Effect of Biochar on Rhizosphere Soil Microbial Diversity and Metabolism in Tobacco-Growing Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
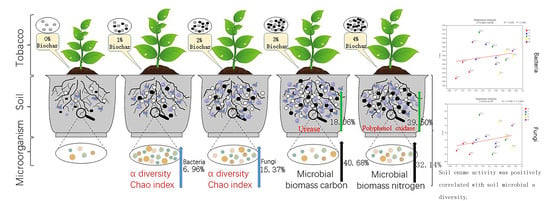
2.2. Experimental Design
2.3. Measurement and Analysis
2.3.1. Sampling Methods
Soil Samples for Rhizosphere Microorganisms
Soil Physical and Chemical Indicators
2.3.2. Measurement Methods
Soil Bacterial DNA Extraction and PCR Amplification
Illumina Miseq Sequencing of Soil Microorganisms
Soil Microbial Data Processing
2.3.3. Data Analysis Methods
3. Results and Analysis
3.1. Species Assessment of Bacteria and Fungi
3.2. Alpha Diversity of Bacteria and Fungi
3.3. Beta Diversity of Bacteria and Fungi
3.4. The Impact of Biochar on Soil Enzyme Activity
3.5. The Impact of Biochar on Soil Microbial Biomass Carbon and Microbial Biomass Nitrogen
3.6. Correlation Analysis of Soil Enzyme Activity and Microbial Diversity
3.7. Correlation Analysis of Soil Microbial Biomass Carbon and Nitrogen and Microbial Diversity
4. Discussion
4.1. The Impact of Biochar on Soil Microbial Diversity
4.1.1. Bacterial Alpha Diversity (α-Diversity)
4.1.2. Bacterial Beta Diversity
4.1.3. Fungal Alpha Diversity
4.1.4. Fungal Beta Diversity
4.2. The Effect of Biochar on Soil Enzyme Activity
4.3. The Effect of Biochar on Soil Microbial Biomass Carbon and Microbial Biomass Nitrogen
4.4. The Relationship between Soil Microbial Diversity and Microbial Biomass Carbon and Nitrogen
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graber, E.R.; Meller, H.Y.; Kolton, M. Biocharimpact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Bandara, T.; Franks, A.; Xu, J.; Bolan, N.; Wang, H.; Tang, C. Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit. Rev. Environ. Sci. Technol. 2020, 50, 903–978. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.L.; Zhang, J.; Zheng, L.; Chen, D.; Wu, Z.; Shaheen, S.M.; Rinklebe, J.; Ok, Y.S.; Wang, H.; et al. Coconut-fiber biochar reduced the bioavailability of lead but increased its translocation rate in rice plants: Elucidation of immobilization mechanisms and significance of iron plaque barrier on roots using spectroscopic techniques. J. Hazard. Mater. 2020, 389, 122117. [Google Scholar] [CrossRef]
- Wei, J.; Tu, C.; Yuan, G.; Zhou, Y.; Wang, H.; Lu, J. Limited Cu(II) binding to biochar DOM: Evidence from C K-edge NEXAFS and EEM-PARAFAC combined with two-dimensional correlation analysis. Sci. Total Environ. 2020, 701, 134919. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yang, X.; Wang, H.; Sarkar, B.; Shaheen, S.M.; Gielen, G.; Bolan, N.; Guo, J.; Che, L.; Sun, H.; et al. Animal carcass- and wood-derived biochars improved nutrient bioavailability, enzyme activity, and plant growth in metal-phthalic acid ester co-contaminated soils: A trial for reclamation and improvement of degraded soils. J. Environ. Manag. 2020, 261, 110246. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ran, J.; Dong, L.; Du, Q.; Ji, M.; Yao, S.; Sun, Y.; Gong, C.; Hou, Q.; Gong, H.; et al. Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef]
