Flow Reactor. Org. Process Res. Dev. 2018, 22, 1823–1827. [Google Scholar] [CrossRef]
- Zimmerman, H.E. Oxa-Di-π-Methane Rearrangement. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; Two Volume Set; CRC Press: Boca Raton, FL, USA, 2019; pp. 527–548. ISBN 9780429100253. [Google Scholar]
- Swenton, J.S.; Madigani, D.M. Competitive hydrogen and carbomethoxy migration in the photochemistry of 7-carbomethoxy-3,4-benzotropilidene. Tetrahedron 1972, 28, 2703–2716. [Google Scholar] [CrossRef]
- Becker, H.G.O. Einführung in Die Photochemie; Deutscher Verlag der Wissenschaften: Berlin, Germany, 1991; ISBN 3136337026. [Google Scholar]
- Fréneau, M.; Hoffmann, N. The Paternò-Büchi reaction—Mechanisms and application to organic synthesis. J. Photochem. Photobiol. C Photochem. Rev. 2017, 33, 83–108. [Google Scholar] [CrossRef]
- D’Auria, M. Paternó-Büchi Reaction. In CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; Two Volume Set; CRC Press: Boca Raton, FL, USA, 2019; pp. 653–681. [Google Scholar]
- D’Auria, M. The Paternò-Büchi reaction—A comprehensive review. Photochem. Photobiol. Sci. 2019, 18, 2297–2362. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.S. Ultraviolet Irradiation of Carbonyl Compounds in Cyclohexene and 1-Hexene. J. Org. Chem. 1966, 31, 237–240. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Stadtmüller, S. Photocycloaddition of Benzaldehyde to Cyclic Olefins: Electronic Control of Endo Stereoselectivity. J. Am. Chem. Soc. 1990, 112, 1281–1283. [Google Scholar] [CrossRef]
- Andreu, I.; Neshchadin, D.; Rico, E.; Griesser, M.; Samadi, A.; Morera, I.M.; Gescheidt, G.; Miranda, M.A. Probing lipid peroxidation by using linoleic acid and benzophenone. Chem.—Eur. J. 2011, 17, 10089–10096. [Google Scholar] [CrossRef]
- Zipse, H. Radical Stability—A Theoretical Perspective. In Radicals in Synthesis I; Gansäuer, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 163–189. ISBN 978-3-540-31330-4. [Google Scholar]
- Yu, H.S.; He, X.; Li, S.L.; Truhlar, D.G. MN15: A Kohn-Sham Global-Hybrid Exchange-Correlation Density Functional with Broad Accuracy for Multi-Reference and Single-Reference Systems and Noncovalent Interactions. Chem. Sci. 2016, 7, 5032–5051. [Google Scholar] [CrossRef] [PubMed]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-Row Atoms. J. Comp. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845. [Google Scholar] [CrossRef]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 14334–14356. [Google Scholar] [CrossRef]
- STOE & Cie GmbH, X-Area. Software Package for Collecting Single-Crystal Data on STOE Area-Detector Diffractometers, for Image Processing, for the Correction and Scaling of Reflection Intensities and for Outlier Rejection; STOE & Cie GmbH: Darmstadt, Germany, 2018. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Cryst. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Cryst. C 2015, 71, 3. [Google Scholar] [CrossRef]
- Kratzert, D. FinalCif. Available online: https://dkratzert.de/finalcif.html (accessed on 23 September 2023).
- Brandenburg, K.; Putz, H. Diamond. Crystal and Molecular Structure Visualization; Crystal Impact: Bonn, Germany, 2020. [Google Scholar]
















| Entry | Solvent | Light Source | Time (h) | Conv. (%) 2 | Additive | Product (%) 3 |
|---|---|---|---|---|---|---|
| 1 | MTBE | UV-B | 6 | <5 | – | – |
| 2 | MTBE | UV-B | 24 | 43 | – | 12a (3) |
| 3 | MTBE | UV-B | 24 | 87 | O2 4 | 12a (8) |
| 4 | MTBE | – | 24 | <5 | O2 4 | – |
| 5 | MTBE | UV-B | 24 | <5 | – 5 | – |
| 6 | MTBE | Rayonet | 6 | 10 | – | – |
| 7 | MTBE | Rayonet | 24 | 44 | – | 13a (6) |
| 8 | MTBE | Rayonet | 120 | 86 | – | 13a (5) |
| 9 | MTBE | Rayonet | 24 | 42 | Cu(i)OTf 6 | – |
| 10 | MTBE | UV-C 7 | 6 | 58 | – | – |
| 11 | CH3CN | UV-C 7 | 12 | 90 | – | 13a (10) |
| 12 | acetone | UV-C 7 | 12 | 12 | – | – |
| 13 | CH3CN | UV-B | 12 | <5 | Xanthone | – |
| 14 | CH3CN | UV-C 7 | 12 | 88 | Isoprene | 13a (9) |
| 15 | CH3CN 8 | UV-C 7 | 5 | 93 | – | 13a (14) |
| 16 | CH3CN | UV-C 7 | 3 | 41 | – | 13a (18) 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fudickar, W.; Metz, M.; Krüger-Braunert, T.; Kelling, A.; Sperlich, E.; Wessig, P.; Linker, T. Photochemistry of β-γ-Unsaturated Spirolactones. Photochem 2023, 3, 408-426. https://doi.org/10.3390/photochem3040025
Fudickar W, Metz M, Krüger-Braunert T, Kelling A, Sperlich E, Wessig P, Linker T. Photochemistry of β-γ-Unsaturated Spirolactones. Photochem. 2023; 3(4):408-426. https://doi.org/10.3390/photochem3040025
Chicago/Turabian StyleFudickar, Werner, Melanie Metz, Tobias Krüger-Braunert, Alexandra Kelling, Eric Sperlich, Pablo Wessig, and Torsten Linker. 2023. "Photochemistry of β-γ-Unsaturated Spirolactones" Photochem 3, no. 4: 408-426. https://doi.org/10.3390/photochem3040025