Fabrication and Characterization of Free-Standing and Flexible Polyaniline Membranes: Role of Graphene Nanoscrolls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Conversion of Graphene Nanoplatelets (GNPs) to Graphene Nanoscrolls (GNS)
2.3. PANI-GNS Flexible Film Preparation
2.4. Immobilization of Glucose Oxidase on Flexible Electrode
2.5. Characterization
2.5.1. Scanning Electron Microscopy: Morphological Analysis
2.5.2. Fourier Transform Infrared Spectroscopy: Chemical Analysis
2.5.3. Nanoindentation: Modulus and Hardness Studies
2.5.4. Four-Point Probe Technique: Resistivity and Conductivity Studies
2.5.5. X-ray Diffraction
3. Results and Discussion
3.1. Preparation of Flexible Electrode
3.2. Spectroscopic Analysis
3.3. Morphological Analysis
3.4. Mechanical Analysis
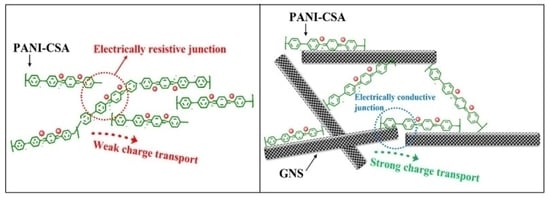
3.5. Conductivity Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kushwaha, C.S.; Singh, P.; Shukla, S.K.; Chemimi, M. Advances in conducting polymers nanocomposites based chemical sensors: An overview. Mater. Sci. Eng. B 2022, 284, 115856. [Google Scholar] [CrossRef]
- Aydemir, N.; Malmström, J.; Travas-Sejdic, J. Conducting Polymer Based Electrochemical Biosensors. Phys. Chem. Chem. Phys. 2016, 18, 8264–8277. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-Based Glucose Biosensor: A Review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Gao, F.; Mu, J.; Bi, Z.; Wang, S.; Li, Z. Recent Advances of Polyaniline Composites in Anticorrosive Coatings: A Review. Prog. Org. Coat. 2021, 151, 106071. [Google Scholar] [CrossRef]
- Lv, D.; Shen, W.; Chen, W.; Tan, R.; Xu, L.; Song, W. PSS-PANI-PVDF Composite based flexible NH3-sensors with sub-PPm detection at room temperature. Sens. Actuators B Chem. 2021, 328, 129085. [Google Scholar] [CrossRef]
- Cho, S.; Lee, J.S.; Joo, H. Recent Developments of the Solution-Processable and Highly Conductive Polyaniline Composites for Optical and Electrochemical Applications. Polymers 2019, 11, 1965. [Google Scholar] [CrossRef] [Green Version]
- Xing, X.; Du, L.; Feng, D.; Wang, C.; Tian, Y.; Li, Z.; Liu, H.; Yang, D. Twistable and tailorable V2O5/PANI/GO nanocomposite textile for wearable ammonia sensing. Sens. Actuators B Chem. 2022, 351, 130944. [Google Scholar] [CrossRef]
- Ajala, O.; Werther, C.; Nikaeen, P.; Singh, R.P.; Depan, D. Influence of Graphene Nanoscrolls on the Crystallization Behavior and Nano-mechanical Properties of Polylactic Acid. Polym. Adv. Technol. 2019, 30, 1825–1835. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, B.; Chen, J.; Gao, C. Highly Efficient Synthesis of Neat Graphene Nanoscrolls from Graphene Oxide by Well-Controlled Lyophilization. Chem. Mater. 2014, 26, 6811–6818. [Google Scholar] [CrossRef]
- Liu, F.; Luo, S.; Liu, D.; Chen, W.; Huang, Y.; Dong, L.; Wang, L. Facile Processing of Free-Standing Polyaniline/SWCNT Film as an Integrated Electrode for Flexible Supercapacitor Application. ACS Appl. Mater. Interfaces 2017, 9, 33791–33801. [Google Scholar] [CrossRef]
- Akyilmaz, E.; Oyman, G.; Cınar, E.; Odabas, G. A New Polyaniline–Catalase–Glutaraldehyde-Modified Biosensor for Hydrogen Peroxide Detection. Prep. Biochem. Biotechnol. 2017, 47, 86–93. [Google Scholar] [CrossRef]
