Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film
Abstract
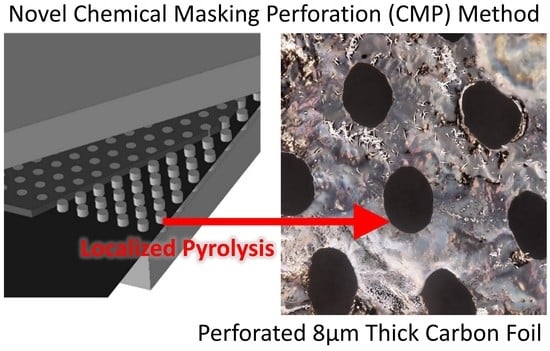
:1. Introduction
2. Materials and Methods
2.1. Sulfornation
2.2. Carbonization
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roch, T.; Beyer, E.; Lasagni, A. Surface modification of thin tetrahedral amorphous carbon films by means of UV direct laser interference patterning. Diam. Relat. Mater. 2010, 19, 1472–1477. [Google Scholar] [CrossRef]
- Lünsdorf, H.; Spiess, E. A rapid method of preparing perforated supporting foils for the thin carbon films used in high resolution transmission electron microscopy. J. Microsc. 1986, 144, 211–213. [Google Scholar] [CrossRef]
- Lei, Y.; Yang, S.; Wu, M.; Wilde, G. Surface patterning using templates: Concept, properties and device applications. Chem. Soc. Rev. 2011, 40, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Park, J.H.; Hong, N.; Bae, J.; Yang, C.-S.; Shin, K. Ultrathin carbon film from carbonization of spin-cast polyacrylonitrile film. J. Ind. Eng. Chem. 2013, 19, 1631–1637. [Google Scholar] [CrossRef]
- Hasebe, H.; Okuno, H.; Kuboki, H.; Ryuto, H.; Fukunishi, N.; Kamigaito, O.; Goto, A.; Kase, M.; Yano, Y. Development of long-life carbon stripper foils for uranium ion beams. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2010, 613, 453–456. [Google Scholar] [CrossRef]
- Wei, L.; Nitta, N.; Yushin, G. Lithographically patterned thin activated carbon films as a new technology platform for on-chip devices. ACS Nano 2013, 7, 6498–6506. [Google Scholar] [CrossRef]
- Schueller, O.J.A.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by pyrolysis of microfabricated polymeric precursors. Adv. Mater. 1997, 9, 477–480. [Google Scholar] [CrossRef]
- Schueller, O.J.; Brittain, S.T.; Whitesides, G.M. Fabrication of glassy carbon microstructures by soft lithography. Sens. Actuators A Phys. 1999, 72, 125–139. [Google Scholar] [CrossRef]
- Fuchs, A.N.; Schoeberl, M.; Tremmer, J.; Zaeh, M.F. Laser Cutting of Carbon Fiber Fabrics. Phys. Procedia 2013, 41, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Sakhavand, N. Mechanics of Platelet-Matrix Composites across Scales: Theory, Multiscale Modeling, and 3D Fabrication. Ph.D. Dissertation, Rice University, Houston, TX, USA, 2015. [Google Scholar]
- Sakhavand, N.; Shahsavari, R. Universal composition-structure-property maps for natural and biomimetic platelet-matrix composites and stacked heterostructures. Nat. Commun. 2015, 6, 6523. [Google Scholar] [CrossRef]
- Rouhana, R.; Stommel, M. Modelling and Experimental Investigation of Hexagonal Nacre-Like Structure Stiffness. J. Compos. Sci. 2020, 4, 91. [Google Scholar] [CrossRef]
- Behr, S.; Köllner, A.; Schneider, G.A. Tailoring Toughness and Mechanical Reliability by Controlled Defects: Nature-Inspired Composite Laminates of Laser-Perforated Yttria-Stabilized Zirconia. Adv. Eng. Mater. 2016, 18, 1877–1883. [Google Scholar] [CrossRef]
- Behr, S.; Vainio, U.; Müller, M.; Schreyer, A.; Schneider, G.A. Large-scale parallel alignment of platelet-shaped particles through gravitational sedimentation. Sci. Rep. 2015, 5, 9984. [Google Scholar] [CrossRef] [Green Version]
- Mirkhalaf, M.; Dastjerdi, A.K.; Barthelat, F. Overcoming the brittleness of glass through bio-inspiration and micro-architecture. Nat. Commun. 2014, 5, 3166. [Google Scholar] [CrossRef] [Green Version]
- Lipperman, F.; Ryvkin, M.; Fuchs, M.B. Crack arresting low-density porous materials with periodic microstructure. Int. J. Eng. Sci. 2008, 46, 572–584. [Google Scholar] [CrossRef]
- Marshall, A.L.; Karandikar, P.G.; Givens, B.P.; Liszkiewicz, A.F.; Segura, R.; Klier, E.M.; Doherty, K.J. Geometrical Effect on Damage in Reaction Bonded Ceramic Composites having Experienced High Strain Rate Impact. Ceram. Eng. Sci. Proc. 2014, 41–52. [Google Scholar] [CrossRef]
- Chan, K.S.; He, M.Y.; Hutchinson, J.W. Cracking and stress redistribution in ceramic layered composites. Mater. Sci. Eng. A 1993, 167, 57–64. [Google Scholar] [CrossRef]
