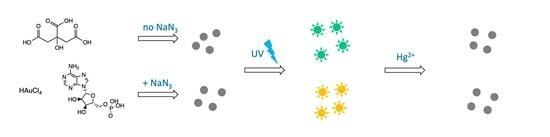
Gold Nanoclusters Prepared in the Presence of Adenosine Monophosphate and Citrate: Factorial-Based Synthesis Optimization and Sensing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. General Synthesis Procedure
2.3. Order of Components Mixing
2.4. Factorial Design of AuNCs Synthesis
2.5. Sensitivity to Metal Ions
2.6. Characterization and Data Processing
3. Results
3.1. Order of Mixing
3.2. Factorial-Based Synthesis Design
3.3. Optimization of the Synthesis Conditions to Maximize the Quantum Yield
3.4. Optimization of the Synthesis Conditions to Maximize the Yellow Emission
3.5. Blue and Yellow AuNCs Prepared under the Optimal Conditions
- Sample A—cAu 0.2 mmol/L, rAMP 40, razide 0, rcitr 200, and rNa/H 0.5;
- Sample B—cAu 0.2 mmol/L, rAMP 5, razide 8.5, rcitr 275, and rNa/H 0.75.
4. Discussion
- If HAuCl4 was first mixed with citrate and then AMP and NaN3 were added, the absorption spectra were weaker in the 300–350 nm characteristic range, showing long-wave scattering (blue and pink curves in Figure 1a). The formation of larger gold species via the reduction of citrate in the absence of an efficient stabilizer (AMP) could explain that fact. Due to low absorbance at 365 nm, the normalized emission intensity was still comparable to other spectra (Figure 1b);
- If NaN3 was added to the mixture after AMP and citrate, the long-wave yellow emission was suppressed (blue and brown curves in Figure 1a). Hence, although the AuNCs formation in the AMP + HAuCl4 + citrate system was complete only within 2–3 h at 90 °C (taking days at room temperature [11,12]), its initial stage was fast, and the formed intermediate was not sensitive to NaN3 which did not impart yellow emission in that case;
- If citrate was the last component introduced prior to dilution and heating, the spectral properties of the product were independent of the order of AMP and NaN3 addition (red and green curves). Hence, the competing coordination of AMP and NaN3 with gold(III) before the reduction was fast, and the mixture came to equilibrium within 15 min after adding the components. On the other hand, our trials showed that the interaction of HAuCl4 with AMP alone was slower (yellow coloration due to the complex formation was developed within hours). Likely, the interaction of HAuCl4 with AMP was multistage: the initial coordination was relatively fast and could affect the AuNCs synthesis, whereas complete ligand exchange leading to the coloration was slower.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Sample No. | cAu | rAMP | rcitr | rNa/H | razide |
|---|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0 | 0 | −1 |
| 5 | 0 | 0 | 0 | 0 | −1 |
| 6 | 0 | 0 | 0 | 0 | −1 |
| 7 | −1 | −1 | −1 | −1 | −1 |
| 8 | −1 | −1 | −1 | −1 | 1 |
| 9 | −1 | −1 | −1 | 1 | −1 |
| 10 | −1 | −1 | −1 | 1 | 1 |
| 11 | −1 | −1 | 1 | −1 | −1 |
| 12 | −1 | −1 | 1 | −1 | 1 |
