Two-Dimensional Materials: From Discovery to Application in Membrane Distillation/Crystallization Processes
Abstract
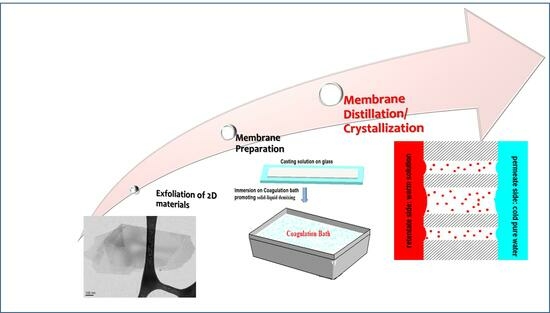
:1. Introduction
1.1. The Advent of 2D Materials
1.1.1. Graphene
1.1.2. Transition Metal Dichalcogenides
1.2. Exfoliation of 2D Materials
2. Preparation of Membranes Functionalized with 2D Materials
- (a)
- Lithographic technique is proposed especially for creating polymeric surfaces with a regular structure and a precise geometric order. The main feature of lithography is the mechanical reproduction of certain images. At the nanometer level (lateral size between the size of a single atom and about 100 nm), lithography can be used in the fabrication of advanced semiconductor integrated circuits or nano electromechanical systems (NEMS). Lithography can also be performed using a support [110] on which the polymer solution is placed, making the final product exhibit certain structures and properties. Patterns are created with mechanical deformation of the impression strength and subsequent processes. The main disadvantages of this process are the high cost of making molds with dimensions of the nano order and the difficulty in obtaining a large resolution. On a larger scale, the creation of well-made surfaces turns out to be more efficient and easier.
- (b)
- Phase separation micro-molding (PSμM) is a highly specialized manufacturing process that produces extremely small, high-precision thermoplastic parts and components with micron tolerances [111]. The process begins in a tooling department where a mold is created that has a cavity shaped like the desired part. Thermoplastic or resin is rapidly injected into the cavity, creating the component or part at high speed. The combination of micro-molding and phase separation techniques is another approach to create nanostructures with hierarchical ordered morphology. It can be seen as an evolution of the traditional phase reversal. A polymer is precipitated from a solution assuming a desired configuration similar to traditional phase inversion. The final configuration can be flat, cylindrical or spherical. The solidification process begins with the transition from a liquid to two liquid phases: one rich in polymer, the other rich in solvent. The first solidifies forming a solid network; the second generates pores or voids in the matrix [112].
- (c)
- Colloidal templates use polymeric nanocapsules composed of a shell or membrane, which is mechanically very strong and separates the internal cavity from the outside medium, thus creating a barrier for various substances that can be encapsulated therein [113]. Scientists found that monodisperse colloidal spheres can self-assemble into arrays of periodic spheres, called colloidal crystals, in a hexagonal arrangement under well-controlled experimental conditions using drop coating, spin coating, dip coating, electrophoretic deposition and self-assembly at the liquid/gas interface. Moreover, in the large hollow spaces, substances such as drugs and biomolecules can be encapsulated and released in a controlled manner, which make the polymeric nanocapsules attractive devices for drug delivery, cancer and gene therapy, protecting enzymes, etc. [114]. The synthesis of monodisperse colloidal spheres offers an opportunity to extend their applications. Membranes with morphological features of high structural order at the nanometric scale may be achieved according to the colloidal template method. Colloid crystalline particles are three-dimensional close-packed crystals of sub-micrometer spheres working as imprinting agents, whose long-ranged ordered structure is replicated in a solid matrix, thus yielding materials with ordered pores. Colloidal crystal structures with this ordered architecture are of great interest for tissue engineering wherein the availability of arrays for cellular proliferation is requested to promote the optimum environment for a good adhesion and consequent cells proliferation [115].
- (d)
- Self-assembly copolymers exploit the ability of some materials to spontaneously form ordered aggregates [116]. This allows to obtain nanostructures, even complex ones, depending on the intrinsic structure and chemistry of the molecules involved. The components most present in these types of assemblies are as follows:
- Lipids, proteins, carbohydrates and nucleic acids;
- Molecular crystals;
- Liquid crystals;
- Semi-crystalline and separate phase polymers.
- -
- The use of block polymers which give rise to a cylindrical morphology;
- -
- The use of a block polymer with a bi-continuous morphology which obviates the need for alignment [117].
- (e)
- Breath figure (BF) for bio-inspired high-defined membranes has been developed in the context of bio, innovative and bio processes in the preparation of micro-porous membranes [118]. This technique allows to obtain membranes with ordered pore geometry. The basic idea for using BF has been developed from the observation of the common phenomenon of fog formation which originates when water vapor comes into contact with a cold surface. During this event, the condensed water droplets tend to rearrange themselves into an ordered geometry that resembles honeycomb patterns. The condensation of water droplets on the surface of dilute polymeric solutions containing immiscible or partially miscible solvents also allows for an easier recovery of the solvent at the end of the process. Furthermore, water is a widely available non-toxic templating agent, so the general approach can be considered as an environmentally friendly production technology. Despite the simplicity with which droplets can be formed, the mechanism that controls the formation of BF geometry can be very complex and not perfectly unique. This may depend on the polymeric materials and solvents used, but also on changes in the surrounding experimental conditions, which make the management of water droplet dynamics somewhat difficult [119].
- (f)
- Deposition for filtration is one of the most common and effective deposition methods. The vertical downtown force, supplied with pressure/vacuum filtration, drives the 2D nanosheets group in a layered interlocking structure on the substrate. The thickness is provided by the membrane that is supported, though it can slightly vary with deposition. Furthermore, other ions, molecules or nanoparticles can be easily mixed and interspersed in the strikers, providing additional flexibility on the tuning of the membrane structure [120].
- (g)
- Coating: Coating is the creation of a thin layer on the surface of the membrane. Various coating methods have been reported to assemble 2D nanosheets on membranes, including drop coating, sterile coating, spin coating and casting, etc. The success of a uniform coating is based on the smoothness of the substrate, the surface tension of the coating solutions, as well as the process of evaporation applied. Among the methods, spin coating could provide centrifugal and cutting forces to control the assembly of nanosheets, producing a well-interloped ordered laminated structure. At present, this technique is also applied to prepare highly ordered labeled membranes [79,121].
- (h)
- Layer-by-layer self-assembly (LBL) refers to the deposition process of different materials on the surface of the substrate. This approach is mainly based on the interactions between adjacent layers, including electrostatic bonding, hydrogen or even covalent interactions. The LBL method can accurately check the thickness of the selective layer by varying the number of deposition cycles and is useful for introducing the intercalary stabilization forces. Therefore, the resulting membranes can remain stable in aqueous or organic media. However, the implementation of this method requires the presence of material interactions and the preparation process takes time [122,123].
