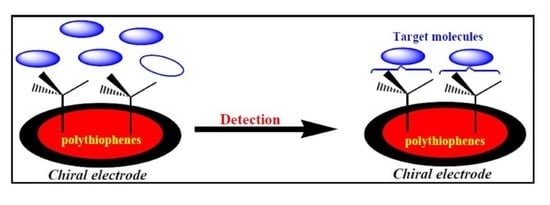
Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors
Abstract
:1. Introduction
2. Electropolymerization
3. Chiral Electrodes Based on Polythiophenes
4. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terán-Alcocer, A.; Bravo-Plascencia, F.; Cevallos-Morillo, C.; Palma-Cando, A. Electrochemical sensors based on conducting polymers for the aqueous detection of biologically relevant molecules. Nanomaterials 2021, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, S.; Grecchi, S.; Magni, M.; Mussini, P. Electroactive chiral oligo- and polymer layers for electrochemical enantiorecognition. Curr. Opin. Electrochem. 2018, 7, 188–199. [Google Scholar] [CrossRef]
- Chahma, M.; Gilroy, J.B.; Hicks, R.G. Linear and branched electroactive polymers based on ethylenedioxythiophene–triarylamine conjugates. J. Mater. Chem. 2007, 17, 4768–4771. [Google Scholar] [CrossRef]
- Ibanez, J.G.; Rincon, M.E.; Gutierrez-Granados, S.; Chahma, M.; Jaramillo-Quintero, O.A.; Frontana-Uribe, B.A. Conducting polymers in the fields of energy, environmental remediation, and chemical–chiral sensors. Chem. Rev. 2018, 118, 4731–4816. [Google Scholar]
- Mandler, D. Chiral self-assembled monolayers in electrochemistry. Curr. Opin. Electrochem. 2018, 7, 42–47. [Google Scholar] [CrossRef]
- Chahma, M.; Lee, J.S.; Kraatz, H.-B. Fc-ssDNA conjugate: Electrochemical properties in a borate buffer and adsorption on gold electrode surfaces. J. Electroanal. Chem. 2004, 567, 283–287. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Sterically hindered diazonium salts for the grafting of a monolayer on metals. J. Am. Chem. Soc. 2008, 130, 8576–8577. [Google Scholar] [CrossRef]
- Adenier, A.; Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Formation of polyphenylene films on metal electrodes by electrochemical reduction of benzenediazonium salts. Chem. Mater. 2006, 18, 2021–2029. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Brousse, T.; Bélanger, D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon 2015, 92, 362–381. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Thompson, L.A.; Kowalik, J.; Josowicz, M.; Janata, J. Label-free DNA hybridization probe based on a conducting polymer. J. Am. Chem. Soc. 2003, 125, 324–325. [Google Scholar] [CrossRef] [PubMed]
- Lakard, B. Electrochemical biosensors based on conducting polymers: A review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Saheb, A.; Patterson, S.; Josowicz, M. Probing for DNA methylation with a voltammetric DNA detector. Analyst 2014, 139, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Arnaboldi, S.; Magni, M.; Mussini, P.R. Enantioselective selectors for chiral electrochemistry and electroanalysis: Stereogenic elements and enantioselection performance. Curr. Opin. Electrochem. 2018, 8, 60–72. [Google Scholar] [CrossRef]
- Kane-Maguire, L.A.P.; Wallace, G.G. Chiral conducting polymers. Chem. Soc. Rev. 2010, 39, 2545–2576. [Google Scholar] [CrossRef]
- Vacek, J.; Zadny, J.; Storch, J.; Hrbac, J. Chiral electrochemistry: Anodic deposition of enantiopure helical molecules. ChemPlusChem 2020, 85, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Trojanowicz, M. Enantioselective electrochemical sensors and biosensors: A mini-review. Electrochem. Commun. 2014, 38, 47–52. [Google Scholar] [CrossRef]