| 13 | −1 | −1 | 1 | 1 | −1 |
| 14 | −1 | −1 | 1 | 1 | 1 |
| 15 | −1 | 1 | −1 | −1 | −1 |
| 16 | −1 | 1 | −1 | −1 | 1 |
| 17 | −1 | 1 | −1 | 1 | −1 |
| 18 | −1 | 1 | −1 | 1 | 1 |
| 19 | −1 | 1 | 1 | −1 | −1 |
| 20 | −1 | 1 | 1 | −1 | 1 |
| 21 | −1 | 1 | 1 | 1 | −1 |
| 22 | −1 | 1 | 1 | 1 | 1 |
| 23 | 1 | −1 | −1 | −1 | −1 |
| 24 | 1 | −1 | −1 | −1 | 1 |
| 25 | 1 | −1 | −1 | 1 | −1 |
| 26 | 1 | −1 | −1 | 1 | 1 |
| 27 | 1 | −1 | 1 | −1 | −1 |
| 28 | 1 | −1 | 1 | −1 | 1 |
| 29 | 1 | −1 | 1 | 1 | −1 |
| 30 | 1 | −1 | 1 | 1 | 1 |
| 31 | 1 | 1 | −1 | −1 | −1 |
| 32 | 1 | 1 | −1 | −1 | 1 |
| 33 | 1 | 1 | −1 | 1 | −1 |
| 34 | 1 | 1 | −1 | 1 | 1 |
| 35 | 1 | 1 | 1 | −1 | −1 |
| 36 | 1 | 1 | 1 | −1 | 1 |
| 37 | 1 | 1 | 1 | 1 | −1 |
| 38 | 1 | 1 | 1 | 1 | 1 |
| Sample No. | rAMP | rcitr | rNa/H | razide |
|---|---|---|---|---|
| 75 | 0 | 0 | 0 | 0 |
| 76 | 0 | 0 | 0 | 0 |
| 77 | 0 | 0 | 0 | 0 |
| 78 | −1 | −1 | −1 | −1 |
| 79 | 1 | −1 | −1 | −1 |
| 80 | −1 | 1 | −1 | −1 |
| 81 | 1 | 1 | −1 | −1 |
| 82 | −1 | −1 | 1 | −1 |
| 83 | 1 | −1 | 1 | −1 |
| 84 | −1 | 1 | 1 | −1 |
| 85 | 1 | 1 | 1 | −1 |
| 86 | −1 | −1 | −1 | 1 |
| 87 | 1 | −1 | −1 | 1 |
| 88 | −1 | 1 | −1 | 1 |
| 89 | 1 | 1 | −1 | 1 |
| 90 | −1 | −1 | 1 | 1 |
| 91 | 1 | −1 | 1 | 1 |
| 92 | −1 | 1 | 1 | 1 |
| 93 | 1 | 1 | 1 | 1 |
Appendix B
| Factor (Interaction) | SS | DF | F | P |
|---|---|---|---|---|
| cAu | 0.352 | 1 | 0.122 | 0.730 |
| rAMP | 1.04 | 1 | 0.362 | 0.553 |
| razide | 73.1 | 1 | 25.5 | 4.7∙10−5 |
| rcitr | 0.071 | 1 | 0.025 | 0.876 |
| rNa/H | 86.9 | 1 | 30.3 | 1.6∙10−5 |
| cAu:rAMP | 0.003 | 1 | 0.001 | 0.974 |
| cAu:razide | 0.077 | 1 | 0.027 | 0.872 |
| cAu:rcitr | 0.073 | 1 | 0.025 | 0.875 |
| cAu:rNa/H | 0.001 | 1 | 0.0004 | 0.985 |
| rAMP:razide | 0.598 | 1 | 0.208 | 0.653 |
| rAMP:rcitr | 0.343 | 1 | 0.119 | 0.733 |
| rAMP:rNa/H | 0.869 | 1 | 0.302 | 0.588 |
| razide:rcitr | 1.321 | 1 | 0.460 | 0.505 |
| razide:rNa/H | 0.493 | 1 | 0.172 | 0.683 |
| rcitr:rNa/H | 2.525 | 1 | 0.879 | 0.359 |
| Residuals | 63.2 | 22 |
| Factor (Interaction) | SS | DF | F | P |
|---|---|---|---|---|
| cAu | 0.001 | 1 | 5.45 | 0.035 |
| rAMP | 0.057 | 1 | 257 | 2∙10−10 |
| razide | 0.720 | 1 | 3233 | <10−15 |
| rcitr | 0.001 | 1 | 3.50 | 0.082 |
| rNa/H | 0.050 | 1 | 227 | 5∙10−10 |
| cAu:rAMP | <0.001 | 1 | 0.602 | 0.450 |
| cAu:razide | <0.001 | 1 | 0.051 | 0.824 |
| cAu:rcitr | <0.001 | 1 | 0.003 | 0.956 |
| cAu:rNa/H | <0.001 | 1 | 1.02 | 0.330 |
| rAMP:razide | 0.051 | 1 | 230 | 4∙10−10 |
| rAMP:rcitr | <0.001 | 1 | 0.262 | 0.617 |
| rAMP:rNa/H | <0.001 | 1 | 1.10 | 0.312 |
| razide:rcitr | 0.003 | 1 | 15.5 | 0.001 |
| razide:rNa/H | 0.092 | 1 | 411 | 9∙10−12 |
| rcitr:rNa/H | <0.001 | 1 | 1.56 | 0.232 |
| Residuals | 0.003 | 14 |
| Factor (Interaction) | SS | DF | F | P |
|---|---|---|---|---|
| rAMP | 2.93 | 1 | 267 | 2∙10−7 |
| razide | 0.023 | 1 | 2.12 | 0.184 |
| rcitr | 0.106 | 1 | 9.66 | 0.014 |
| rNa/H | 0.653 | 1 | 59.6 | 6∙10−5 |
