Evaluation of Enamel Volume Loss after Exposure to Energy Drinks
Abstract
:1. Introduction
2. Materials and Methods
3. Evaluation of Volume Loss
3.1. Sample Preparation
3.2. Scanning
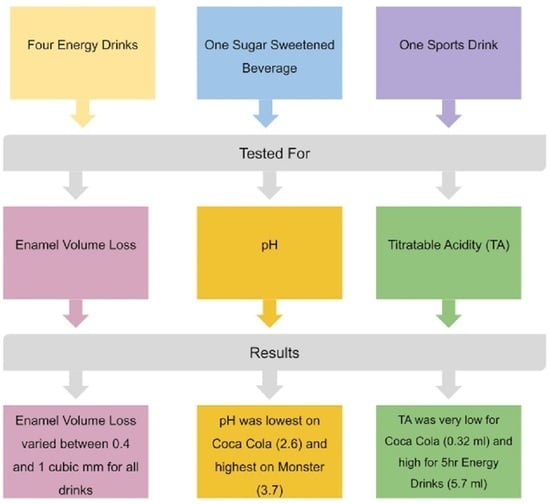
3.3. Drink Selection
3.4. Volume Loss Test
3.5. Evaluation of pH
3.6. Evaluation of Titratable Acidity
4. Results
4.1. Volume Loss Evaluation
4.2. Evaluation of pH
4.3. Evaluation of Titratable Acidity
4.4. Correlation Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, J.G.; Martins, J.P.; de Sousa, E.B.; Fernandes, N.L.; Meira, I.A.; Sampaio, F.C.; de Oliveira, A.F.; Pereira, A.M. Influence of energy drinks on enamel erosion: In vitro study using different assessment techniques. J. Clin. Exp. Dent. 2021, 11, e1076–e1082. [Google Scholar] [CrossRef]
- Ali, F.; Rehman, H.; Babayan, Z.; Stapleton, D.; Joshi, D.D. Energy drinks and their adverse health effects: A systematic review of the current evidence. Postgrad. Med. 2015, 127, 308–322. [Google Scholar] [CrossRef]
- Alsunni, A.A. Energy drink consumption: Beneficial and adverse health effects. Int. J. Health Sci. 2015, 4, 468–474. [Google Scholar] [CrossRef]
- Statista. Available online: https://www.statista.com/topics/10313/energy-drinks-worldwide/#topicOverview (accessed on 30 November 2023).
- Bevsource. Available online: https://www.bevsource.com/news/examining-the-9-latest-trends-in-energy-drinks (accessed on 30 November 2023).
- Oregon Consulting Group. Available online: https://business.uoregon.edu/sites/default/files/media/energy-drink-industry-report.pdf (accessed on 30 November 2023).
- Investopedia. Available online: https://www.investopedia.com/articles/investing/022315/energy-drinks-industry.asp (accessed on 30 November 2023).
- Healthy Food America. Available online: https://www.healthyfoodamerica.org/sugartoolkit_kahuna (accessed on 30 November 2023).
- Higgins, J.P.; Babu, K.; Deuster, P.A.; Shearer, J. Energy drinks: A contemporary issues paper. Curr. Sports Med. Rep. 2018, 2, 65–72. [Google Scholar] [CrossRef]
- Kumar, G.; Park, S.; Onufrak, S. Perceptions about energy drinks are associated with energy drink intake among US youth. Am. J. Health Promot. 2015, 4, 238–244. [Google Scholar] [CrossRef]
- Clapp, O.; Morgan, M.Z.; Fairchild, R.M. The top five selling UK energy drinks: Implications for dental and general health. Br. Dent. J. 2019, 7, 493–497. [Google Scholar] [CrossRef]
- Higgins, J.P.; Tuttle, T.D.; Higgins, C.L. Energy beverages: Content and safety. Mayo Clin. Proc. 2010, 11, 1033–1041. [Google Scholar] [CrossRef]
- Seifert, S.M.; Seifer, S.A.; Schaechter, J.L.; Bronstein, A.C.; Benson, B.E.; Hershorin, E.R.; Arheart, K.L.; Franco, V.I.; Lipshultz, S.E. An analysis of energy drink toxicity in the National Poison Data System. Clin. Toxicol. 2013, 7, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.A.; Cotter, B.V.; Merchant, R.C.; Babu, K.M.; Baird, J.R.; Nirenberg, T. Behavioral and physiologic adverse effects in adolescent and young adult emergency department patients reporting use of energy drinks and caffeine. Clin. Toxicol. 2013, 7, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Occiano, A.; Nguyen, T.A.; Chan, A.; Sky, J.C.; Battacharya, M.; O’Dell, K.M.; Shek, A.; Nguyen, N.N. Electrocardiographic and blood pressure effects of energy drinks and Panax ginseng in healthy volunteers: A randomized clinical trial. Int. J. Cardiol. 2016, 218, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kaminer, Y. Problematic use of energy drinks by adolescents. Child Adolesc. Psychiatr. Clin. N. Am. 2010, 19, 643–650. [Google Scholar] [CrossRef]
- Duchan, E.; Patel, N.D.; Feucht, C. Energy drinks: A review of use and safety for athletes. Physician Sports Med. 2010, 38, 171–179. [Google Scholar] [CrossRef]
- Chrysant, S.G.; Chrysant, G.S. Cardiovascular complications from consumption of high energy drinks: Recent evidence. J. Hum. Hypertens. 2015, 29, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, C.; Waigandt, A.; Beck, N.C. Athletes and energy drinks: Reported risk-taking and consequences from the combined use of alcohol and energy drinks. J. Appl. Sports Psychol. 2010, 22, 65–71. [Google Scholar] [CrossRef]
- Monsterenergy. Available online: https://www.monsterenergy.com/us/en/products/monster-energy/monster-energy (accessed on 30 November 2023).