- (i)
- Honeycomb membranes possess a specific distribution of the pores on the surface of the partition walls and an enlarged porosity in addition to subtle dividing walls of a prescribed value. The preparation of these particular structures is based on the preparation of lithographic precision membranes according to a BF biospirated process [119]. The condensation waterdrops act as the imprinting agents on the polymer surface and with the balance between solvent evaporation and humid air condensation in a 3D construction, bee nest membranes can be obtained in a single pass. After condensation, the dripping water drills grow and self-assemble in ordered arrays, producing a highly defined hexagonal geometry as a result of their imprinting action, different from what is observed for the separation techniques of conventional phases. The main limitation is using this process to produce commercially large-scale films is the lack of control over the long-range structural order through the surface of the films created [124].
3. Application of 2D Materials in Membrane Distillation
3.1. Graphene in Membrane Distillation
3.2. TMDC in Membrane Processes
3.3. MXenes in Membrane Processes
3.4. Silica Nanoparticles and MOFs in Membrane Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-economic assessment and environmental impacts of desalination technologies. Desalination 2011, 266, 263–273. [Google Scholar] [CrossRef]
- Naseer, M.N.; Zaidi, A.A.; Khan, H.; Kumar, S.; Bin Owais, M.T.; Wahab, Y.A.; Dutta, K.; Jaafar, J.; Uzair, M.; Johan, M.R.; et al. Desalination technology for energy-efficient and low-cost water production: A bibliometric analysis. Green Process. Synth. 2022, 11, 306–315. [Google Scholar] [CrossRef]
- Naidu, G.; Tijing, L.; Johir, M.A.H.; Shon, H.; Vigneswaran, S. Hybrid membrane distillation: Resource, nutrient and energy recovery. J. Membr. Sci. 2020, 599, 117832. [Google Scholar] [CrossRef]
- Tan, Y.Z.; Wang, H.; Han, L.; Tanis-Kanbur, M.B.; Pranav, M.V.; Chew, J.W. Photothermal-enhanced and fouling-resistant membrane for solar-assisted membrane distillation. J. Membr. Sci. 2018, 565, 254–265. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. A Forward Osmosis–Membrane Distillation Hybrid Process for Direct Sewer Mining: System Performance and Limitations. Environ. Sci. Technol. 2013, 47, 13486–13493. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization technology for zero liquid discharge and resource recovery: Opportunities, challenges and futuristic perspectives. Sci. Total. Environ. 2021, 806, 150692. [Google Scholar] [CrossRef]
- Ananthoji, R.; Eubank, J.F.; Nouar, F.; Mouttaki, H.; Eddaoudi, M.; Harmon, J.P. Symbiosis of zeolite-like metal–organic frameworks (rho-ZMOF) and hydrogels: Composites for controlled drug release. J. Mater. Chem. 2011, 21, 9587–9594. [Google Scholar] [CrossRef]
- Hayashi, H.; Côté, A.P.; Furukawa, H.; O’keeffe, M.; Yaghi, O.M. Zeolite A imidazolate frameworks. Nat. Mater. 2007, 6, 501–506. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z. A comprehensive review on anti-fouling nanocomposite membranes for pressure driven membrane separation processes. Desalination 2016, 379, 137–154. [Google Scholar] [CrossRef]
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane synthesis for membrane distillation: A review. Sep. Purif. Technol. 2017, 182, 36–51. [Google Scholar] [CrossRef]
- Xiong, P.; Ma, R.; Wang, G.; Sasaki, T. Progress and perspective on two-dimensional unilamellar metal oxide nanosheets and tailored nanostructures from them for electrochemical energy storage. Energy Storage Mater. 2018, 19, 281–298. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3C2Tx MXene Interlayered Forward Osmosis Membranes for Enhanced Water Purification and Organic Solvent Recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Shafie, Z.M.H.M.; Ahmad, A.L. Juxtaposition of PES based hollow fiber membrane: Antifouling and antibacterial potential of LiCl mediated PVA–ZnO blend. J. Ind. Eng. Chem. 2018, 62, 273–283. [Google Scholar] [CrossRef]
- Gude, V.G. Desalination and water reuse to address global water scarcity. Rev. Environ. Sci. Bio/Technol. 2017, 16, 591–609. [Google Scholar] [CrossRef]
- Kumar, C.; Das, S.; Jit, S. Device physics and device integration of two-dimensional heterostructures. In 2D Nanoscale Heterostructured Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–214. [Google Scholar] [CrossRef]
- Song, C.; Huang, S.; Wang, C.; Luo, J.; Yan, H. The optical properties of few-layer InSe. J. Appl. Phys. 2020, 128, 060901. [Google Scholar] [CrossRef]
- Er, D.; Ghatak, K. Atomistic modeling by density functional theory of two-dimensional materials. In Synthesis, Modeling, and Characterization of 2D Materials, and Their Heterostructures; Elsevier: Amsterdam, The Netherlands, 2020; pp. 113–123. [Google Scholar] [CrossRef]
- Senapati, S.; Maiti, P. Emerging bio-applications of two-dimensional nanoheterostructure materials. In 2D Nanoscale Heterostructured Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 243–255. [Google Scholar] [CrossRef]
- Hasan, M.N.; Nafiujjaman, M.; Lee, Y.-K. 2D Nanomaterials for Gene Delivery. In Biomedical Applications of Graphene and 2D Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–104. [Google Scholar] [CrossRef]
- Frappa, M.; Castillo, A.E.D.R.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. A few-layer graphene for advanced composite PVDF membranes dedicated to water desalination: A comparative study. Nanoscale Adv. 2020, 2, 4728–4739. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Politano, A.; Drioli, E. The advent of graphene and other two-dimensional materials in membrane science and technology. Curr. Opin. Chem. Eng. 2017, 16, 78–85. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, X.; Wu, C.; Zhang, Q. Flux enhancement in membrane distillation by incorporating AC particles into PVDF polymer matrix. J. Membr. Sci. 2016, 500, 46–54. [Google Scholar] [CrossRef]