- Hu, W.; Hou, Q.; Delgado-Baquerizo, M.; Stegen, J.C.; Du, Q.; Dong, L.; Ji, M.; Sun, Y.; Yao, S.; Gong, H.; et al. Continental-scale niche differentiation of dominant topsoil archaea in drylands. Environ. Microbio 2022, 24, 5483–5497. [Google Scholar] [CrossRef]
- Díaz, L.M.; Nicolás, E.; López, M.R.; Galera, L.; Garrido, I.; Fenoll, J.; Bastida, F. Combined ozonation and solarization for the removal of pesticides from soil: Combined ozonation and solarization for the removal of pesticides from soil: Effects on soil microbial communities. Sci. Total Environ. 2020, 758, 143950. [Google Scholar] [CrossRef]
- Van, Z.L.; Kimber, S.; Morris, S.; Chan, K.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil 40 Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 3rd ed.; FAO: Rome, Italy, 2014. [Google Scholar]
- Ward, M.W.; Owens, C.W.; Rennie, M.J. Nitrogen estimation in biological samples by use of chemiluminescence. Clin. Chem. 1980, 26, 1336–1339. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Sriram, V.; Yogeeswaran, G. Sensitive spectrophotometric assay for 3-hydroxy-substituted flavonoids, based on their binding with molybdenum, antimony, or bismuth. J. Agric. Food Chem. 2000, 48, 2802–2806. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.C.; Khor, E.; Tan, T.K.; Wong, S.M. The degree of deacetylation of chitosan: Advocating the first derivative UV-spectrophotometry method of determination. Talanta 1998, 45, 713–719. [Google Scholar] [CrossRef]
- Nielsen, S.; Joseph, S.; Ye, J.; Chia, C.; Munroe, P.; van Zwieten, L.; Thomas, T. Cropseason and residual effects of sequentially applied mineral enhanced biochar and N fertiliser on crop yield, soil chemistry and microbial communities. Agric. Ecosyst. Environ. 2018, 255, 52–61. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, M.J.; Lu, X.; Tang, C.; Liu, X.M.; Brookes Philip, C.; Xu, J.M. Combined application of biochar and nitrogen fertilizer benefits nitrogen retention in the rhizosphere of soybean by increasing microbial biomass but not altering microbial community structure. Sci. Total Environ. 2018, 640–641, 1221–1230. [Google Scholar]
- Ma, Q.; Wen, Y.; Wang, D.; Sun, X.; Hill, P.W.; Macdonald, A.; Chadwick, D.R.; Wu, L.; Jones, D.L. Farmyard manure applications stimulate soil carbon and nitrogen cycling by boosting microbial biomass rather than changing its community composition. Soil Biol. Biochem. 2020, 144, 107760. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, K.; Sun, Y.H. Carbon, nitrogen mineralization and effcets on yield and quality of tomato in greenhouse of biochar made by sludge. Chin. Agric. Sci. Bull. 2014, 30, 215–220. [Google Scholar]
- Tian, D.; Gao, M.; Huang, R.; Lu, S.; Xu, C. Response of soil respiration and heterotrophic respiration to returning of straw and biochar in rape-maize rotation systems. Acta Sci. Circumst 2017, 38, 2988–2999. [Google Scholar]
- Wang, H.; Ren, T.; Yang, H.; Feng, Y.; Feng, H.; Liu, G.; Yin, Q.; Shi, H. Research and Application of Biochar in Soil CO2 Emission, Fertility, and Microorganisms: A Sustainable Solution to Solve China’s Agricultural Straw Burning Problem. Sustainability 2020, 12, 1922. [Google Scholar] [CrossRef] [Green Version]