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health effects of energy drinks on children and adolescents and young adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Trapp, G.S.; Allen, K.L.; O’Sullivan, T.; Robinson, M.; Jacoby, P.; Oddy, W.H. Energy drink consumption among young Australian adults: Associations with alcohol and illicit drug use. Drug Alcohol Depend. 2014, 134, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bonar, E.E.; Cunningham, R.M.; Polshkova, S.; Chermack, S.T.; Blow, F.C.; Walton, W.A. Alcohol and energy use among adolescents seeking emergency department care. Addict. Behav. 2015, 43, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Bigard, A.X. Risks of energy drinks in youths. Arch. Pediatr. 2010, 17, 1624–1631. [Google Scholar]
- Marczinski, C.A. Can energy drinks increase the desire for more alcohol? Adv. Nutr. 2014, 6, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Jaeggi, T.; Lussi, A. Prevalence incidence and distribution of erosion. Monogr. Oral Sci. 2014, 25, 55–73. [Google Scholar]
- Ganss, C. Definition of erosion and links to tooth wear. Monogr. Oral Sci. 2006, 20, 9–16. [Google Scholar] [PubMed]
- Moazzez, R.; Bartlett, D. Intrinsic causes of erosion. Monogr. Oral Sci. 2014, 25, 180–196. [Google Scholar]
- Barbour, M.E.; Lussi, A. Erosion in relation to nutrition and the environment. Monogr. Oral Sci. 2014, 25, 143–154. [Google Scholar] [PubMed]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental erosion—An overview with emphasis in chemical and histopathological aspects. Caries Res. 2011, 45 (Suppl. SI), 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, M.; Owens, B.M. Effect of carbonated beverages, coffee, sports and high energy drinks, and bottled water on the in vitro erosion characteristics of dental enamel. J. Clin. Pediatr. Dent. 2007, 31, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.; Duncan, J.F.; Cutress, T.W. A mechanism for dental caries based on chemical processes and diffusion phenomena during in vitro caries simulation on human tooth enamel. Arch. Oral Biol. 1979, 24, 101–112. [Google Scholar] [CrossRef]
- Lussi, A.; Jaeggi, T.; Schaerer, S. The influence of different factors on in vitro enamel erosion. Caries Res. 1993, 27, 387–393. [Google Scholar] [CrossRef]
- Huysmans, M.C.; Chew, H.P.; Ellwood, R.P. Clinical studies of dental erosion and erosive wear. Caries Res. 2011, 45 (Suppl. S1), 60–68. [Google Scholar] [CrossRef]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization-remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- Attin, T.; Wegehaupt, F.J. Methods for assessment of dental erosion. Monogr. Oral Sci. 2014, 25, 123–142. [Google Scholar]
- Vaidya, N.; Kumar, P.; Pathak, K.; Punia, S.K.; Choudhary, A.; Patnana, A.K. Comparative Evaluation of the Influence of Different Sports/Energy Drinks and Alcoholic Beverages on the Surface Roughness of Three Different Flowable Esthetic Restorative Materials: An In Vitro Analysis. J. Int. Soc. Prev. Community Dent. 2020, 5, 585–590. [Google Scholar]
- Featherstone, J.D.; Rogers, B.E. Effects of acetic, lactic and other organic acids on the formation of artificial caries lesions. Caries Res. 1981, 15, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.D.; Lussi, A. Understanding the chemistry of dental erosion. Monogr. Oral Sci. 2006, 20, 66–76. [Google Scholar] [PubMed]
- Shellis, R.P.; Barbour, M.E.; Jones, S.B.; Addy, M. Effects of pH and acid concentration on erosive dissolution of enamel, dentine, and compressed hydroxyapatite. Eur. J. Oral Sci. 2010, 118, 475–482. [Google Scholar] [CrossRef]