- Attia, H.; Osman, M.S.; Johnson, D.J.; Wright, C.; Hilal, N. Modelling of air gap membrane distillation and its application in heavy metals removal. Desalination 2017, 424, 27–36. [Google Scholar] [CrossRef]
- Attia, H.; Alexander, S.; Wright, C.J.; Hilal, N. Superhydrophobic electrospun membrane for heavy metals removal by air gap membrane distillation (AGMD). Desalination 2017, 420, 318–329. [Google Scholar] [CrossRef]
- Li, J.; Gunister, E.; Barsoum, I. Effect of graphene oxide as a filler material on the mechanical properties of LLDPE nanocomposites. J. Compos. Mater. 2019, 53, 2761–2773. [Google Scholar] [CrossRef]
- Tang, B.; Hu, G.; Gao, H.; Hai, L. Application of graphene as filler to improve thermal transport property of epoxy resin for thermal interface materials. Int. J. Heat Mass Transf. 2015, 85, 420–429. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, H.; Xue, Y.; Li, B.; Cai, W. Liquid-Phase Exfoliation of Graphene: An Overview on Exfoliation Media, Techniques, and Challenges. Nanomaterials 2018, 8, 942. [Google Scholar] [CrossRef] [PubMed]
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef]
- Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D nanostructures for water purification: Graphene and beyond. Nanoscale 2016, 8, 15115–15131. [Google Scholar] [CrossRef] [PubMed]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef]
- Gusakova, J.; Wang, X.; Shiau, L.L.; Krivosheeva, A.; Shaposhnikov, V.; Borisenko, V.; Gusakov, V.; Tay, B.K. Electronic Properties of Bulk and Monolayer TMDs: Theoretical Study within DFT Framework (GVJ-2e Method). Phys. Status Solidi. 2017, 214, 1700218. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Zheng, Y.; Peng, T.; Yao, K.; Xie, S.; Zhang, X.; Xia, X.; Li, J.; Jiang, H. Development of a quantitative fluorescence-based lateral flow immunoassay for determination of chloramphenicol, thiamphenicol and florfenicol in milk. Food Agric. Immunol. 2018, 29, 56–66. [Google Scholar] [CrossRef]
- Dai, H.; Feng, N.; Li, J.; Zhang, J.; Li, W. Chemiresistive humidity sensor based on chitosan/zinc oxide/single-walled carbon nanotube composite film. Sens. Actuators B Chem. 2019, 283, 786–792. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, C.; Zhao, J.; Liu, X.; Ji, D.; Zhang, H. One-step facile fabrication of PVDF/graphene composite nanofibrous membrane with enhanced oil affinity for highly efficient gravity-driven emulsified oil/water separation and selective oil absorption. Sep. Purif. Technol. 2021, 254, 117576. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Cranford, S.W.; Brommer, D.B.; Buehler, M.J. Extended graphynes: Simple scaling laws for stiffness, strength and fracture. Nanoscale 2012, 4, 7797–7809. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, W.; Xu, N. Two-Dimensional-Material Membranes: A New Family of High-Performance Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 13384–13397. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Macedonio, F.; Politano, A.; Drioli, E. Prospects of 2D materials-based membranes in water desalination. Chem. Eng. Trans. 2019, 73, 265–270. [Google Scholar] [CrossRef]
- Kauling, A.P.; Seefeldt, A.T.; Pisoni, D.P.; Pradeep, R.C.; Bentini, R.; Oliveira, R.V.B.; Novoselov, K.S.; Neto, A.H.C. The Worldwide Graphene Flake Production. Adv. Mater. 2018, 30, e1803784. [Google Scholar] [CrossRef]
- Castillo, A.E.D.R.; Pellegrini, V.; Ansaldo, A.; Ricciardella, F.; Sun, H.; Marasco, L.; Buha, J.; Dang, Z.; Gagliani, L.; Lago, E.; et al. High-yield production of 2D crystals by wet-jet milling. Mater. Horizons. 2018, 5, 890–904. [Google Scholar] [CrossRef]
- Dawlaty, J.M.; Shivaraman, S.; Chandrashekhar, M.; Rana, F.; Spencer, M.G. Measurement of ultrafast carrier dynamics in epitaxial graphene. Appl. Phys. Lett. 2008, 92, 042116. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y.; Kim, J.M.; Kim, K.S.; Ahn, J.-H.; Kim, P.; Choi, J.-Y.; Hong, B.H. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Nikkho, S.; Mirzaei, M.; Sabet, J.K.; Moosavian, M.A.; Hedayat, S.M. Enhanced quality of transfer-free graphene membrane for He/CH4 separation. Sep. Purif. Technol. 2020, 232, 115972. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Gu, X.; Zhao, Y.; Sun, K.; Vieira, C.L.; Jia, Z.; Cui, C.; Wang, Z.; Walsh, A.; Huang, S. Method of ultrasound-assisted liquid-phase exfoliation to prepare graphene. Ultrason. Sonochem. 2019, 58, 104630. [Google Scholar] [CrossRef]
- Sitko, R.; Zawisza, B.; Malicka, E. Graphene and Derivatives: Sample Handling. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Woo, Y.C.; Kim, S.-H.; Shon, H.K.; Tijing, L.D. Introduction: Membrane Desalination Today, Past, and Future. In Current Trends and Future Developments on (Bio-) Membranes; Elsevier: Amsterdam, The Netherlands, 2019; pp. xxv–xlvi. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, X.; Yang, W.; Wang, Y.; Simon, G.P.; Li, D. Controllable corrugation of chemically converted graphene sheets in water and potential application for nanofiltration. Chem. Commun. 2011, 47, 5810–5812. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Farrusseng, D. Metal-Organic Frameworks: Applications from Catalysis to Gas Storage; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Raza, A.; Hassan, J.Z.; Mahmood, A.; Nabgan, W.; Ikram, M. Recent advances in membrane-enabled water desalination by 2D frameworks: Graphene and beyond. Desalination 2022, 531, 115684. [Google Scholar] [CrossRef]
- Macedonio, F.; Politano, A.; Drioli, E.; Gugliuzza, A. Bi2Se3-assisted membrane crystallization. Mater. Horizons 2018, 5, 912–919. [Google Scholar] [CrossRef]