- Birk, J.J.; Steiner, C.; Teixiera, W.C. Microbial Response to charcoal amendments and fertilization of a highly weathered tropical soil. In Amazonian Dark Earths: Wim Sombroek’s Vision; Woods, W.I., Teixeira, W.G., Lehmann, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 45, pp. 309–324. [Google Scholar]
- Wang, L.; Ok, Y.S.; Tsang, D.C.W.; Alessi, D.S.; Rinklebe, J.; Wang, H.; Mašek, O.; Hou, R.; O’Connor, D.; Hou, D. New Trends in Biochar Pyrolysis and Modification Strategies: Feedstock, Pyrolysis Conditions, Sustainability Concerns and Implications for Soil Amendment. Soil Use Manag. 2020, 36, 358–386. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.G.; Chen, Z.; Yin, H.; Guo, W.; Cao, Y.; Wang, G.J.; Sun, B.; Yan, X.F.; Li, J.D.; Zhao, T.H.; et al. The Responses of Forest Fine Root Biomass/Necromass Ratio to Environmental Factors Depend on Mycorrhizal Type and Latitudinal Region. J. Geophys. Res. Biogeosci. 2018, 123, 1769–1788. [Google Scholar] [CrossRef] [Green Version]
- Mooshammer, M.; Wanek, W.; Zechmeister-Boltenstern, S.; Richter, A. Stoichiometric imbalances between terrestrial decomposer communities and their resources: Mechanisms and implications of microbial adaptations to their resources. Front. Microbiol. 2014, 5, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, F.; Li, G.T.; Lin, Q.M.; Zhao, X.R. Crop Yield and Soil Properties in the First 3 Years After Biochar Application to a Calcareous Soil. J. Integr. Agric. 2014, 13, 525–532. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Fidel, R.B.; Laird, D.A.; Spokas, K.A. Sorption of ammonium and nitrate to biochars is electrostatic and pH-dependent. Sci. Rep. 2018, 8, 17627. [Google Scholar] [CrossRef] [Green Version]
- Ihara, H.; Kumagai, A.; Hori, T.; Nanba, K.; Aoyagi, T.; Takasaki, M.; Katayama, Y. Direct comparison of bacterial communities in soils contaminated with different levels of radioactive cesium from the first Fukushima nuclear power plant accident. Sci. Total Environ. 2021, 756, 143844. [Google Scholar] [CrossRef]
- Joseph, T.; Kehinde, O.; Tolulope, G.; Zhao, H.Y. Effect of biochar on soil structure and storage of soil organic carbon and nitrogen in the aggregate fractions of an Albic soil. Arch. Agron. Soil Sci. 2020, 66, 1–12. [Google Scholar] [CrossRef]
- Durenkamp, M.; Luo, Y.; Brookes, P.C. Impact of black carbon addition to soil on the determination of soil microbial biomass by fumigation extraction. Soil Biol. Biochem. 2010, 42, 2026–2029. [Google Scholar] [CrossRef]
- Han, G.; Meng, J.; Cao, T.; Liu, Z.; Chen, W. Effects of biochar on spinach rhizosphere microorganisms and soil physical and chemical properties. J. Shenyang Agric. Univ. 2012, 43, 515–520. [Google Scholar]
- Fisk, M.; Santangelo, S.; Minick, K. Carbon mineralization is promoted by phosphorus and reduced by nitrogen addition in the organic horizon of northern hardwood forests. Soil Biol. Biochem. 2015, 81, 212–218. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Zhang, Q.Z. The effect of land use change on soil carbon, nitrogen, and phosphorus contents and their stoichiometry in temperate sapling stands in northeastern China. Acta Ecol. Sin. 2015, 35, 6694–6702. [Google Scholar]
