Photocatalytic Reduction of CO2 over Iron-Modified g-C3N4 Photocatalysts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Characterization
2.3. Photocatalytic Test
3. Results and Discussion
3.1. Crystalline Structure
3.2. Surface Area and Porosity
3.3. Chemical Composition
3.4. Optical Characteristics
3.5. Mössbauer and EPR Spectroscopy
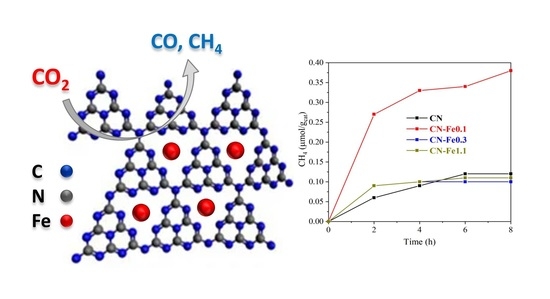
3.6. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- European Commission. EU Energy in Figures-Statistical Pocket Book 2019; Drukarnia INTERAK SP ZOO: Czarnków, Poland, 2019. [Google Scholar]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Garlisi, C.; Pagliaro, M.; Al-Ali, K.; Palmisano, G. Metal-organic frameworks for photocatalytic CO2 reduction under visible radiation: A review of strategies and applications. Catal. Today 2020, 340, 209–224. [Google Scholar] [CrossRef]
- Yuan, L.; Xu, Y.J. Photocatalytic conversion of CO2 into value-added and renewable fuels. Appl. Surf. Sci. 2015, 342, 154–167. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew. Chem. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Nikokavoura, A.; Trapalis, C. Alternative photocatalysts to TiO2 for the photocatalytic reduction of CO2. Appl. Surf. Sci. 2017, 391, 149–174. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Akhter, P.; Farkhondehfal, M.A.; Hernández, S.; Hussain, M.; Fina, A.; Saracco, G.; Khan, A.U.; Russo, N. Environmental issues regarding CO2 and recent strategies for alternative fuels through photocatalytic reduction with titania-based materials. J. Environ. Chem. Eng. 2016, 4, 3934–3953. [Google Scholar] [CrossRef]
- Kočí, K.; Van, H.D.; Edelmannová, M.; Reli, M.; Wu, J.C.S. Photocatalytic reduction of CO2 using Pt/C3N4 photocatalyts. Appl. Surf. Sci. 2020, 503, 144426. [Google Scholar] [CrossRef]
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloys Compd. 2019, 802, 196–209. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Chem. Int. Ed. Engl. 2015, 54, 11350–11366. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Effect of phosphorus doping on electronic structure and photocatalytic performance of g-C3N4: Insights from hybrid density functional calculation. J. Alloys Compd. 2016, 672, 271–276. [Google Scholar] [CrossRef]
- Wu, H.Z.; Liu, L.M.; Zhao, S.J. The effect of water on the structural, electronic and photocatalytic properties of graphitic carbon nitride. Phys. Chem. Chem. Phys. 2014, 16, 3299–3304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Guo, Y.; Chen, Z.; Zhang, Z.; Fang, X. Constructing a novel ternary Fe(III)/graphene/g-C3N4 composite photocatalyst with enhanced visible-light driven photocatalytic activity via interfacial charge transfer effect. Appl. Catal. B 2016, 183, 231–241. [Google Scholar] [CrossRef]
- Prasad, C.; Tang, H.; Bahadur, I. Graphitic carbon nitride based ternary nanocomposites: From synthesis to their applications in photocatalysis: A recent review. J. Mol. Liq. 2019, 281, 634–654. [Google Scholar] [CrossRef]
- Xiao, M.; Luo, B.; Wang, S.; Wang, L. Solar energy conversion on g-C3N4 photocatalyst: Light harvesting, charge separation, and surface kinetics. J. Energy Chem. 2018, 27, 1111–1123. [Google Scholar] [CrossRef] [Green Version]
- Praus, P.; Svoboda, L.; Dvorský, R.; Reli, M.; Kormunda, M.; Mančík, P. Synthesis and properties of nanocomposites of WO3 and exfoliated g-C3N4. Ceram. Int. 2017, 43, 13581–13591. [Google Scholar] [CrossRef]