- Lemaire, M.; Delabouglise, D.; Garreau, R.; Guy, A.; Roncali, J. Enantioselective chiral poly(thiophenes). Chem. Commun. 1988, 10, 658–661. [Google Scholar] [CrossRef]
- Mondal, P.C.; Kantor-Uriel, N.; Mathew, S.P.; Tassinari, F.; Fontanesi, C. Chiral conductive polymers as spin filters. Adv. Mater. 2015, 27, 1924–1927. [Google Scholar] [CrossRef]
- Salmón, M.; Bidan, G. Chiral polypyrroles from optically active pyrrole monomers. J. Electrochem. Soc. 1985, 132, 1897–1899. [Google Scholar] [CrossRef]
- Ramos, J.C.; Dias, J.M.M.; Souto-Maior, R.M.; Ribeiro, A.S.; Tonholo, J.; Barbier, V.; Penelle, J.; Navarro, M. Synthesis and characterization of poly[(R)-(−) and (S)-(+)-3-(1′-pyrrolyl)propyl-N-(3″,5″-dinitrobenzoyl)-α-phenylglycinate]s as chiral oligomers of pyrrole. Synth. Met. 2010, 160, 1920–1924. [Google Scholar] [CrossRef]
- Takano, N.; Seki, C. Preparation of chiral polypyrrole film-coated electrode incorporating palladium metal and asymmetric hydrogenation of α-keto esters. Electrochemistry 2006, 74, 596–598. [Google Scholar] [CrossRef]
- Deore, B.; Yakabe, H.; Shiigi, H.; Nagaok, T. Enantioselective uptake of amino acids using an electromodulated column packed with carbon fibres modified with overoxidised polypyrrole. Analyst 2002, 127, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Syritski, V.; Reut, J.; Menaker, A.; Gyurcsányi, R.E.; Öpik, A. Electrosynthesized molecularly imprinted polypyrrole films for enantioselective recognition of l-aspartic acid. Electrochim. Acta 2008, 53, 2729–2736. [Google Scholar] [CrossRef]
- Yang, J.; Yu, Y.; Wu, D.; Tao, Y.; Deng, L.; Kong, Y. Coinduction of a chiral microenvironment in polypyrrole by overoxidation and camphorsulfonic acid for electrochemical chirality sensing. Anal. Chem. 2018, 90, 9551–9558. [Google Scholar] [CrossRef]
- Zhao, Q.; Yang, J.; Zhang, J.; Wu, D.; Tao, Y.; Kong, Y. Single-template molecularly imprinted chiral sensor for simultaneous recognition of alanine and tyrosine enantiomers. Anal. Chem. 2019, 91, 12546–12552. [Google Scholar] [CrossRef] [PubMed]
- Basozabal, I.; Gómez-Caballero, A.; Unceta, N.; Goicolea, M.A.; Barrio, R.J. Voltammetric sensors with chiral recognition capability: The use of a chiral inducing agent in polyaniline electrochemical synthesis for the specific recognition of the enantiomers of the pesticide dinoseb. Electrochim. Acta 2011, 58, 729–735. [Google Scholar] [CrossRef]
- He, S.; Shang, X.; Lu, W.; Tian, Y.; Xu, Z.; Zhang, W. Electrochemical enantioselective sensor for effective recognition of tryptophan isomers based on chiral polyaniline twisted nanoribbon. Anal. Chim. Acta 2021, 1147, 155–164. [Google Scholar] [CrossRef]
- Feng, Z.; Li, M.; Yan, Y.; Jihai, T.; Xiao, L.; Wei, Q. Several novel and effective methods for chiral polyaniline to recognize the configuration of alanine. Tetrahedron Asymmetry 2012, 23, 411–414. [Google Scholar] [CrossRef]
- Tourillon, G.; Garnier, F. Stability of conducting polythiophene and derivatives. J. Electrochem. Soc. 1983, 130, 2042–2044. [Google Scholar] [CrossRef]
- Joergensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of Polymer Solar Cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef] [PubMed]