- Verma, S.; Das, T.; Pandey, V.K.; Verma, B. Nanoarchitectonics of GO/PANI/CoFe2O4 (Graphene oxide/polyaniline/Cobalt Ferrite) based hybrid composite and its use in fabricating symmetric supercapacitor devices. J. Mol. Struct. 2022, 1266, 133515. [Google Scholar] [CrossRef]
- Zheng, B.; Gao, C. Preparation of Graphene Nanoscroll/Polyaniline Composites and Their Use in High Performance Supercapacitors. New Carbon Mater. 2016, 31, 315–320. [Google Scholar] [CrossRef]
- Zheng, X.; Ali Mohsin, M.E.; Arsad, A.; Hassan, A. Polymerization of Polyaniline under Various Concentrations of Ammonium Peroxydisulfate and Hydrochloric Acid by Ultrasonic Irradiation. J. Appl. Polym. Sci. 2021, 138, 50637. [Google Scholar] [CrossRef]
- Cho, S.; Shin, K.-H.; Jang, J. Enhanced Electrochemical Performance of Highly Porous Supercapacitor Electrodes Based on Solution Processed Polyaniline Thin Films. ACS Appl. Mater. Interfaces 2013, 5, 9186–9193. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-L.; Wang, Y.Z.; Long, S.M.; Bobeczko, C.; Epstein, A.J. Synthesis and Physical Properties of Highly Sulfonated Polyaniline. J. Am. Chem. Soc. 1996, 118, 2545–2555. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.M.V.; Rajendran, V.; Mishra, R.K.; Jayraman, M. Recent advances and perspectives in sweat based wearable electrochemical sensors. TrAc Trends Anal. Chem. 2020, 131, 116024. [Google Scholar] [CrossRef]
- Kim, Y.G.; Nguyen, H.L.; Kinlen, P. Secondary dopants of electrically conducting polyaniline. Polymers 2021, 13, 2904. [Google Scholar] [CrossRef]
- Nagaraja, M.; Mahesh, H.M.; Manjanna, J.; Rajanna, K.; Kurian, M.Z.; Lokesh, S.V. Effect of Multiwall Carbon Nanotubes on Electrical and Structural Properties of Polyaniline. J. Electron. Mater. 2012, 41, 1882–1885. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; He, D.; Ju, C.; Gao, Q.; Gao, L.; Fu, M. Synthesis and Properties of Nano-Polyaniline Films Doped with Camphor Sulfonic Acid. J. Nanosci. Nanotechnol. 2014, 14, 3417–3421. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.S.; Kim, C.; Lee, T.H.; Lee, Y.W.; Do, K.; Ko, J.; Im, S.S. Camphorsulfonic Acid-Doped Polyaniline Transparent Counter Electrode for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 22743–22748. [Google Scholar] [CrossRef]
- Awata, R.; Shehab, M.; El Tahan, A.; Soliman, M.; Ebrahim, S. High Performance Supercapacitor Based on Camphor Sulfonic Acid Doped Polyaniline/Multiwall Carbon Nanotubes Nanocomposite. Electrochim. Acta 2020, 347, 136229. [Google Scholar] [CrossRef]
- Qiu, H.; Wang, J.; Qi, S.; He, Z.; Fan, X.; Dong, Y. Microwave Absorbing Properties of Multi-Walled Carbon Nanotubes/Polyaniline Nanocomposites. J. Mater. Sci. Mater. Electron. 2015, 26, 564–570. [Google Scholar] [CrossRef]
- Cho, S.; Kim, M.; Lee, J.S.; Jang, J. Polypropylene/Polyaniline Nanofiber/Reduced Graphene Oxide Nanocomposite with Enhanced Electrical, Dielectric, and Ferroelectric Properties for a High Energy Density Capacitor. ACS Appl. Mater. Interfaces 2015, 7, 22301–22314. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Guang, S.; Xu, H.; Su, X. Facile Fabrication of Three-Dimensional Highly Ordered Structural Polyaniline–Graphene Bulk Hybrid Materials for High Performance Supercapacitor Electrodes. J. Mater. Chem. A 2014, 2, 813–823. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Mani, V.; Chen, S.