| rAMP:razide | <0.001 | 1 | 0.026 | 0.875 |
| rAMP:rcitr | 0.004 | 1 | 0.409 | 0.540 |
| rAMP:rNa/H | 0.181 | 1 | 16.5 | 0.003 |
| razide:rcitr | 0.017 | 1 | 1.55 | 0.248 |
| razide:rNa/H | 0.003 | 1 | 0.268 | 0.619 |
| rcitr:rNa/H | 0.001 | 1 | 0.081 | 0.784 |
| Residuals | 0.088 | 8 |
References
- Qu, X.; Li, Y.; Li, L.; Wang, Y.; Liang, J.; Liang, J. Fluorescent gold nanoclusters: Synthesis and recent biological application. J. Nanomater. 2015, 2015, 784097. [Google Scholar] [CrossRef]
- van de Looij, S.M.; Hebels, E.R.; Viola, M.; Hembury, M.; Oliveira, S.; Vermonden, T. Gold nanoclusters: Imaging, therapy, and theranostic roles in biomedical applications. Bioconjugate Chem. 2022, 33, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Shao, Z.-S.; Song, Z.; Wang, Y.-B.; Wang, H.-S. Development of gold nanoclusters: From preparation to applications in the field of biomedicine. J. Mater. Chem. C 2020, 8, 14312–14333. [Google Scholar] [CrossRef]
- Zheng, J.; Petty, J.T.; Dickson, R.M. High quantum yield blue emission from water-soluble Au8 nanodots. J. Am. Chem. Soc. 2003, 125, 7780–7781. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, J.; Jiang, H.; Wang, X. Gold nanoclusters for theranostic applications. Coord. Chem. Rev. 2021, 431, 213689. [Google Scholar] [CrossRef]
- He, Z.; Shu, T.; Su, L.; Zhang, X. Strategies of luminescent gold nanoclusters for chemo-/bio-sensing. Molecules 2019, 24, 3045. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y.; Ying, J.Y. Highly selective and ultrasensitive detection of Hg2+ based on fluorescence quenching of Au nanoclusters by Hg2+–Au+ interactions. Chem. Commun. 2010, 46, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Tseng, W.-L. Ultrasensitive sensing of Hg2+ and CH3Hg+ based on the fluorescence quenching of lysozyme type VI-stabilized gold nanoclusters. Anal. Chem. 2010, 82, 9194–9200. [Google Scholar] [CrossRef]
- Shojaeifard, Z.; Hemmateenejad, B.; Shamsipur, M. Efficient on–off ratiometric fluorescence probe for cyanide ion based on perturbation of the interaction between gold nanoclusters and a copper(II)-phthalocyanine complex. ACS Appl. Mater. Interfaces 2016, 8, 15177–15186. [Google Scholar] [CrossRef]
- Li, H.; Huang, H.; Feng, J.-J.; Luo, X.; Fang, K.-M.; Wang, Z.-G.; Wang, A.-J. A polypeptide-mediated synthesis of green fluorescent gold nanoclusters for Fe3+ sensing and bioimaging. J. Colloid Interface Sci. 2017, 506, 386–392. [Google Scholar] [CrossRef]
- Lopez, A.; Liu, J. Light-activated metal-coordinated supramolecular complexes with charge-directed self-assembly. J. Phys. Chem. C 2013, 117, 3653–3661. [Google Scholar] [CrossRef]