- So, R.C.; Carreon-Asok, A.C. Molecular design, synthetic strategies, and applications of cationic polythiophenes. Chem. Rev. 2019, 119, 11442–11509. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From fundamental perspectives to applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Yamamoto, T.; Koizumi, T.A. Synthesis of π-conjugated polymers bearing electronic and optical functionalities by organometallic polycondensations and their chemical properties. Polymer 2007, 48, 5449–5472. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, K.P.R.; Johan, D.; Olsson, J.D.; Konradsson, P.; Inganas, O. Enantiomeric substituents determine the chirality of luminescent conjugated polythiophenes. Macromolecules 2004, 37, 6316–6321. [Google Scholar] [CrossRef]
- Chahma, M.; Myles, D.J.T.; Hicks, R.G. Synthesis and characterization of a conducting organomain group polymer, poly[bis((3,4-ethylenedioxy)-2-thienyl) sulfide]: A heteroaromatic relative of poly(p-phenylene sulfide). Macromolecules 2004, 37, 2010–2012. [Google Scholar] [CrossRef]
- Roncali, J. Conjugated poly (thiophenes): Synthesis, functionalization, and applications. Chem. Rev. 1992, 92, 711–738. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3, 4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv. Mater. 2000, 12, 481–534. [Google Scholar] [CrossRef]
- Genies, E.M.; Bidan, G.; Diaz, A.F. Spectroelectrochemical study of polypyrrole films. J. Electroanal. Chem. 1983, 149, 101–113. [Google Scholar] [CrossRef]
- Amatore, C.; Savéant, J.-M. ECE and disproportionation: Part, V. Stationary state general solution application to linear sweep voltammetry. J. Electroanal. Chem. 1977, 85, 27–46. [Google Scholar]
- Andrieux, C.P.; Audebert, P.; Hapiot, P.; Savéant, J.-M. Identification of the first steps of the electrochemical polymerization of pyrroles by means of fast potential step techniques. J. Phys. Chem. 1991, 95, 10158–10164. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Chahma, M. Synthesis and characterization of alanine functionalized oligo/polythiophenes. New J. Chem. 2010, 34, 1417–1423. [Google Scholar] [CrossRef]
- McTiernan, C.D.; Omri, K.; Chahma, M. Chiral conducting surfaces via electrochemical oxidation of l-leucine-oligothiophenes. J. Org. Chem. 2010, 75, 6096–6103. [Google Scholar] [CrossRef] [PubMed]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A practical beginner’s guide to cyclic voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Chahma, M.; McTiernan, C.D.; Abbas, S.A. Characterization of phenomena occurring at the interface of chiral conducting surfaces. New J. Chem. 2014, 38, 3379–3385. [Google Scholar] [CrossRef]
- Hu, D.; Lu, B.; Duan, X.; Xu, J.; Zhang, L.; Zhang, K.; Zhang, S.; Zhen, S. Synthesis of novel chiral l-leucine grafted PEDOT derivatives with excellent electrochromic performances. RSC Adv. 2014, 4, 35597–35608. [Google Scholar] [CrossRef]
- Grenier, R.G.; George, S.J.; Joncheray, T.J.; Meijer, E.W.; Reynolds, J.R. Chiral ethylhexyl substituents for optically active aggregates of π-conjugated polymers. J. Am. Chem. Soc. 2007, 129, 10694–10699. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Lu, B.; Zhang, K.; Sun, X.; Xu, J.; Duan, X.; Dong, L.; Sun, H.; Zhu, X.; Zhen, S. Synthesis of novel chiral l-phenylalanine grafted PEDOT derivatives with electrochemical chiral sensor for 3,4-dihydroxyphenylalanine discrimination. Int. J. Electrochem. Sci. 2015, 10, 3065–3081. [Google Scholar]