-M.; Dinesh, B.; Huang, S.-T. The Immobilization of Glucose Oxidase at Manganese Dioxide Particles-Decorated Reduced Graphene Oxide Sheets for the Fabrication of a Glucose Biosensor. Ind. Eng. Chem. Res. 2014, 53, 15582–15589. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in Preparation, Processing and Applications of Polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Rahmani, M.; Ghafoori Fard, H.; Ahmadi, M.T.; Rahmani, K. Analytical Prediction of Carbon Nanoscroll-Based Electrochemical Glucose Biosensor Performance. Int. J. Environ. Anal. Chem. 2017, 97, 1024–1036. [Google Scholar] [CrossRef]
- Meng, L.; Xia, Y.; Liu, W.; Zhang, L.; Zou, P.; Zhang, Y. Hydrogen Microexplosion Synthesis of Platinum Nanoparticles/Nitrogen Doped Graphene Nanoscrolls as New Amperometric Glucose Biosensor. Electrochim. Acta 2015, 152, 330–337. [Google Scholar] [CrossRef]
- Peng, X.; Meng, L.; Zhang, W.; Liu, W.; Zhang, L.; Zhang, Y. Facile Preparation of Nitrogen-Doped Graphene Scrolls via Acoustic Cavitation as Electrocatalyst for Glucose Biosensing. J. Solid State Electrochem. 2016, 20, 439–447. [Google Scholar] [CrossRef]
- Ajala, O.; Werther, C.; Mahmudzade, R.; Nikaeen, P.; Depan, D. Crystallization Kinetics of Poly(Lactic Acid)–Graphene Nanoscroll Nanocomposites: Role of Tubular, Planar, and Scrolled Carbon Nanoparticles. C 2021, 7, 75. [Google Scholar] [CrossRef]
- Li, H.; Papadakis, R.; Jafri, S.H.M.; Thersleff, T.; Michler, J.; Ottosson, H.; Leifer, K. Superior Adhesion of Graphene Nanoscrolls. Commun. Phys. 2018, 1, 44. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.-Y.; Hung, H.-L.; Han, C.-C.; Chiu, H.-C. Correlation between Nanoscale Elasticity, Semiconductivity, and Structural Order in Functionalized Polyaniline Thin Films. Langmuir 2020, 36, 4153–4164. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, E.M.; Kabel, K.I.; Farag, A.A.; Al-Gamal, A.G. Comparative Study on Doping of Polyaniline with Graphene and Multi-Walled Carbon Nanotubes. J. Nanostruct. Chem. 2017, 7, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liang, Y.; Liu, Y.; Liu, S.; Li, P.; He, C. Engineering Doping Level for Enhanced Thermoelectric Performance of Carbon Nanotubes/Polyaniline Composites. Compos. Sci. Technol. 2021, 210, 108797. [Google Scholar] [CrossRef]
- Mergen, Ö.B.; Umut, E.; Arda, E.; Kara, S. A Comparative Study on the AC/DC Conductivity, Dielectric and Optical Properties of Polystyrene/Graphene Nanoplatelets (PS/GNP) and Multi-Walled Carbon Nanotube (PS/MWCNT) Nanocomposites. Polym. Test. 2020, 90, 106682. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmudzade, R.; Depan, D. Fabrication and Characterization of Free-Standing and Flexible Polyaniline Membranes: Role of Graphene Nanoscrolls. Macromol 2022, 2, 543-553. https://doi.org/10.3390/macromol2040035
Mahmudzade R, Depan D. Fabrication and Characterization of Free-Standing and Flexible Polyaniline Membranes: Role of Graphene Nanoscrolls. Macromol. 2022; 2(4):543-553. https://doi.org/10.3390/macromol2040035
Chicago/Turabian StyleMahmudzade, Rauf, and Dilip Depan. 2022. "Fabrication and Characterization of Free-Standing and Flexible Polyaniline Membranes: Role of Graphene Nanoscrolls" Macromol 2, no. 4: 543-553. https://doi.org/10.3390/macromol2040035