- Ajayan, P.; Kim, P.; Banerjee, K. Two-dimensional van der Waals materials. Phys. Today 2016, 69, 38–44. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Ma, Q.; Zhang, H. Novel structured transition metal dichalcogenide nanosheets. Chem. Soc. Rev. 2018, 47, 3301–3338. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Duan, Y.; Wei, Y.; Dong, C.; Ding, L.; Qiao, Z.; Wang, H. Selective gas diffusion in two-dimensional MXene lamellar membranes: Insights from molecular dynamics simulations. J. Mater. Chem. A 2018, 6, 11734–11742. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Achari, A.; Sahana, S.; Eswaramoorthy, M. High performance MoS2 membranes: Effects of thermally driven phase transition on CO2 separation efficiency. Energy Environ. Sci. 2016, 9, 1224–1228. [Google Scholar] [CrossRef]
- Keshebo, D.L.; Hu, C.-P.; Hu, C.-C.; Hung, W.-S.; Wang, C.-F.; Tsai, H.-C.; Lee, K.-R.; Lai, J.-Y. Effect of composition of few-layered transition metal dichalcogenide nanosheets on separation mechanism of hydrogen selective membranes. J. Membr. Sci. 2021, 634, 119419. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of nanomaterials using various top-down and bottom-up approaches, influencing factors, advantages, and disadvantages: A review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef] [PubMed]
- Kolahalam, L.A.; Viswanath, I.K.; Diwakar, B.S.; Govindh, B.; Reddy, V.; Murthy, Y. Review on nanomaterials: Synthesis and applications. Mater. Today: Proc. 2019, 18, 2182–2190. [Google Scholar] [CrossRef]
- Liu, F. Mechanical exfoliation of large area 2D materials from vdW crystals. Prog. Surf. Sci. 2021, 96, 100626. [Google Scholar] [CrossRef]
- Castillo, A.E.D.R.; Reyes-Vazquez, C.D.; Rojas-Martinez, L.E.; Thorat, S.B.; Serri, M.; Martinez-Hernandez, A.L.; Velasco-Santos, C.; Pellegrini, V.; Bonaccorso, F. Single-step exfoliation and functionalization of few-layers black phosphorus and its application for polymer composites. FlatChem 2019, 18, 100131. [Google Scholar] [CrossRef]
- Murphy, G.W. Desalination by Photoelectrodialysis. II. J. Electrochem. Soc. 1981, 128, 1819–1821. [Google Scholar] [CrossRef]
- Peng, Y.-H.; Kashale, A.A.; Lai, Y.; Hsu, F.-C.; Chen, I.-W.P. Exfoliation of 2D materials by saponin in water: Aerogel adsorption/photodegradation organic dye. Chemosphere 2021, 274, 129795. [Google Scholar] [CrossRef] [PubMed]
- Raccichini, R.; Varzi, A.; Passerini, S.; Scrosati, B. The role of graphene for electrochemical energy storage. Nat. Mater. 2015, 14, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Sakthivel, R.; Keerthi, M.; Chung, R.-J.; He, J.-H. Heterostructures of 2D materials and their applications in biosensing. Prog. Mater. Sci. 2023, 132, 101024. [Google Scholar] [CrossRef]
- Foller, T.; Wang, H.; Joshi, R. Rise of 2D materials-based membranes for desalination. Desalination 2022, 536, 115851. [Google Scholar] [CrossRef]
- Karagiannidis, P.G.; Hodge, S.A.; Lombardi, L.; Tomarchio, F.; Decorde, N.; Milana, S.; Goykhman, I.; Su, Y.; Mesite, S.V.; Johnstone, D.N.; et al. Microfluidization of Graphite and Formulation of Graphene-Based Conductive Inks. ACS Nano 2017, 11, 2742–2755. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Rafea, M.A.; Roushdy, N.; Youssef, M.E.; Gouda, M.H. Development and evaluation of cost-effective and green Bi-functional nickel oxide decorated graphene electrocatalysts for alkaline fuel cells. Results Eng. 2023, 17, 100871. [Google Scholar] [CrossRef]
- Huang, H.-H.; Joshi, R.K.; De Silva, K.K.H.; Badam, R.; Yoshimura, M. Fabrication of reduced graphene oxide membranes for water desalination. J. Membr. Sci. 2019, 572, 12–19. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Xia, Y.; Kang, Y.; Yang, J.; Uddin, H.; Liu, H.; Selomulya, C.; Zhang, X. Minimizing Non-selective Nanowrinkles of Reduced Graphene Oxide Laminar Membranes for Enhanced NaCl Rejection. Environ. Sci. Technol. Lett. 2020, 7, 273–279. [Google Scholar] [CrossRef]
- Buha, J.; Gaspari, R.; Castillo, A.E.D.R.; Bonaccorso, F.; Manna, L. Thermal Stability and Anisotropic Sublimation of Two-Dimensional Colloidal Bi2Te3 and Bi2Se3 Nanocrystals. Nano Lett. 2016, 16, 4217–4223. [Google Scholar] [CrossRef]
- Huang, X.; Marsh, K.L.; McVerry, B.T.; Hoek, E.M.V.; Kaner, R.B. Low-Fouling Antibacterial Reverse Osmosis Membranes via Surface Grafting of Graphene Oxide. ACS Appl. Mater. Interfaces 2016, 8, 14334–14338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, H.; Yan, Y. Catalytic oxidation of ethyl acetate over CuO/ZSM-5 zeolite membrane coated on stainless steel fibers by chemical vapor deposition. Chem. Eng. Res. Des. 2020, 157, 13–24. [Google Scholar] [CrossRef]
- Lang, H.; Dietrich, S. Metals—Gas-Phase Deposition and Applications. In Comprehensive Inorganic Chemistry II.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 211–269. [Google Scholar] [CrossRef]
- JOKOH. Available online: https://jokoh.com/en/ (accessed on 1 February 2011).
- Loh, Z.H.; Samanta, A.K.; Heng, P.W.S. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 255–274. [Google Scholar] [CrossRef]
- Del Rio-Castillo, A.E.; Merino, C.; Díez-Barra, E.; Vázquez, E. Selective suspension of single layer graphene mechanochemically exfoliated from carbon nanofibres. Nano Res. 2014, 7, 963–972. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Fu, B.; Sun, J.; Wang, G.; Shang, C.; Ma, Y.; Ma, J.; Xu, L.; Scardaci, V. Solution-processed two-dimensional materials for ultrafast fiber lasers (invited). Nanophotonics 2020, 9, 2169–2189. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Tzanakis, I.; Morton, J.; Mi, J.; Porfyrakis, K.; Maciejewska, B.M.; Grobert, N.; Eskin, D.G. Ultrasonic exfoliation of graphene in water: A key parameter study. Carbon 2020, 168, 737–747. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Hu, J.; Hou, J.; Huang, S.; Zong, L.; Li, X.; Zhang, Z.; Duan, Y.; Zhang, J. One-pot preparation of zwitterionic graphene nanosheets with exceptional redispersibility and its application in pickering emulsions. Carbon 2020, 157, 448–456. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Kumar, D.S.; Kumar, B.J.; Mahesh, H. Quantum Nanostructures (QDs): An Overview. In Synthesis of Inorganic Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 59–88. [Google Scholar] [CrossRef]
