Characterization Techniques Coupled with Mathematical Tools for Deepening Asphaltene Structure
Abstract
:1. Introduction
2. Materials and Methods
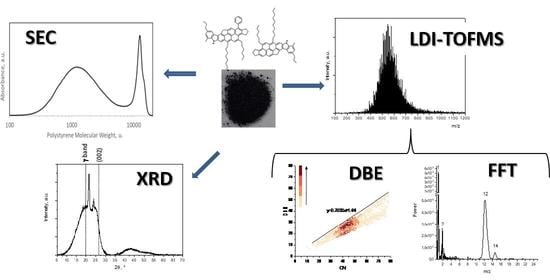
2.1. Laser Desorption Ionization-Time of Flight Mass Spectrometry (LDI-TOFMS)
2.2. Size Exclusion Chromatography (SEC)
2.3. X-ray Diffraction (XRD)
3. Results
4. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Speight, J.G. Petroleum Asphaltenes—Part 1: Asphaltenes, Resins and the Structure of Petroleum. Oil Gas Sci. Technol. 2004, 59, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Mullins, O.C.; Sheu, E.Y. Structures and Dynamics of Asphaltenes; Springer Science and Business Media: New York, NY, USA, 2013. [Google Scholar]
- Wu, S.-P.; Pang, L.; Mo, L.-T.; Chen, Y.-C.; Zhu, G.-J. Influence of aging on the evolution of structure, morphology and rheology of base and SBS modified bitumen. Constr. Build. Mater. 2009, 23, 1005–1010. [Google Scholar] [CrossRef]
- Loeber, L.; Muller, G.; Morel, J.; Sutton, O. Bitumen in colloid science: A chemical, structural and rheological approach. Fuel 1998, 77, 1443–1450. [Google Scholar] [CrossRef]
- Rahim, A.; Milad, A.; Yusoff, N.I.M.; Airey, G.; Thom, N. Stiffening Effect of Fillers Based on Rheology and Micromechanics Models. Appl. Sci. 2021, 11, 6521. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sheu, E.Y.; Hammami, A.; Marshall, A.G. Asphaltenes, Heavy Oils, and Petroleomics; Springer Science and Business Media: New York, NY, USA, 2007. [Google Scholar]
- Vargas, F.M.; Tavakkoli, M. Asphaltene Deposition: Fundamentals, Prediction, Prevention, and Remediation; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Mullins, O.C.; Martinez-Haya, B.; Marshall, A.G. Contrasting perspective on asphaltene molecular weight. This comment vs the overview of Herod, A.A., Bartle, K.D., and Kandiyoti, R. Energy Fuels 2008, 22, 1765–1773. [Google Scholar] [CrossRef]
- Herod, A.A.; Bartle, K.D.; Kandiyoti, R. Comment on a paper by Mullins, Martinez-Haya and Marshall “Contrasting perspective on asphaltene molecular weight. This comment vs the overview of Herod, A.A., Bartle, K.D., and Kandiyoti, R.”. Energy Fuels 2008, 22, 4312–4317. [Google Scholar] [CrossRef]
- Wen, C.S.; Chilingarian, G.; Yen, T.F. Properties and structure of bitumens. In Bitumens, Asphalts and Tar Sands; Elsevier: Amsterdam, The Netherlands, 1978; Volume 7, pp. 155–190. [Google Scholar]
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Andrews, A.B.; Gross, L.; Pauchard, V.; Banerjee, S.; Mullins, O.C. Overview of asphaltene nanostructures and thermodynamic applications. Energy Fuels 2020, 34, 15082–15105. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Gray, M.R.; Rüger, C.; Smith, D.F.; Glattke, T.J.; Niles, S.F.; Neumann, A.; Weisbrod, C.R.; Yen, A.; McKenna, A.M.; et al. Lessons Learned from a Decade-Long Assessment of Asphaltenes by Ultrahigh-Resolution Mass Spectrometry and Implications for Complex Mixture Analysis. Energy Fuels 2021, 35, 16335–16376. [Google Scholar] [CrossRef]
- Badre, S.; Carla Goncalves, C.; Norinaga, K.; Gustavson, G.; Mullins, O.C. Molecular size and weight of asphaltenes and asphaltene solubility fractions from coals, crude oils and bitumen. Fuel 2006, 85, 1–11. [Google Scholar] [CrossRef]
- Przybilla, L.; Brand, J.D.; Yoshimura, K.; Rader, H.J.; Müllen, K. MALDI-TOF mass spectrometry of insoluble giant polycyclic aromatic hydrocarbons by a new method of sample preparation. Anal. Chem. 2000, 72, 4591–4597. [Google Scholar] [CrossRef]
- Rizzi, A.; Cosmina, P.; Flego, C.; Montanari, L.; Seraglia, R.; Traldi, P. Laser desorption/ionization techniques in the characterization of high molecular weight oil fractions. Part 1: Asphaltenes. J. Mass Spectrom. 2006, 41, 1232–1241. [Google Scholar] [CrossRef]
- Apicella, B.; Carpentieri, A.; Alf‘e, M.; Barbella, R.; Tregrossi, A.; Pucci, P.; Ciajolo, A. Mass spectrometric analysis of large PAH in a fuel-rich ethylene flame. Proc. Combust. Inst. 2007, 31, 547–553. [Google Scholar] [CrossRef]
- Apicella, B.; Bruno, A.; Wang, X.; Spinelli, N. Fast Fourier Transform and autocorrelation function for the analysis of complex mass spectra. Int. J. Mass Spec. 2013, 338, 30–38. [Google Scholar] [CrossRef]