- Dong, L.; Lu, B.; Duan, X.; Xu, J.; Hu, D.; Zhang, K.; Zhu, X.; Sun, H.; Ming, S.; Wang, Z.; et al. Novel chiral PEDOTs for selective recognition of 3, 4-dihydroxyphenylalanine enantiomers: Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 2238–2251. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Y.; Duan, X.; Zhu, X.; Su, H.; Xu, J. Chiral PEDOT-based enantioselective electrode modification material for chiral electrochemical sensing: Mechanism and model of chiral recognition. Anal. Chem. 2017, 89, 9695–9702. [Google Scholar] [CrossRef]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Grecchi, S.; Santagostini, L.; Sannicolò, F.; Mussini, P.R. “Inherently chiral” thiophene-based electrodes at work: A screening of enantioselection ability toward a series of pharmaceutically relevant phenolic or catecholic amino acids, amino esters, and amine. Anal. Bioanal. Chem. 2016, 408, 7243–7254. [Google Scholar] [CrossRef] [PubMed]
- Arnaboldi, S.; Benincori, T.; Cirilli, R.; Kutner, W.; Magni, M.; Mussini, P.R.; Noworyta, K.; Sannicolò, F. Inherently chiral electrodes: The tool for chiral voltammetry. Chem. Sci. 2015, 6, 1706–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wattanakit, C.; Côme, Y.; Lapeyre, V.; Bopp, P.A.; Heim, M.; Yadnum, S.; Nokbin, S.; Warakulwit, C.; Limtrakul, J.; Kuhn, A. Enantioselective recognition at mesoporous chiral metal surfaces. Nat. Commun. 2014, 5, 3325. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, X.; Liu, Z.; Sun, L.; Fang, G.; Liu, J.; Wang, S. Electrochemical detection of organophosphorus pesticides based on amino acids-conjugated P3TAA-modified electrodes. Analyst 2020, 145, 8068–8076. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of pesticide residues in food using surface-enhanced Raman spectroscopy: A review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [CrossRef] [PubMed]
- Pernites, R.B.; Venkata, S.K.; Tiu, B.D.B.; Yago, A.C.C.; Advincula, R.C. Nanostructured, molecularly imprinted, and template-patterned polythiophenes for chiral sensing and differentiation. Small 2012, 8, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Duan, D.; Yang, H.; Ding, Y.; Li, L.; Ma, G. A three-dimensional conductive molecularly imprinted electrochemical sensor based on MOF derived porous carbon/carbon nanotubes composites and prussian blue nanocubes mediated amplification for chiral analysis of cysteine enantiomers. Electrochim. Acta 2019, 302, 137–144. [Google Scholar] [CrossRef]
- Shoja, Y.; Rafati, A.A.; Ghodsi, J. Polythiophene supported MnO2 nanoparticles as nano-stabilizer for simultaneously electrostatically immobilization of d-amino acid oxidase and hemoglobin as efficient bio-nanocomposite in fabrication of dopamine bi-enzyme biosensor. Mater. Sci. Eng. C 2017, 76, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Duay, J.; Lee, S.B. Redox exchange induced MnO2 nanoparticle enrichment in poly(3,4-ethylenedioxythiophene) nanowires for electrochemical energy storage. ACS Nano 2010, 4, 4299–4307. [Google Scholar] [CrossRef]
- Li, Q.; Xia, Y.; Wan, X.; Yang, S.; Cai, Z.; Ye, Y.; Li, G. Morphology-dependent MnO2/nitrogen-doped graphene nanocomposites for simultaneous detection of trace dopamine and uric acid. Mater. Sci. Eng. C 2020, 109, 110615. [Google Scholar] [CrossRef] [PubMed]