- Zhang, Y.; Ng, Y.L.; Goh, K.-L.; Chow, Y.; Wang, S.; Zivkovic, V. Fluidization of fungal pellets in a 3D-printed micro-fluidized bed. Chem. Eng. Sci. 2021, 236, 116466. [Google Scholar] [CrossRef]
- Zivkovic, V.; Kashani, M.N.; Biggs, M.J. Experimental and theoretical study of a micro-fluidized bed. AIP Conf. Proc. 2013, 1542, 93–96. [Google Scholar] [CrossRef]
- Maa, Y.-F.; Hsu, C.C. Performance of Sonication and Microfluidization for Liquid–Liquid Emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Osong, S.H.; Norgren, S.; Engstrand, P. Processing of wood-based microfibrillated cellulose and nanofibrillated cellulose, and applications relating to papermaking: A review. Cellulose 2016, 23, 93–123. [Google Scholar] [CrossRef]
- Yurdacan, H.M.; Sari, M.M. Functional green-based nanomaterials towards sustainable carbon capture and sequestration. In Sustainable Materials for Transitional and Alternative Energy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 125–177. [Google Scholar] [CrossRef]
- Ansaldo, A.; Bondavalli, P.; Bellani, S.; Castillo, A.E.D.R.; Prato, M.; Pellegrini, V.; Pognon, G.; Bonaccorso, F. High-Power Graphene–Carbon Nanotube Hybrid Supercapacitors. Chemnanomat 2017, 3, 436–446. [Google Scholar] [CrossRef]
- Magesa, F.; Wu, Y.; Tian, Y.; Vianney, J.-M.; Buza, J.; He, Q.; Tan, Y. Graphene and graphene like 2D graphitic carbon nitride: Electrochemical detection of food colorants and toxic substances in environment. Trends Environ. Anal. Chem. 2019, 23, e00064. [Google Scholar] [CrossRef]
- Zhu, J.; Hou, J.; Uliana, A.; Zhang, Y.; Tian, M.; Van der Bruggen, B. The rapid emergence of two-dimensional nanomaterials for high-performance separation membranes. J. Mater. Chem. A 2018, 6, 3773–3792. [Google Scholar] [CrossRef]
- Saidin, N.; Zen, D.I.M.; Hamida, A.B.; Khan, S.; Ahmad, H.; Dimyati, K.; Harun, S.W. AQ-switched thulium-doped fiber laser with a graphene thin film based saturable absorber. Laser Phys. 2013, 23, 115102. [Google Scholar] [CrossRef]
- Calabrò, V.; Basile, A. Economic analysis of membrane use in industrial applications. In Advanced Membrane Science and Technology for Sustainable Energy and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Garciadiego, A.; Luo, T.; Dowling, A.W. Molecular design targets and optimization of low-temperature thermal desalination systems. Desalination 2021, 504, 114941. [Google Scholar] [CrossRef]
- Lund, S.; Kauppila, J.; Sirkiä, S.; Palosaari, J.; Eklund, O.; Latonen, R.-M.; Smått, J.-H.; Peltonen, J.; Lindfors, T. Fast high-shear exfoliation of natural flake graphite with temperature control and high yield. Carbon 2021, 174, 123–131. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.S.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Varrla, E.; Paton, K.R.; Backes, C.; Harvey, A.; Smith, R.J.; McCauley, J.; Coleman, J.N. Turbulence-assisted shear exfoliation of graphene using household detergent and a kitchen blender. Nanoscale 2014, 6, 11810–11819. [Google Scholar] [CrossRef] [PubMed]
- Asli, A.E.N.; Guo, J.; Lai, P.L.; Montazami, R.; Hashemi, N.N. High-Yield Production of Aqueous Graphene for Electrohydrodynamic Drop-on-Demand Printing of Biocompatible Conductive Patterns. Biosensors 2020, 10, 6. [Google Scholar] [CrossRef]
- Frappa, M.; Castillo, A.D.R.; Macedonio, F.; Di Luca, G.; Drioli, E.; Gugliuzza, A. Exfoliated Bi2Te3-enabled membranes for new concept water desalination: Freshwater production meets new routes. Water Res. 2021, 203, 117503. [Google Scholar] [CrossRef] [PubMed]
- Frappa, M.; Macedonio, F.; Gugliuzza, A.; Jin, W.; Drioli, E. Performance of PVDF Based Membranes with 2D Materials for Membrane Assisted-Crystallization Process. Membranes 2021, 11, 302. [Google Scholar] [CrossRef]
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
- Lee, H.-J.; Yoon, T.-H.; Park, J.-H.; Perumal, J.; Kim, D.-P. Characterization and fabrication of polyvinylsilazane glass microfluidic channels via soft lithographic technique. J. Ind. Eng. Chem. 2008, 14, 45–51. [Google Scholar] [CrossRef]
- Tüzün-Antepli, B.; Elçin, A.E.; Elçin, Y.M. Construction of micro-grooved PCL/nanohydroxyapatite membranes by non-solvent induced phase separation method and its evaluation for use as a substrate for human periodontal ligament fibroblasts. Chem. Eng. Sci. 2022, 248, 117120. [Google Scholar] [CrossRef]
- Vogelaar, L.; Barsema, J.; van Rijn, C.; Nijdam, W.; Wessling, M. Phase Separation Micromolding—PSμM. Adv. Mater. 2003, 15, 1385–1389. [Google Scholar] [CrossRef]
- Li, Y.; Koshizaki, N.; Cai, W. Periodic one-dimensional nanostructured arrays based on colloidal templates, applications, and devices. Co-ord. Chem. Rev. 2011, 255, 357–373. [Google Scholar] [CrossRef]
- Haladjova, E.; Rangelov, S.; Tsvetanov, C.; Simon, P. Preparation of polymeric nanocapsules via nano-sized poly(methoxydiethyleneglycol methacrylate) colloidal templates. Polymer 2014, 55, 1621–1627. [Google Scholar] [CrossRef]
- Velev, O.D.; Kaler, E.W. Structured Porous Materials via Colloidal Crystal Templating: From Inorganic Oxides to Metals. Adv. Mater. 2000, 12, 531–534. [Google Scholar] [CrossRef]
- Marques, D.S.; Vainio, U.; Chaparro, N.M.; Calo, V.M.; Bezahd, A.R.; Pitera, J.W.; Peinemann, K.-V.; Nunes, S.P. Self-assembly in casting solutions of block copolymer membranes. Soft Matter 2013, 9, 5557–5564. [Google Scholar] [CrossRef]