- Pellegrin, V. Molecular formulas of organic compounds: The nitrogen rule and degree of unsaturation. J. Chem. Educ. 1983, 60, 626–633. [Google Scholar] [CrossRef]
- Russo, C.; Ciajolo, A.; Stanzione, F.; Tregrossi, A.; Oliano, M.M.; Carpentieri, A.; Apicella, B. Investigation on chemical and structural properties of coal- and petroleum-derived pitches and implications on physico-chemical properties (solubility, softening and coking). Fuel 2019, 245, 478–487. [Google Scholar] [CrossRef]
- Russo, C.; Stanzione, F.; Tregrossi, A.; Ciajolo, A. Infrared spectroscopy of some carbon-based materials relevant in combustion: Qualitative and quantitative analysis of hydrogen. Carbon 2014, 74, 127–138. [Google Scholar] [CrossRef]
- Apicella, B.; Russo, C.; Carpentieri, A.; Tregrossi, A.; Ciajolo, A. PAHs and fullerenes as structural and compositional motifs tracing and distinguishing organic carbon from soot. Fuel 2021, 309, 122356. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, Y.H.; Kim, S. Planar Limit-Assisted Structural Interpretation of Saturates/Aromatics/Resins/Asphaltenes Fractionated Crude Oil Compounds Observed by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal. Chem. 2011, 83, 6068–6073. [Google Scholar] [CrossRef]
- Zhang, W.; Andersson, J.T.; Rader, H.J.; Müllen, K. Molecular characterization of large polycyclic aromatic hydrocarbons in solid petroleum pitch and coal tar pitch by high resolution MALDI TOF MS and insights from ion mobility separation. Carbon 2015, 95, 672–680. [Google Scholar] [CrossRef]
- Niyonsaba, E.; Manheim, J.M.; Yerabolu, R.; Kenttämaa, H.I. Recent advances in petroleum analysis by mass spectrometry. Anal. Chem. 2019, 91, 156–177. [Google Scholar] [CrossRef]
- Apicella, B.; Alfè, M.; Ciajolo, A. Mass Spectrometric Advances in the Analysis of Large Aromatic Fractions of Heavy Fuel Oils and Carbon Particulates. Combust. Sci. Tech. 2010, 182, 640–652. [Google Scholar] [CrossRef]
- Acevedo, S.; Gutiérrez, L.; Negrin, G.; Pereira, J.; Mendez, B.; Broseta, D. Molecular Weight of Petroleum Asphaltenes: A Comparison between Mass Spectrometry and Vapor Pressure Osmometry. J. Energy Fuels 2005, 19, 1548–1560. [Google Scholar] [CrossRef]
- Al-Muhareb, E.; Morgan, T.J.; Herod, A.A.; Kandiyoti, R. Characterization of petroleum asphaltenes by size exclusion chromatography, UV-fluorescence and mass spectrometry. Pet. Sci. Technol. 2007, 25, 81–91. [Google Scholar] [CrossRef]
- Gargiulo, V.; Apicella, B.; Russo, C.; Stanzione, F.; Tregrossi, A.; Millan, M.; Ciajolo, A. Structural Characterization of Large Polycyclic Aromatic Hydrocarbons. Part 2: Solvent-Separated Fractions of Coal Tar Pitch and Naphthalene-Derived Pitch. Energy Fuels 2016, 30, 2574–2583. [Google Scholar] [CrossRef]
- Strausz, O.; Safarik, I.; Lown, E.M. Cause of asphaltenes fluorescence intensity variation with molecular weight and its ramification for laser ionization mass spectrometry. Energy Fuels 2009, 23, 1555–1562. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Kong, C.; Harris, D. Quantitative X-ray diffraction analysis and its application to various coals. Carbon 2001, 39, 1821–1833. [Google Scholar] [CrossRef]
- Manoj, B.; Kunjomana, A.G. Study of Stacking Structure of Amorphous Carbon by X-ray Diffraction Technique. Int. J. Electrochem. Sci. 2012, 7, 3127–3134. [Google Scholar]
- Andersen, S.I.; Jensen, J.O.; Speight, J.G. X-ray Diffraction of Subfractions of Petroleum Asphaltenes. Energy Fuels 2005, 19, 2371–2377. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apicella, B.; Ciajolo, A.; Carpentieri, A.; Popa, C.; Russo, C. Characterization Techniques Coupled with Mathematical Tools for Deepening Asphaltene Structure. Fuels 2022, 3, 75-84. https://doi.org/10.3390/fuels3010005
Apicella B, Ciajolo A, Carpentieri A, Popa C, Russo C. Characterization Techniques Coupled with Mathematical Tools for Deepening Asphaltene Structure. Fuels. 2022; 3(1):75-84. https://doi.org/10.3390/fuels3010005
Chicago/Turabian StyleApicella, Barbara, Anna Ciajolo, Andrea Carpentieri, Ciprian Popa, and Carmela Russo. 2022. "Characterization Techniques Coupled with Mathematical Tools for Deepening Asphaltene Structure" Fuels 3, no. 1: 75-84. https://doi.org/10.3390/fuels3010005