- Huang, J.; Zhang, Q.Z.; Zhang, D.J. Research advances in effects of biochar application on agroecosystem. Chin. J. Agrometeorol. 2012, 33, 232–239. [Google Scholar]
- Zhou, X.; Wu, S.B.; Wang, R.G.; Wu, H.M. Nitrogen removal in response to the varying C/N ratios in subsurface flow constructed wetland microcosms with biochar addition. Environ. Sci. Pollut. Res. 2019, 26, 3382–3391. [Google Scholar] [CrossRef] [PubMed]
- Buchkowski, R.W.; Shaw, A.N.; Sihi, D.; Smith, G.R.; Keiser, A.D. Constraining Carbon and Nutrient Flows in Soil with Ecological Stoichiometry. Front. Ecol. Evol. 2019, 7, 382. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Li, S.Q.; Geng, Y.J.; Miao, Y.C.; Ding, Y.; Liu, S.W.; Zou, J.W. Differential responses of soil N2O to biochar depend on the predominant microbial pathway. Appl. Soil Ecol. 2020, 145, 103348. [Google Scholar]
- Rondon, M.A.; Lehmann, J.; Ramírez, J. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wu, M. Effect of methamidophos on soil fungi community in microcosms by plate count, DGGE and clone library analysis. J. Environ. Sci. China 2008, 20, 619–625. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Grossman, J.M.; O’neill, B.E.; Tsai, S.M. Amazonian anthrosols support similar microbial communities that differ distinctly from those extant in adjacent, unmodified soils of the same mineralogy. Microb. Ecol. 2010, 60, 192–205. [Google Scholar] [CrossRef]
- Grunwald, D.; Kaiser, M.; Junker, S.; Marhan, S.; Piepho, H.-P.; Poll, C.; Bamminger, C.; Ludwig, B. Influence of elevated soil temperature and biochar application on organic matter associated with aggregate-size and density fractions in an arable soil. Agric. Ecosyst. Environ. 2017, 241, 79–87. [Google Scholar] [CrossRef]
- Singh, B.P.; Hatton, B.J.; Singh, B.; Cowie, A.L.; Kathuria, A. Influence of bio-charson nitrous oxide emission and nitrogen learning learning for bio-charson nitrous ossion and nitrogen learning jiaocontrasting sol. J. Environ. Qual. 2010, 39, 1224–1235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.W.; Liu, X.R. Effects of biochar on the key soil nitrogen transformation processes in agricultural soil. Chin. Agrometeorol. 2015, 36, 709–716. [Google Scholar]







| Sample | Sequence Number/Piece | Base Number/bp | Average Length/bp | Minimum Sequence Length/bp | Longest Sequence Length/bp |
|---|---|---|---|---|---|
| T4_1 | 62,756 | 26,173,971 | 417.08 | 230 | 521 |
| T3_3 | 70,429 | 29,342,325 | 416.62 | 203 | 510 |
| T4_3 | 72,504 | 30,244,323 | 417.14 | 215 | 496 |
| T3_1 | 74,752 | 31,109,636 | 416.17 | 225 | 486 |
| T0_1 | 62,998 | 26,240,464 | 416.53 | 214 | 469 |
| T0_3 | 63,403 | 26,412,256 | 416.58 | 208 | 486 |
| T0_2 | 72,663 | 30,259,304 | 416.43 | 232 | 469 |
| T2_3 | 71,010 | 29,592,302 | 416.73 | 214 | 507 |
| T2_2 | 69,747 | 29,083,027 | 416.98 | 208 | 461 |
| T2_1 | 66,276 | 27,600,565 | 416.45 | 203 | 465 |
| T3_2 | 73,127 | 30,444,850 | 416.33 | 216 | 466 |
| T4_2 | 71,090 | 29,636,375 | 416.89 | 234 | 466 |
| T1_1 | 67,919 | 28,328,289 | 417.09 | 235 | 469 |
| T1_2 | 74,381 | 30,995,232 | 416.71 | 202 | 494 |
| T1_3 | 68,860 | 28,675,557 | 416.43 | 203 | 499 |
| Sample | Sequence Number/Piece | Base Number/bp | Average Length/bp | Minimum Sequence Length/bp | Longest Sequence Length/bp |