- Oss-Ronen, L.; Schmidt, J.; Abetz, V.; Radulescu, A.; Cohen, Y.; Talmon, Y. Characterization of Block Copolymer Self-Assembly: From Solution to Nanoporous Membranes. Macromolecules 2012, 45, 9631–9642. [Google Scholar] [CrossRef]
- Pingitore, V.; Gugliuzza, A. Fabrication of Porous Semiconductor Interfaces by pH-Driven Assembly of Carbon Nanotubes on Honeycomb Structured Membranes. J. Phys. Chem. C 2013, 117, 26562–26572. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Perrotta, M.L.; Drioli, E. Controlled Bulk Properties of Composite Polymeric Solutions for Extensive Structural Order of Honeycomb Polysulfone Membranes. Membranes 2016, 6, 27. [Google Scholar] [CrossRef]
- Ye, W.; Liu, H.; Lin, F.; Lin, J.; Zhao, S.; Yang, S.; Hou, J.; Zhou, S.; Van der Bruggen, B. High-flux nanofiltration membranes tailored by bio-inspired co-deposition of hydrophilic g-C3N4 nanosheets for enhanced selectivity towards organics and salts. Environ. Sci. Nano 2019, 6, 2958–2967. [Google Scholar] [CrossRef]
- Kotoka, F.; Merino-Garcia, I.; Velizarov, S. Surface Modifications of Anion Exchange Membranes for an Improved Reverse Electrodialysis Process Performance: A Review. Membranes 2020, 10, 160. [Google Scholar] [CrossRef]
- Rahaman, S.; Thérien-Aubin, H.; Ben-Sasson, M.; Ober, C.K.; Nielsen, M.; Elimelech, M. Control of biofouling on reverse osmosis polyamide membranes modified with biocidal nanoparticles and antifouling polymer brushes. J. Mater. Chem. B 2014, 2, 1724–1732. [Google Scholar] [CrossRef]
- Hung, W.-S.; Tsou, C.-H.; De Guzman, M.; An, Q.-F.; Liu, Y.-L.; Zhang, Y.-M.; Hu, C.-C.; Lee, K.-R.; Lai, J.-Y. Cross-Linking with Diamine Monomers To Prepare Composite Graphene Oxide-Framework Membranes with Varying d-Spacing. Chem. Mater. 2014, 26, 2983–2990. [Google Scholar] [CrossRef]
- Perrotta, M.; Saielli, G.; Casella, G.; Macedonio, F.; Giorno, L.; Drioli, E.; Gugliuzza, A. An ultrathin suspended hydrophobic porous membrane for high-efficiency water desalination. Appl. Mater. Today 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Mortazavi, M.; Tajiri, K. Liquid water breakthrough pressure through gas diffusion layer of proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2014, 39, 9409–9419. [Google Scholar] [CrossRef]
- Bakshi, A.; Bustamante, H.; Sui, X.; Joshi, R. Structure Dependent Water Transport in Membranes Based on Two-Dimensional Materials. Ind. Eng. Chem. Res. 2021, 60, 10917–10959. [Google Scholar] [CrossRef]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-assisted transport of water vapour in super-hydrophobic membranes filled with multilayer graphene platelets. Nanoscale 2019, 11, 11521–11529. [Google Scholar] [CrossRef] [PubMed]
- Gontarek-Castro, E.; Di Luca, G.; Lieder, M.; Gugliuzza, A. Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance. Membranes 2022, 12, 511. [Google Scholar] [CrossRef]
- Zahirifar, J.; Karimi-Sabet, J.; Moosavian, S.M.A.; Hadi, A.; Khadiv-Parsi, P. Fabrication of a novel octadecylamine functionalized graphene oxide/PVDF dual-layer flat sheet membrane for desalination via air gap membrane distillation. Desalination 2018, 428, 227–239. [Google Scholar] [CrossRef]
- Hou, D.; Fan, H.; Jiang, Q.; Wang, J.; Zhang, X. Preparation and characterization of PVDF flat-sheet membranes for direct contact membrane distillation. Sep. Purif. Technol. 2014, 135, 211–222. [Google Scholar] [CrossRef]
- Grasso, G.; Galiano, F.; Yoo, M.; Mancuso, R.; Park, H.; Gabriele, B.; Figoli, A.; Drioli, E. Development of graphene-PVDF composite membranes for membrane distillation. J. Membr. Sci. 2020, 604, 118017. [Google Scholar] [CrossRef]
- Camacho, L.M.; Pinion, T.A.; Olatunji, S.O. Behavior of mixed-matrix graphene oxide—Polysulfone membranes in the process of direct contact membrane distillation. Sep. Purif. Technol. 2020, 240, 116645. [Google Scholar] [CrossRef]
- Qiu, H.; Peng, Y.; Ge, L.; Hernandez, B.V.; Zhu, Z. Pore channel surface modification for enhancing anti-fouling membrane distillation. Appl. Surf. Sci. 2018, 443, 217–226. [Google Scholar] [CrossRef]
- Mansour, S.; Giwa, A.; Hasan, S. Novel graphene nanoplatelets-coated polyethylene membrane for the treatment of reject brine by pilot-scale direct contact membrane distillation: An optimization study. Desalination 2018, 441, 9–20. [Google Scholar] [CrossRef]
- Chen, T.; Soroush, A.; Rahaman, S. Highly Hydrophobic Electrospun Reduced Graphene Oxide/Poly(vinylidene fluoride-co-hexafluoropropylene) Membranes for Use in Membrane Distillation. Ind. Eng. Chem. Res. 2018, 57, 14535–14543. [Google Scholar] [CrossRef]
- Athanasekou, C.; Sapalidis, A.; Katris, I.; Savopoulou, E.; Beltsios, K.; Tsoufis, T.; Kaltzoglou, A.; Falaras, P.; Bounos, G.; Antoniou, M.; et al. Mixed Matrix PVDF/Graphene and Composite-Skin PVDF/Graphene Oxide Membranes Applied in Membrane Distillation. Polym. Eng. Sci. 2019, 59, E262–E278. [Google Scholar] [CrossRef]
- Bhadra, M.; Roy, S.; Mitra, S. Desalination across a graphene oxide membrane via direct contact membrane distillation. Desalination 2016, 378, 37–43. [Google Scholar] [CrossRef]
- Han, G.; Zhou, J.; Wan, C.; Yang, T.; Chung, T.-S. Investigations of inorganic and organic fouling behaviors, antifouling and cleaning strategies for pressure retarded osmosis (PRO) membrane using seawater desalination brine and wastewater. Water Res. 2016, 103, 264–275. [Google Scholar] [CrossRef]
- Lou, M.; Li, J.; Zhu, X.; Chen, J.; Zhang, X.; Fang, X.; Li, F. Difunctional MOF-wrapped graphene membranes for efficient photothermal membrane distillation and VOCs interception. J. Membr. Sci. 2023, 676, 121592. [Google Scholar] [CrossRef]