- Borazjani, M.; Mehdinia, A.; Jabbari, A. An enantioselective electrochemical sensor for simultaneous determination of mandelic acid enantiomers using dexamethasone-based chiral nanocomposite coupled with chemometrics method. J. Electroanal. Chem. 2017, 805, 83–90. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Mo, Z.; Pan, Z.; Liu, Z.; Shuai, C.; Gao, Q.; Liu, N.; Guo, R. Electrochemical chiral recognition at molecular imprinting Au surfaces. J. Electrochem. Soc. 2019, 166, B1126–B1130. [Google Scholar] [CrossRef]
- Ning, G.; Wang, H.; Fu, M.; Liu, J.; Sun, Y.; Lu, H.; Fan, X.; Zhang, Y.; Wang, H. Dual signals electrochemical biosensor for point-of-care testing of amino acids enantiomers. Electroanalysis 2021. [Google Scholar] [CrossRef]
- Zeng, X.; Li, N.; Wang, J. Electrochemical synthesis of (poly) dimethoxyaniline on glassy carbon electrodes and their applications in the detection of l and d-glutamic acids. J. Electrochem. Soc. 2019, 166, B3066–B3071. [Google Scholar] [CrossRef]
- Pandey, I.; Kant, R. Electrochemical impedance based chiral analysis of anti-ascorbutic drug: L-Ascorbic acid and d-ascorbic acid using C-dots decorated conductive polymer nano-composite electrode. Biosens. Bioelectron. 2016, 77, 715–724. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Peng, C.; Zhang, Z.; Chen, J.; Zhou, X.; Jiang, H. Enantiorecognition of tyrosine based on a novel magnetic electrochemical chiral sensor. Electrochim. Acta 2017, 241, 386–394. [Google Scholar] [CrossRef]
- Zou, J.; Yu, J.G. Chiral recognition of tyrosine enantiomers on a novel bis-aminosaccharides composite modified glassy carbon electrode. Anal. Chim. Acta 2019, 1088, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Zhou, Y.; Zhang, G.; Zhang, Y.; Zhang, C.; Hong, S.; Yang, Y.; Dong, C.; Shuang, S. A highly efficient chiral sensing platform for tryptophan isomers based on a coordination self-assembly. Talanta 2019, 195, 306–312. [Google Scholar] [CrossRef] [PubMed]














| Electrode Construction | Detection | LOD | Ref |
|---|---|---|---|
| Leu/PTh/Pt | LeuOMe/AlaOMe | 1 mM | [47] |
| PTh/GCE | 1,4-Dihydroxyphenylalanine | 0.50 mM | [51] |
| Norephedrine/PTh/QCM | Norephedrine | 0.25 mM | [58] |
| His-Ser-Glu/PTh/GCE | Organophosphorus | 0.50 µM | [56] |
| DAAO-Hb/MnO2 NPs/PTh/GCE | Dopamine | 40 nM | [60] |
| DAAO-Hb/MnO2 NPs/GCE | Dopamine | 0.039 µM | [62] |
| PPy-DEX/GR/GCE | Mandelic acid | 0.25 mM | [63] |
| Trp/PPy/Au | l-Tryptophan, d-Tryptophan | 0.012 μM, 0.009 μM | [64] |
| L/D-CNT/PPy/Pt | Amino acids (Tryptophan) | 0.107 nM | [65] |
| PANi/GCE | l-Glutamic acid | 0.011 mM | [66] |
| PANI-FSA/PGE | l-Ascorbic acid | 7.3–4.5 10−4 nM | [67] |
| L-Cys-Au/Fe3O4-NP | l-and d-Tyrosine | 0.021–0.084 μM | [68] |
| GCE | l-Tyrosine, d-Tyrosine | 0.65, 0.86 mM | [69] |
| Cu-β-CD/PLA/MWCN/GCE. | Tryptophan | 3.3 10−7 M | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chahma, M. Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors. Electrochem 2021, 2, 677-688. https://doi.org/10.3390/electrochem2040042
Chahma M. Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors. Electrochem. 2021; 2(4):677-688. https://doi.org/10.3390/electrochem2040042
Chicago/Turabian StyleChahma, M’hamed. 2021. "Doped Polythiophene Chiral Electrodes as Electrochemical Biosensors" Electrochem 2, no. 4: 677-688. https://doi.org/10.3390/electrochem2040042