- Todorova, N.; Papailias, I.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Dallas, P.; Edelmannová, M.; Reli, M.; Kočí, K.; Trapalis, C. Photocatalytic H2 Evolution, CO2 Reduction, and NOx Oxidation by Highly Exfoliated g-C3N4. Catalysts 2020, 10, 1147. [Google Scholar] [CrossRef]
- Mohamed, N.A.; Safaei, J.; Ismail, A.F.; Noh, M.F.M.; Arzaee, N.A.; Mansor, N.N.; Ibrahim, M.A.; Ludin, N.A.; Sagu, J.S.; Teridi, M.A.M. Fabrication of exfoliated graphitic carbon nitride, (g-C3N4) thin film by methanolic dispersion. J. Alloys Compd. 2020, 818, 152916. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Boukos, N.; Athanasekou, C.P.; Dimotikali, D.; Trapalis, C. Chemical vs thermal exfoliation of g-C3N4 for NOx removal under visible light irradiation. Appl. Catal. B 2018, 239, 16–26. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Masih, D.; Ma, Y.; Rohani, S. Graphitic C3N4 based noble-metal-free photocatalyst systems: A review. Appl. Catal. B 2017, 206, 556–588. [Google Scholar] [CrossRef]
- Heidarpour, H.; Padervand, M.; Soltanieh, M.; Vossoughi, M. Enhanced decolorization of rhodamine B solution through simultaneous photocatalysis and persulfate activation over Fe/C3N4 photocatalyst. Chem. Eng. Res. Des. 2020, 153, 709–720. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, P.; Cui, J.; An, W.; Liu, L.; Liang, Y.; Yang, Q.; Yang, H.; Cui, W. High-efficiency removal of phenol and coking wastewater via photocatalysis-Fenton synergy over a Fe-g-C3N4 graphene hydrogel 3D structure. J. Ind. Eng. Chem. 2020, 84, 305–314. [Google Scholar] [CrossRef]
- Ma, T.; Shen, Q.; Xue, B.Z.J.; Guan, R.; Liu, X.; Jia, H.; Xu, B. Facile synthesis of Fe-doped g-C3N4 for enhanced visible-light photocatalytic activity. Inorg. Chem. Commun. 2019, 107, 107451. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wu, Z.; Wang, L. g-C3N4 based composite photocatalysts for photocatalytic CO2 reduction. Catal. Today 2018, 300, 160–172. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef]
- Ge, L. Synthesis and photocatalytic performance of novel metal-free g-C3N4 photocatalysts. Mater. Lett. 2011, 65, 2652–2654. [Google Scholar] [CrossRef]
- Baudys, M.; Paušová, Š.; Praus, P.; Brezová, V.; Dvoranová, D.; Barbieriková, Z.; Krýsa, J. Graphitic Carbon Nitride for Photocatalytic Air Treatment. Materials 2020, 13, 3038. [Google Scholar] [CrossRef] [PubMed]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Yan, H.; Chen, Y.; Xu, S. Synthesis of graphitic carbon nitride by directly heating sulfuric acid treated melamine for enhanced photocatalytic H2 production from water under visible light. Int. J. Hydrogen Energy 2012, 37, 125–133. [Google Scholar] [CrossRef]
- Ho, W.; Zhang, Z.; Xu, M.; Zhang, X.; Wang, X.; Huang, Y. Enhanced visible-light-driven photocatalytic removal of NO: Effect on layer distortion on g-C3N4 by H2 heating. Appl. Catal. B 2015, 179, 106–112. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, X.; Jia, C.; Zhou, T.; Qu, X.; Jian, J.; Xu, Y.; Zhou, T. A facile mechanochemical way to prepare g-C3N4. Mater. Sci. Eng. B 2005, 122, 90–93. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y.; Zhao, H.; Wang, H. Fabrication of atomic single layer graphitic-C3N4 and its high performance of photocatalytic disinfection under visible light irradiation. Appl. Catal. B 2014, 153, 46–50. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Ioannidis, N.; Dallas, P.; Dimotikali, D.; Trapalis, C. Novel torus shaped g-C3N4 photocatalysts. Appl. Catal. B 2020, 268, 118733. [Google Scholar] [CrossRef]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Karapati, S.; Boukos, N.; Dimotikali, D.; Trapalis, C. Enhanced NO2 abatement by alkaline-earth modified g-C3N4 nanocomposites for efficient air purification. Appl. Surf. Sci. 2018, 430, 225–233. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Luo, Q.; Dong, F.; Li, H.; Ho, W.K. Mass-Controlled Direct Synthesis of Graphene-like Carbon Nitride Nanosheets with Exceptional High Visible Light Activity. Less is Better. Sci. Rep. 2015, 5, 14643. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Q.; Wang, W. Construction of a crossed-layer-structure MoS2/g-C3N4 heterojunction with enhanced photocatalytic performance. RSC Adv. 2017, 7, 6131–6139. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, H.; Liu, P.; Wang, D.; Li, Y.; Zhao, H. Cross-Linked g-C3N4/rGO Nanocomposites with Tunable Band Structure and Enhanced Visible Light Photocatalytic Activity. Small 2013, 9, 3336–3344. [Google Scholar] [CrossRef] [Green Version]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite hydroxyapatite/TiO2 materials for photocatalytic oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]
- Hu, S.; Jin, R.; Lu, G.; Liu, D.; Gui, J. The properties and photocatalytic performance comparison of Fe3+-doped g-C3N4 and Fe2O3/g-C3N4 composite catalysts. RSC Adv. 2014, 4, 24863–24869. [Google Scholar] [CrossRef]
- Oliveira, A.A.S.; Teixeira, I.F.; Ribeiro, L.P.; Lorençon, E.; Ardisson, J.D.; Fernandez-Outon, L.; Macedo, W.A.A.; Moura, F.C.C. Magnetic amphiphilic nanocomposites produced via chemical vapor deposition of CH4 on Fe-Mo/nano-Al2O3. Appl. Catal. A 2013, 456, 126–134. [Google Scholar] [CrossRef]
- Cunha, I.T.; Teixeira, I.F.; Albuquerque, A.S.; Ardisson, J.D.; Macedo, W.A.A.; Oliveira, H.S.; Tristão, J.C.; Sapag, K.; Lago, R.M. Catalytic oxidation of aqueous sulfide in the presence of ferrites (MFe2O4, M=Fe, Cu, Co). Catal. Today 2016, 259, 222–227. [Google Scholar] [CrossRef]
- Cuello, N.I.; Elías, V.R.; Winkler, E.; Pozo-López, G.; Oliva, M.I.; Eimer, G.A. Magnetic behavior of iron-modified MCM-41 correlated with clustering processes from the wet impregnation method. J. Magn. Magn. Mater. 2016, 407, 299–307. [Google Scholar] [CrossRef]
- Wang, N.; Han, Z.; Fan, H.; Ai, S. Copper nanoparticles modified graphitic carbon nitride nanosheets as a peroxidase mimetic for glucose detection. RSC Adv. 2015, 5, 91302–91307. [Google Scholar] [CrossRef]
- Li, Z.; Kong, C.; Lu, G. Visible Photocatalytic Water Splitting and Photocatalytic Two-Electron Oxygen Formation over Cu- and Fe-Doped g-C3N4. J. Phys. Chem. C 2016, 120, 56–63. [Google Scholar] [CrossRef]
- Bicalho, H.A.; Lopez, J.L.; Binatti, I.; Batista, P.F.R.; Ardisson, J.D.; Resende, R.R.; Lorençon, E. Facile synthesis of highly dispersed Fe(II)-doped g-C3N4 and its application in Fenton-like catalysis. Mol. Catal. 2017, 435, 156–165. [Google Scholar] [CrossRef]
- Guzmán, H.; Farkhondehfal, M.A.; Tolod, K.R.; Hernández, S.; Russo, N. Chapter 11-Photo/electrocatalytic hydrogen exploitation for CO2 reduction toward solar fuels production. In Solar Hydrogen Production; Calise, F., D’Accadia, M.D., Santarelli, M., Lanzini, A., Ferrero, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 365–418. [Google Scholar]