- Jafari, A.; Kebria, M.R.S.; Rahimpour, A.; Bakeri, G. Graphene quantum dots modified polyvinylidenefluride (PVDF) nanofibrous membranes with enhanced performance for air Gap membrane distillation. Chem. Eng. Process. Process Intensif. 2018, 126, 222–231. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Luque-Alled, J.M.; Leaper, S.; Alberto, M.; Fan, X.; Vijayaraghavan, A.; Gad-Allah, T.A.; El-Kalliny, A.S.; Szekely, G.; Ahmed, S.I.; et al. PVDF membranes containing reduced graphene oxide: Effect of degree of reduction on membrane distillation performance. Desalination 2019, 452, 196–207. [Google Scholar] [CrossRef]
- Li, H.; Shi, W.; Zeng, X.; Huang, S.; Zhang, H.; Qin, X. Improved desalination properties of hydrophobic GO-incorporated PVDF electrospun nanofibrous composites for vacuum membrane distillation. Sep. Purif. Technol. 2020, 230, 115889. [Google Scholar] [CrossRef]
- Luque-Alled, J.M.; Leaper, S.; Abdel-Karim, A.; Skuse, C.; Gorgojo, P. PVDF membranes containing alkyl and perfluoroalkyl-functionalized graphene nanosheets for improved membrane distillation. J. Environ. Chem. Eng. 2023, 11, 109898. [Google Scholar] [CrossRef]
- Yang, S.; Jiang, Q.; Zhang, K. Few-layers 2D O–MoS2 TFN nanofiltration membranes for future desalination. J. Membr. Sci. 2020, 604, 118052. [Google Scholar] [CrossRef]
- Heiranian, M.; Farimani, A.B.; Aluru, N.R. Water desalination with a single-layer MoS2 nanopore. Nat. Commun. 2015, 6, 8616. [Google Scholar] [CrossRef] [PubMed]
- Abdikheibari, S.; Lei, W.; Dumée, L.F.; Milne, N.; Baskaran, K. Thin film nanocomposite nanofiltration membranes from amine functionalized-boron nitride/polypiperazine amide with enhanced flux and fouling resistance. J. Mater. Chem. A 2018, 6, 12066–12081. [Google Scholar] [CrossRef]
- Abdikheibari, S.; Lei, W.; Dumée, L.F.; Barlow, A.J.; Baskaran, K. Novel thin film nanocomposite membranes decorated with few-layered boron nitride nanosheets for simultaneously enhanced water flux and organic fouling resistance. Appl. Surf. Sci. 2019, 488, 565–577. [Google Scholar] [CrossRef]
- Sun, L.; Ying, Y.; Huang, H.; Song, Z.; Mao, Y.; Xu, Z.; Peng, X. Ultrafast Molecule Separation through Layered WS2 Nanosheet Membranes. ACS Nano 2014, 8, 6304–6311. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Few-layers MoS2 nanosheets modified thin film composite nanofiltration membranes with improved separation performance. J. Membr. Sci. 2020, 595, 117526. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, X.; Bao, S.; Chang, H.; Liu, H.; Fan, G.; Wang, Q.; Fu, X.; Qu, F.; Liang, H. Integration of in situ Fenton-like self-cleaning and photothermal membrane distillation for wastewater treatment via Co-MoS2/CNT catalytic membrane. Sep. Purif. Technol. 2022, 303, 122207. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Optimizing polyamide thin film composite membrane covalently bonded with modified mesoporous silica nanoparticles. J. Membr. Sci. 2013, 428, 341–348. [Google Scholar] [CrossRef]
- Witting, I.T.; Ricci, F.; Chasapis, T.C.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of n-Type Bismuth Telluride: Bismuth Selenide Alloys Bi2Te3−xSex. Research 2020, 2020, 4361703. [Google Scholar] [CrossRef]
- Veis, A.N.; Lukyanova, L.N.; Kutasov, V.A. On the conduction-band structure of bismuth telluride: Optical absorption data. Semiconductors 2017, 51, 836–839. [Google Scholar] [CrossRef]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Arora, S.; Jaimini, V.; Srivastava, S.; Vijay, Y.K. Properties of Nanostructure Bismuth Telluride Thin Films Using Thermal Evaporation. J. Nanotechnol. 2017, 2017, 4276506. [Google Scholar] [CrossRef]
- Feng, H.; Li, H.; Li, M.; Zhang, X. Construction of omniphobic PVDF membranes for membrane distillation: Investigating the role of dimension, morphology, and coating technology of silica nanoparticles. Desalination 2022, 525, 115498. [Google Scholar] [CrossRef]
- Al-Hamadani, Y.A.J.; Jun, B.-M.; Yoon, M.; Taheri-Qazvini, N.; Snyder, S.A.; Jang, M.; Heo, J.; Yoon, Y. Applications of MXene-based membranes in water purification: A review. Chemosphere 2020, 254, 126821. [Google Scholar] [CrossRef] [PubMed]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional, Ordered, Double Transition Metals Carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef]
- Zhao, S.; Huang, L.; Tong, T.; Zhang, W.; Wang, Z.; Wang, J.; Wang, S. Antifouling and antibacterial behavior of polyethersulfone membrane incorporating polyaniline@silver nanocomposites. Environ. Sci. Water Res. Technol. 2017, 3, 710–719. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Wang, J.; Cao, B.; Tang, C.Y. In Situ Reduction of Silver by Polydopamine: A Novel Antimicrobial Modification of a Thin-Film Composite Polyamide Membrane. Environ. Sci. Technol. 2016, 50, 9543–9550. [Google Scholar] [CrossRef] [PubMed]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Chung, T.-S. Metal–Organic Framework-Functionalized Alumina Membranes for Vacuum Membrane Distillation. Water 2016, 8, 586. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Q.; Mirza, N.R.; Huang, Z.; Zhang, J.; Zheng, Y.-M.; Xiang, J.; Xie, Z. Enhanced desalination performance of aluminium fumarate MOF-incorporated electrospun nanofiber membrane with bead-on-string structure for membrane distillation. Desalination 2021, 520, 115338. [Google Scholar] [CrossRef]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal–Organic Frameworks Supported on Nanofiber for Desalination by Direct Contact Membrane Distillation. ACS Appl. Mater. Interfaces 2018, 10, 11251–11260. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, G.; Zhang, J.; Gray, S.; Xie, Z. Dual-layer membranes with a thin film hydrophilic MOF/PVA nanocomposite for enhanced antiwetting property in membrane distillation. Desalination 2021, 518, 115268. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, L.; Li, N.; Smith, S.J.; Wu, D.; Zhang, J.; Ng, D.; Wu, C.; Martinez, M.R.; Batten, M.P.; et al. Aluminum fumarate MOF/PVDF hollow fiber membrane for enhancement of water flux and thermal efficiency in direct contact membrane distillation. J. Membr. Sci. 2019, 588, 117204. [Google Scholar] [CrossRef]