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, Á.; Dékány, I. Nucleotide-directed syntheses of gold nanohybrid systems with structure-dependent optical features: Selective fluorescence sensing of Fe3+ ions. Colloids Surf. B 2017, 155, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Ga, L.; Ai, J. One-pot synthesis of nucleoside-templated fluorescent silver nanoparticles and gold nanoparticles. ACS Omega 2019, 4, 7643–7649. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, N.K.; Karpushkin, E.A.; Lopatina, L.I.; Sergeyev, V.G. DNA as a template for synthesis of fluorescent gold nanoclusters. Mendeleev Commun. 2023, 33, 346–348. [Google Scholar] [CrossRef]
- Karpushkin, E.A.; Ivanova, N.K.; Mesnyankina, E.A.; Sergeyev, V.G. Synthesis of fluorescent gold nanoclusters in the presence of adenosine monophosphate: Effect of azide ions. Mendeleev Commun. 2023, submitted.
- Lichstein, H.C. Studies of the effect of sodium azide on microbic growth and respiration: III. The effect of sodium azide on the gas metabolism of B. subtilis and P. aeruginosa and the influence of pyocyanine on the gas exchange of a pyocyanine-free strain of P. aeruginosa in the presence of sodium azide. J. Bacteriol. 1944, 47, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef]
- Turkevich, J. Colloidal gold. Part I: Historical and preparative aspects, morphology and structure. Gold Bull. 1985, 18, 86–91. [Google Scholar] [CrossRef]
- Usher, A.; McPhail, D.C.; Brugger, J. A spectrophotometric study of aqueous Au(III) halide–hydroxide complexes at 25–80 °C. Geochim. Cosmochim. Acta 2009, 73, 3359–3380. [Google Scholar] [CrossRef]
- Tribolet, R.; Sigel, H. Self-association and protonation of adenosine 5′-monophosphate in comparison with its 2′- and 3′-analogues and tubercidin 5′-monophosphate (7-deaza-AMP). Eur. J. Biochem. 1987, 163, 353–363. [Google Scholar] [CrossRef]
- Tribolet, R.; Sigel, H. Self-association of adenosine 5′-monophosphate (5′-AMP) as a function of pH and in comparison with adenosine, 2′-AMP and 3′-AMP. Biophys. Chem. 1987, 27, 119–130. [Google Scholar] [CrossRef]
- Karpushkin, E.A.; Mesnyankina, E.A.; Tagirova, M.R.; Zaborova, O.V.; Sergeyev, V.G. How to enhance sensitivity of carbonaceous ultrafine particles to metal ions. Russ. J. Gen. Chem. 2022, 92, 2042–2046. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpushkin, E.; Mesnyankina, E.; Sergeyev, V. Gold Nanoclusters Prepared in the Presence of Adenosine Monophosphate and Citrate: Factorial-Based Synthesis Optimization and Sensing Properties. Analytica 2023, 4, 415-431. https://doi.org/10.3390/analytica4040030
Karpushkin E, Mesnyankina E, Sergeyev V. Gold Nanoclusters Prepared in the Presence of Adenosine Monophosphate and Citrate: Factorial-Based Synthesis Optimization and Sensing Properties. Analytica. 2023; 4(4):415-431. https://doi.org/10.3390/analytica4040030
Chicago/Turabian StyleKarpushkin, Evgeny, Ekaterina Mesnyankina, and Vladimir Sergeyev. 2023. "Gold Nanoclusters Prepared in the Presence of Adenosine Monophosphate and Citrate: Factorial-Based Synthesis Optimization and Sensing Properties" Analytica 4, no. 4: 415-431. https://doi.org/10.3390/analytica4040030