- Choi, D.; Yeo, J.-S.; Joh, H.-I.; Lee, S. Carbon Nanosheet from Polyethylene Thin Film as a Transparent Conducting Film: “Upcycling” of Waste to Organic Photovoltaics Application. ACS Sustain. Chem. Eng. 2018, 6, 12463–12470. [Google Scholar] [CrossRef]
- Ali, Z.; Gao, Y.; Tang, B.; Wu, X.; Wang, Y.; Li, M.; Hou, X.; Li, L.; Jiang, N.; Yu, J. Preparation, Properties and Mechanisms of Carbon Fiber/Polymer Composites for Thermal Management Applications. Polymers 2021, 13, 169. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, J.S. Preparation of carbon fibers from linear low density polyethylene. Carbon 2015, 94, 524–530. [Google Scholar] [CrossRef]
- Kim, K.-W.; Lee, H.-M.; An, J.-H.; Kim, B.-S.; Min, B.-G.; Kang, S.-J.; An, K.-H.; Kim, B.-J. Effects of cross-linking methods for polyethylene-based carbon fibers: Review. Carbon Lett. 2015, 16, 147–170. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.A.; Weisenberger, M.C.; Bradley, S.B.; Abdallah, M.G.; Mecham, S.J.; Pisipati, P.; McGrath, J.E. Synthesis, spinning, and properties of very high molecular weight poly(acrylonitrile-co-methyl acrylate) for high performance precursors for carbon fiber. Polymer 2014, 55, 6471–6482. [Google Scholar] [CrossRef] [Green Version]
- Ball, L.E. Process for Preparing Biaxially Oriented Acrylonitrile Polymer Film. U.S. Patent US3418406, 24 December 1968. [Google Scholar]
- Karacan, I.; Benli, H. Use of sulfonation procedure for the development of thermally stabilized isotactic polypropylene fibers prior to carbonization. J. Appl. Polym. Sci. 2012, 123, 234–245. [Google Scholar] [CrossRef]
- Singh Gill, A.; Visotsky, D.; Mears, L.; Summers, J.D. Cost Estimation Model for Polyacrylonitrile-Based Carbon Fiber Manufacturing Process. J. Manuf. Sci. Eng. 2017, 139, 268. [Google Scholar] [CrossRef]
- Pereña, J.; Lorenzo, V.; Zamfirova, G.; Dimitrova, A. Microhardness of polyethylene surface modified by chlorosulphonic acid. Polym. Test. 2000, 19, 231–236. [Google Scholar] [CrossRef]
- Carl Roth GmbH + Co. KG. Clear Cling Film LLDPE. 2022. Available online: https://www.carlroth.com (accessed on 5 August 2022).
- Sigma-Aldrich Chemie GmbH. Chlorosulphonic Acid 571024-1KG. 2022. Available online: https://www.sigmaaldrich.com (accessed on 13 October 2022).
- Postema, A.R.; de Groot, H.; Pennings, A.J. Amorphous carbon fibres from linear low density polyethylene. J. Mater. Sci. 1990, 25, 4216–4222. [Google Scholar] [CrossRef]
- Palmenaer, A.; Wortberg, G.; Drissen, F.; Seide, G.; Gries, T. Production of Polyethylene Based Carbon Fibres. Chem. Eng. Trans. 2015, 1699–1704. [Google Scholar] [CrossRef]
- Gordon, J.L.; Jones, D.P.; Banas, D.; Hutula, D.N. A Collapse Surface for a Perforated Plate With an Equilateral Triangular Array of Penetrations. J. Press. Vessel Technol. 2002, 124, 201–206. [Google Scholar] [CrossRef]
- Jones, D.P.; Gordon, J.L. Elasto-Plastic Analysis of Perforated Plates Containing Triangular Penetration Patterns of 10 Percent Ligament Efficiency. J. Press. Vessel Technol. 1979, 101, 210–215. [Google Scholar] [CrossRef]
- Penning, J.P.; Lagcher, R.; Pennings, A.J. The effect of diameter on the mechanical properties of amorphous carbon fibres from linear low density polyethylene. Polym. Bull. 1991, 25, 405–412. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouhana, R.; Stommel, M.; Stanko, M.; Muth, M. Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film. Macromol 2022, 2, 554-561. https://doi.org/10.3390/macromol2040036
Rouhana R, Stommel M, Stanko M, Muth M. Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film. Macromol. 2022; 2(4):554-561. https://doi.org/10.3390/macromol2040036
Chicago/Turabian StyleRouhana, Rami, Markus Stommel, Michael Stanko, and Markus Muth. 2022. "Novel Method of Carbon Precursor Masking to Generate Controlled Perforations in a Carbon Film" Macromol 2, no. 4: 554-561. https://doi.org/10.3390/macromol2040036