- Di Luca, G.; Chen, G.; Jin, W.; Gugliuzza, A. Aliquots of MIL-140 and Graphene in Smart PNIPAM Mixed Hydrogels: A Nanoenvironment for a More Eco-Friendly Treatment of NaCl and Humic Acid Mixtures by Membrane Distillation. Membranes 2023, 13, 437. [Google Scholar] [CrossRef]
- Santoro, S.; Avci, A.H.; Politano, A.; Curcio, E. The advent of thermoplasmonic membrane distillation. Chem. Soc. Rev. 2022, 51, 6087–6125. [Google Scholar] [CrossRef]
- Abramovich, S.; Dutta, D.; Rizza, C.; Santoro, S.; Aquino, M.; Cupolillo, A.; Occhiuzzi, J.; La Russa, M.F.; Ghosh, B.; Farias, D.; et al. NiSe and CoSe Topological Nodal-Line Semimetals: A Sustainable Platform for Efficient Thermoplasmonics and Solar-Driven Photothermal Membrane Distillation. Small 2022, 18, e2201473. [Google Scholar] [CrossRef]
- Alessandro, F.; Macedonio, F.; Drioli, E. Plasmonic Phenomena in Membrane Distillation. Membranes 2023, 13, 254. [Google Scholar] [CrossRef]
- Perrotta, M.L.; Macedonio, F.; Tocci, E.; Giorno, L.; Drioli, E.; Gugliuzza, A. Graphene stimulates the nucleation and growth rate of NaCl crystals from hypersaline solution via membrane crystallization. Environ. Sci. Water Res. Technol. 2020, 6, 1723–1736. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, K.; Panda, A.B.; Labhasetwar, P.K.; Shahi, V.K. Membrane distillation crystallization for simultaneous recovery of water and salt from tannery industry wastewater using TiO2 modified poly(vinylidene fluoride-co-hexafluoropropylene) nanocomposite membranes. J. Water Process. Eng. 2021, 44, 102393. [Google Scholar] [CrossRef]
- Santoro, S.; Aquino, M.; Seo, D.H.; Van Der Laan, T.; Lee, M.; Yun, J.S.; Park, M.J.; Bendavid, A.; Shon, H.K.; Avci, A.H.; et al. Dimensionally controlled graphene-based surfaces for photothermal membrane crystallization. J. Colloid Interface Sci. 2022, 623, 607–616. [Google Scholar] [CrossRef] [PubMed]



| Reduction of Graphene Oxide | Chemical Vapor Deposition | Liquid-Phase Exfoliation | Synthesis on SiC | Mechanical Exfoliation | |
|---|---|---|---|---|---|
| Quality | Low | High | High | High | High |
| Cost aspect | Average | None | Average | None | Average |
| Scalability | High | Average | High | Low | None |
| Purity | Low | Average | Average | Average | Average |
| Yield | High | Low | Low | Low | Low |
| Membrane | Feed | Feed/Perm Temp (°C) | Porosity (%) | Thickness (μm) | Ref. |
|---|---|---|---|---|---|
| PVDF | 0.6 M NaCl | 50/20 | 41.5 | 138.1 | [130] |
| PVDF + Non-woven | 0.6 M NaCl | 50/20 | 49.16 | 207.3 | [130] |
| PVDF-f | 0.5 M NaCl | 70/20 | n.a. | 19.6 | [131] |
| 2% GO-PSF | 0.6 M NaCl | 90/20 | 26.6 | 26 | [132] |
| GO-PVP/PVDF | 0.6 M NaCl | 65/20 | n.a. | 56.5 | [133] |
| GP2 (0.16%) | 0.6 M NaCl | 85 | 56 | n.a. | [134] |
| rGO/PVDF-HFP | 0.6 M NaCl | 75/25 | 75 | n.a. | [135] |
| PVDF/Graphene 0.87 wt% | 0.6 M NaCl | 75/20 | 73.8 | 390 | [136] |
| PTFE | 0.6 M NaCl | 70/20 | 70 | 35 | [137] |
| PTFE-PVDF GOIM | 0.6 M NaCl | 70/20 | 70 | 35 | [138] |
| PSF | 0.6 M NaCl | 90/20 | 55.9 | 34 | [132] |
| PSF-GO 1% | 0.6 M NaCl | 90/20 | 48.4 | 30 | [132] |
| PES-LIG25 | 0.6 M NaCl | 25/20 | 75 | 185 | [138] |
| GO-PTFE | 0.6 M NaCl | 60/20 | 80 | 50 | [137] |
| PTFE-GO | 34,000 ppm NaCl | 80/20 | 70 | 35 | [34] |
| PP on PTFE-GO | 10,000 ppm NaCl | 80/18 | 70 | 129 | [3] |
| PVDF-HFP-rGO | 0.6 M NaCl | 75/25 | 76 | 390 | [138] |
| PVDF-rGO and GO | 0.6 M NaCl | 65/20 | n.a. | n.a. | [139] |
| PVDF-NBA-GO | 0.6 M NaCl | 80/20 | n.a. | n.a. | [140] |
| PSF-GO | 2.5 wt% NaCl | 90/20 | 30 | 49 | [141] |
| PVDF-PES-GNPs 2 wt% | 10,000 ppm NaCl | 65 | 88 | 142 | [142] |
| PTFE-G | n.a. | 40/20 | n.a. | 240 | [143] |
| PVDF-PDA-GO(70 °C) | 1000 ppm NaCl | 60/20 | n.a. | n.a. | [144] |
| Ni-Nafion-rGO | 5 g/L NaCl | 60 | 95 | n.a. | [145] |
| Membrane | Tfeed [°C] | Flow Rate [mLmin−1] | Flux [Lm−2h−1] | Rejection [%] | Reference |
|---|---|---|---|---|---|
| PVDF-HFP/AlFu-2 | 50 | 500 | 22.7 | 99.9 | [166] |
| PVDF-HFP/AlFu-0.1 | 50 | 500 | 17.0 | 99.9 | [166] |
| PVDF | 48 | 1500 | 1.83 | 99.98 | [167] |
| MOF/PVDF (PV-5) | 48 | 1500 | 3.26 | 99.98 | [167] |
| Pristine PTFE | 50 | 500 | 43.5 | 99.9 | [168] |
| PTFE-PS/AlFu MOF | 50 | 500 | 45.1 | 99.9 | [168] |
| 1% AlFu MOF/PVDF | 50 | 450 | 8.04 | 99.9 | [169] |
| 1% AlFu MOF/PVDF | 70 | 450 | 15.64 | 99.9 | [169] |
| PVDFLANM | 45 | 100 | 11.7 | 99.99 | [170] |
| Membranes | Feed | Feed Flow Rate [L/h] | Temperature Feed [°C] | Lower CV [%] | Flux [Lm−2h−1] | Ref. |
|---|---|---|---|---|---|---|
| PVDF/Bi2Se3 | 5M NaCl | 6 | 21.96 | 36 | 3.6 | [56] |
| PVDF–GP 0.5 | 5M NaCl | 15 | 36.5 | 32.2 | 3.8 | [171] |
| PVDF–GP 5 | 5M NaCl | 15 | 36.5 | 26.7 | 6 | |
| PVDF–GP10 | 5M NaCl | 15 | 36.5 | 35.8 | 5.5 | |
| PVDF/G 0.5% | 5M NaCl | 15 | 42 | 27.5 | 2.7 | [108] |
| PVDF/BT 0.5% | 5M NaCl | 15 | 42 | 43 | 2.7 | |
| PVDF/BT 7% | 5M NaCl | 15 | 42 | 29.5 | 3.9 | |
| PVDF 3D-G | 5.3M NaCl | 0.6 | 40 | 27 | 0.77 | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frappa, M.; Alessandro, F.; Macedonio, F.; Drioli, E. Two-Dimensional Materials: From Discovery to Application in Membrane Distillation/Crystallization Processes. Chemistry 2023, 5, 2205-2228. https://doi.org/10.3390/chemistry5040148
Frappa M, Alessandro F, Macedonio F, Drioli E. Two-Dimensional Materials: From Discovery to Application in Membrane Distillation/Crystallization Processes. Chemistry. 2023; 5(4):2205-2228. https://doi.org/10.3390/chemistry5040148
Chicago/Turabian StyleFrappa, Mirko, Francesca Alessandro, Francesca Macedonio, and Enrico Drioli. 2023. "Two-Dimensional Materials: From Discovery to Application in Membrane Distillation/Crystallization Processes" Chemistry 5, no. 4: 2205-2228. https://doi.org/10.3390/chemistry5040148