- Choi, W. Pure and modified TiO2 photocatalysts and their environmental applications. Catal. Surv. Asia 2006, 10, 16–28. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Viswanathan, B. Energy Sources: Fundamentals of Chemical Conversion Processes and Applications; Elsevier Science: Amsterdam, Netherlands, 2016. [Google Scholar]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef] [PubMed]
- Di, L.; Yang, H.; Xian, T.; Chen, X. Enhanced Photocatalytic Degradation Activity of BiFeO3 Microspheres by Decoration with g-C3N4 Nanoparticles. Mater. Res. 2018, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Matos, J.; Arcibar-Orozco, J.; Poon, P.S.; Pecchi, G.; Rangel-Mendez, J.R. Influence of phosphorous upon the formation of DMPO-•OH and POBN-O2•− spin-trapping adducts in carbon-supported P-promoted Fe-based photocatalysts. J. Photochem. Photobiol. A 2020, 391, 112362. [Google Scholar] [CrossRef]
- Matos, J.; Poon, P.S.; Montaña, R.; Romero, R.; Gonçalves, G.R.; Schettino, M.A., Jr.; Passamani, E.C.; Freitas, J.C.C. Photocatalytic activity of P-Fe/activated carbon nanocomposites under artificial solar irradiation. Catal. Today 2020, 356, 226–240. [Google Scholar] [CrossRef]
- Matos, J.; Rosales, M.; Garcia, A.; Nieto-Delgado, C.; Rangel-Mendez, J.R. Hybrid photoactive materials from municipal sewage sludge for the photocatalytic degradation of methylene blue. Green Chem. 2011, 13, 3431–3439. [Google Scholar] [CrossRef]
- Casado, J.; Fornaguera, J. Pilot-Scale Degradation of Organic Contaminants in a Continuous-flow Reactor by the Helielectro-Fenton Method. Clean 2008, 36, 53–58. [Google Scholar] [CrossRef]
- Sayama, K.; Yoshida, R.; Kusama, H.; Okabe, K.; Abe, Y.; Arakawa, H. Photocatalytic decomposition of water into H2 and O2 by a two-step photoexcitation reaction using a WO3 suspension catalyst and an Fe3+/Fe2+ redox system. Chem. Phys. Lett. 1997, 277, 387–391. [Google Scholar] [CrossRef]
- Khaing, K.K.; Yin, D.; Xiao, S.; Deng, L.; Zhao, F.; Liu, B.; Chen, T.; Li, L.; Guo, X.; Liu, J.; et al. Efficient Solar Light Driven Degradation of Tetracycline by Fe-EDTA Modified g-C3N4 Nanosheets. J. Phys. Chem. C 2020, 124, 11831–11843. [Google Scholar] [CrossRef]









| Sample | BET Surface Area (m2/g) | Average Pore Size (nm) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| CN | 16 | 13.7 | 0.11 |
| CN-Fe0.1 | 13 | 19 | 0.12 |
| CN-Fe0.3 | 11 | 18.7 | 0.11 |
| CN-Fe1.1 | 7 | 38.5 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelmannová, M.; Reli, M.; Kočí, K.; Papailias, I.; Todorova, N.; Giannakopoulou, T.; Dallas, P.; Devlin, E.; Ioannidis, N.; Trapalis, C. Photocatalytic Reduction of CO2 over Iron-Modified g-C3N4 Photocatalysts. Photochem 2021, 1, 462-476. https://doi.org/10.3390/photochem1030030
Edelmannová M, Reli M, Kočí K, Papailias I, Todorova N, Giannakopoulou T, Dallas P, Devlin E, Ioannidis N, Trapalis C. Photocatalytic Reduction of CO2 over Iron-Modified g-C3N4 Photocatalysts. Photochem. 2021; 1(3):462-476. https://doi.org/10.3390/photochem1030030
Chicago/Turabian StyleEdelmannová, Miroslava, Martin Reli, Kamila Kočí, Ilias Papailias, Nadia Todorova, Tatiana Giannakopoulou, Panagiotis Dallas, Eamonn Devlin, Nikolaos Ioannidis, and Christos Trapalis. 2021. "Photocatalytic Reduction of CO2 over Iron-Modified g-C3N4 Photocatalysts" Photochem 1, no. 3: 462-476. https://doi.org/10.3390/photochem1030030