|---|---|---|---|---|---|
| T4_1 | 50,217 | 19,151,231 | 381.37 | 299 | 424 |
| T3_3 | 56,778 | 21,652,473 | 381.35 | 371 | 431 |
| T4_3 | 37,399 | 14,264,267 | 381.41 | 372 | 388 |
| T3_1 | 49,453 | 18,857,698 | 381.33 | 256 | 397 |
| T0_1 | 40,795 | 15,557,905 | 381.37 | 257 | 424 |
| T0_3 | 52,791 | 20,143,900 | 381.58 | 371 | 431 |
| T0_2 | 35,104 | 13,391,715 | 381.49 | 371 | 430 |
| T2_3 | 50,257 | 19,161,969 | 381.28 | 254 | 425 |
| T2_2 | 46,804 | 17,848,691 | 381.35 | 254 | 410 |
| T2_1 | 47,635 | 18,166,809 | 381.38 | 254 | 409 |
| T3_2 | 49,138 | 18,744,568 | 381.47 | 254 | 424 |
| T4_2 | 50,099 | 19,109,284 | 381.43 | 254 | 408 |
| T1_1 | 40,631 | 15,503,840 | 381.58 | 254 | 427 |
| T1_2 | 43,672 | 16,660,443 | 381.49 | 254 | 411 |
| T1_3 | 49,951 | 19,059,580 | 381.57 | 261 | 431 |
| Diversity Index | Treatment | Bacteria | Fungi |
|---|---|---|---|
| Chao | T0 | 3860.20 ± 636.4 b | 137.24 ± 10.59 c |
| T1 | 4128.91 ± 329.5 a | 155.78 ± 37.4 a | |
| T2 | 4105.01 ± 311.8 a | 158.33 ± 21.9 a | |
| T3 | 4084.85 ± 562.7 a | 148.62 ± 45.1 b | |
| T4 | 4108.54 ± 219.7 a | 147.03 ± 36.2 b | |
| ACE | T0 | 3841.91 ± 672.6 c | 138.56 ± 37.5 b |
| T1 | 4133.20 ± 233.5 a | 147.46 ± 25.1 b | |
| T2 | 4132.00 ± 492.7 a | 156.28 ± 42.9 a | |
| T3 | 4079.00 ± 433.8 b | 147.74 ± 14.8 b | |
| T4 | 4123.70 ± 294.6 a | 143.88 ± 25.6 b | |
| Shannon | T0 | 6.64 ± 0.7 a | 3.01 ± 0.5 b |
| T1 | 6.71 ± 1.5 a | 2.96 ± 0.3 b | |
| T2 | 6.70 ± 0.9 a | 3.08 ± 0.5 ab | |
| T3 | 6.59 ± 3.6 a | 3.11 ± 0.7 a | |
| T4 | 6.76 ± 2.1 a | 3.12 ± 0.6 a | |
| Coverage | T0 | 0.9805 ± 0.05 a | 0.9997 ± 0.04 a |
| T1 | 0.9795 ± 0.31 ab | 0.9997 ± 0.02 a | |
| T2 | 0.9812 ± 0.27 a | 0.9996 ± 0.06 a | |
| T3 | 0.9789 ± 0.07 b | 0.9997 ± 0.04 a | |
| T4 | 0.9787 ± 0.05 b | 0.9997 ± 0.05 a |
| Treatment | MBC (mg kg−1) | MBN (mg kg−1) | TOC (g kg−1) | TN (g kg−1) | MBC/TOC | MBN/TN |
|---|---|---|---|---|---|---|
| T0 | 236.15 ± 28.4 d | 10.36 ± 1.5 d | 8.27 ± 2.8 c | 0.74 ± 0.31 c | 28.56 ± 6.7 b | 14.00 ± 2.4 c |
| T1 | 277.51 ± 79.5 c | 12.48 ± 4.8 c | 10.15 ± 4.1 b | 0.86 ± 0.15 b | 27.34 ± 5.2 c | 14.51 ± 5.2 c |
| T2 | 315.63 ± 73.6 b | 14.52 ± 6.9 a | 10.79 ± 3.5 b | 0.83 ± 0.24 b | 29.25 ± 2.8 a | 17.49 ± 1.9 a |
| T3 | 332.74 ± 35.9 a | 14.78 ± 7.3 a | 11.34 ± 1.4 a | 0.92 ± 0.33 a | 29.34 ± 3.7 a | 16.07 ± 3.6 b |
| T4 | 327.52 ± 44.8 a | 13.69 ± 2.5 b | 11.17 ± 2.7 ab | 0.87 ± 0.18 b | 29.32 ± 5.2 a | 15.74 ± 1.7 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, R.; Zhao, Y.; Shi, H.; Liu, G. Effect of Biochar on Rhizosphere Soil Microbial Diversity and Metabolism in Tobacco-Growing Soil. Ecologies 2022, 3, 539-556. https://doi.org/10.3390/ecologies3040040
Wang H, Zhang R, Zhao Y, Shi H, Liu G. Effect of Biochar on Rhizosphere Soil Microbial Diversity and Metabolism in Tobacco-Growing Soil. Ecologies. 2022; 3(4):539-556. https://doi.org/10.3390/ecologies3040040
Chicago/Turabian StyleWang, Huanhuan, Rui Zhang, Yuanyuan Zhao, Hongzhi Shi, and Guoshun Liu. 2022. "Effect of Biochar on Rhizosphere Soil Microbial Diversity and Metabolism in Tobacco-Growing Soil" Ecologies 3, no. 4: 539-556. https://doi.org/10.3390/ecologies3040040