- Meurman, J.H.; Ten Cate, J.M. Pathogenesis and modifying factors of dental erosion. Eur. J. Oral Sci. 1997, 104, 199–206. [Google Scholar] [CrossRef]
- Mullin, F.; Austin, R.S.; Parkinson, C.R.; Bartlett, D.W. An in situ study to investigate the native clinical resistance of enamel to erosion. J. Dent. 2018, 70, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Jager, D.H.; Vieira, A.M.; Ruben, J.L.; Huysmans, M.C. Estimated erosive potential depends on exposure time. J. Dent. 2012, 40, 1103–1108. [Google Scholar] [CrossRef]
- Eisenburger, M.; Addy, M. Evaluation of pH and erosion time on demineralization. Clin. Oral Investig. 2001, 5, 108–111. [Google Scholar]
- von Fraunhofer, J.A.; Rogers, M.M. Effects of sports drinks and other beverages on dental enamel. Gen. Dent. 2005, 53, 28–31. [Google Scholar]
- Johansson, A.K.; Lingström, P.; Imfeld, T.; Birkhed, D. Influence of drinking method on tooth-surface pH in relation to dental erosion. Eur. J. Oral Sci. 2004, 112, 484–489. [Google Scholar] [CrossRef]
- Lussi, A.; Hellwig, E. Risk assessment and preventive measures. Monogr. Oral Sci. 2006, 20, 190–199. [Google Scholar]
- Barbour, M.E.; Finke, M.; Parker, D.M.; Hughes, J.A.; Allen, G.C.; Addy, M. The relationship between enamel softening and erosion caused by soft drinks at a range of temperatures. J. Dent. 2006, 34, 207–213. [Google Scholar] [CrossRef]
- Eisenburger, M.; Addy, M. Influence of liquid temperature and flow rate on enamel erosion and surface softening. J. Oral Rehabil. 2003, 30, 1076–1080. [Google Scholar] [CrossRef]
- Hannig, M.; Fiebiger, M.; Güntzer, M.; Döbert, A.; Zimehl, R.; Nekrashevych, Y. Protective effect of the in situ formed short-term salivary pellicle. Arch. Oral Biol. 2004, 49, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Vukosavljevic, D.; Custodio, W.; Buzalaf, M.A.; Hara, A.T.; Siqueira, W.L. Acquired pellicle as a modulator for dental erosion. Arch. Oral Biol. 2014, 59, 631–638. [Google Scholar] [CrossRef]
- Hara, A.T.; Zero, D.T. The potential of saliva in protecting against dental erosion. Monogr. Oral Sci. 2014, 25, 197–205. [Google Scholar]
- Ganss, C.; Lussi, A.; Schlueter, N. The histological features and physical properties of eroded dental hard tissues. Monogr. Oral Sci. 2014, 25, 99–107. [Google Scholar]
- Lussi, A.; Carvalho, T.S. Erosive tooth wear: A multifactorial condition of growing concern and Increasing Knowledge. Monogr. Oral Sci. 2014, 25, 1–15. [Google Scholar]
- Lussi, A.; Jaeggi, T. Erosion-diagnosis and risk factors. Clin. Oral Investig. 2008, 12 (Suppl. S1), S5–S13. [Google Scholar] [CrossRef]
- Søvik, J.B.; Skudutyte-Rysstad, R.; Tveit, A.B.; Sandvik, L.; Mulic, A. Sour Sweets and Acidic Beverage Consumption Are Risk Indicators for Dental Erosion. Caries Res. 2015, 49, 243–250. [Google Scholar] [CrossRef]
- West, N.X.; He, T.; Zou, Y.; Biesbrock, A.; Davies, M. Bioavailable gluconate chelated stannous fluoride toothpaste meta-analysis: Effects on dentin hypersensitivity and enamel erosion. J. Dent. 2021, 105, 103566. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, K.A.; Santucci, N.M.; Surti, B.; Habelitz, S.; Bhattacharyya, M.; Noble, W. Evaluation of Enamel Volume Loss after Exposure to Energy Drinks. Oral 2024, 4, 101-112. https://doi.org/10.3390/oral4010009
Schulze KA, Santucci NM, Surti B, Habelitz S, Bhattacharyya M, Noble W. Evaluation of Enamel Volume Loss after Exposure to Energy Drinks. Oral. 2024; 4(1):101-112. https://doi.org/10.3390/oral4010009
Chicago/Turabian StyleSchulze, Karen A., Noëlle M. Santucci, Bina Surti, Stefan Habelitz, Mouchumi Bhattacharyya, and Warden Noble. 2024. "Evaluation of Enamel Volume Loss after Exposure to Energy Drinks" Oral 4, no. 1: 101-112. https://doi.org/10.3390/oral4010009