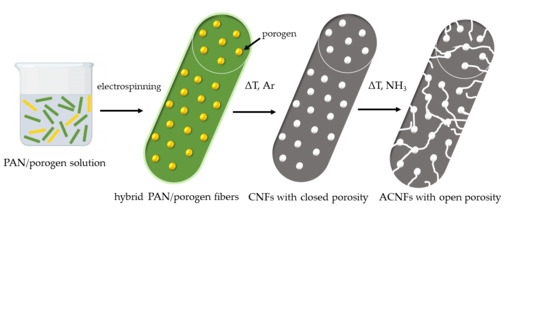
Strategies to Hierarchical Porosity in Carbon Nanofiber Webs for Electrochemical Applications
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Porogen-PAN Fibrous Webs After Carbonization in Ar
3.2. Characterization of Porogen-PAN Fibrous Webs After Carbonization and NH3 Activation
4. Conclusions
- The templating approach of PAN fibers with polymer or inorganic porogens (PMMA, Nafion®, PVP, ZnCl2) resulted in CNFs with closed porosity after carbonization in Ar.
- Subsequent activation in ammonia at 900 °C opened this porosity, resulting in specific surface area in the range of 325–1083 m2·g−1.
- Ammonia activation systematically decreased the electric conductivity of the ACNF webs by a factor of approximately two to three.
- A negative linear correlation between electric conductivity of ammonia-activated ACNF webs and their BET area is revealed.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Shao, Y.; Sun, J.; Yin, G.; Liu, J.; Wang, Y. Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 2016, 29, 314–322. [Google Scholar] [CrossRef]
- Cavaliere, S.; Subianto, S.; Savych, I.; Jones, D.J.; Rozière, J. Electrospinning: Designed architectures for energy conversion and storage devices. Energy Environ. Sci. 2011, 4, 4761–4785. [Google Scholar] [CrossRef]
- Peng, S.; Li, L.; Kong Yoong, J.L.; Tian, L.; Srinivasan, M.; Adams, S.; Ramakrishna, S. Electrospun carbon nanofibers and their hybrid composites as advanced materials for energy conversion and storage. Nano Energy 2016, 22, 361–395. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, L.Z.; Jiao, L. Graphene highly scattered in porous carbon nanofibers: A binder-free and high-performance anode for sodium-ion batteries. J. Mater. Chem. A 2017, 5, 1698–1705. [Google Scholar] [CrossRef]
- Peng, Y.; Lo, C. Electrospun porous carbon nanofibers as lithium ion battery anodes. J. Solid State Electrochem. 2015, 19, 3401–3410. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Bai, Y.; Zhao, X.; Wang, R.; Huang, Y.; Liang, Q.; Huang, Z. A Non-Woven Network of Porous Nitrogen-doping Carbon Nanofibers as a Binder-free Electrode for Supercapacitors. Electrochim. Acta 2017, 230, 445–453. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Sahatiya, P.; Badhulika, S. Template-Assisted Electrospinning of Bubbled Carbon Nanofibers as Binder-Free Electrodes for High-Performance Supercapacitors. ChemElectroChem 2018, 5, 531–539. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Sun, Z.; You, T. Free-standing nitrogen-doped carbon nanofiber films as highly efficient electrocatalysts for oxygen reduction. Nanoscale 2013, 5, 9528–9531. [Google Scholar] [CrossRef] [PubMed]
- Ercolano, G.; Farina, F.; Cavaliere, S.; Jones, D.J.; Rozière, J. Towards ultrathin Pt films on nanofibres by surface-limited electrodeposition for electrocatalytic applications. J. Mater. Chem. A 2017, 5, 3974–3980. [Google Scholar] [CrossRef]
- Kayarkatte, M.K.; Delikaya, Ö.; Roth, C. Freestanding Catalyst Layers: A Novel Electrode Fabrication Technique for PEM Fuel Cells via Electrospinning. ChemElectroChem 2017, 4, 404–411. [Google Scholar] [CrossRef]
- Chan, S.; Jankovic, J.; Susac, D.; Saha, M.S.; Tam, M.; Yang, H.; Ko, F. Electrospun carbon nanofiber catalyst layers for polymer electrolyte membrane fuel cells: Structure and performance. J. Power Sources 2018, 392, 239–250. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, L.; Jiang, H.R.; Chao, C.Y.H.; Zhao, T.S. Formation of electrodes by self-assembling porous carbon fibers into bundles for vanadium redox flow batteries. J. Power Sources 2018, 405, 106–113. [Google Scholar] [CrossRef]
- Cao, Y.; Lu, H.; Hong, Q.; Bai, J.; Wang, J.; Li, X. Co decorated N-doped porous carbon nanofibers as a free-standing cathode for Li-O2 battery: Emphasis on seamlessly continuously hierarchical 3D nano-architecture networks. J. Power Sources 2017, 368, 78–87. [Google Scholar] [CrossRef]
- Liu, C.-K.; Lai, K.; Liu, W.; Yao, M.; Sun, R.-J. Preparation of carbon nanofibres through electrospinning and thermal treatment. Polym. Int. 2009, 58, 1341–1349. [Google Scholar] [CrossRef]
- Nan, D.; Wang, J.-G.; Huang, Z.-H.; Wang, L.; Shen, W.; Kang, F. Highly porous carbon nanofibers from electrospun polyimide/SiO2 hybrids as an improved anode for lithium-ion batteries. Electrochem. Commun. 2013, 34, 52–55. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Wang, L.; Zhang, C.; Liu, S. Hierarchical porous carbon nanofibers as binder-free electrode for high-performance supercapacitor. Electrochim. Acta 2016, 196, 189–196. [Google Scholar] [CrossRef]
- Alothman, Z.A. A review: Fundamental aspects of silicate mesoporous materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Ma, C.; Cao, E.; Li, J.; Fan, Q.; Wu, L.; Song, Y.; Shi, J. Synthesis of mesoporous ribbon-shaped graphitic carbon nanofibers with superior performance as efficient supercapacitor electrodes. Electrochim. Acta 2018, 292, 364–373. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Z.; Zhou, L.; Mai, Y.-W.; Huang, H. In situ formation of hollow graphitic carbon nanospheres in electrospun amorphous carbon nanofibers for high-performance Li-based batteries. Nanoscale 2012, 4, 6800. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Khan, S.; Rojas, O.J.; Parsons, G.N. Control of Micro- and Mesopores in Carbon Nanofibers and Hollow Carbon Nanofibers Derived from Cellulose Diacetate via Vapor Phase Infiltration of Diethyl Zinc. ACS Sustain. Chem. Eng. 2018, 6, 13844–13853. [Google Scholar] [CrossRef]
- Ma, C.; Li, Z.; Li, J.; Fan, Q.; Wu, L.; Shi, J.; Song, Y. Lignin-based hierarchical porous carbon nanofiber films with superior performance in supercapacitors. Appl. Surf. Sci. 2018, 456, 568–576. [Google Scholar] [CrossRef]
- Kim, C.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Kim, Y.J.; Endo, M.; Yang, S.C. Self-Sustained Thin Webs Consisting of Porous Carbon Nanofibers for Supercapacitors via the Electrospinning of Polyacrylonitrile Solutions Containing Zinc Chloride. Adv. Mater. 2007, 19, 2341–2346. [Google Scholar] [CrossRef]
- Yin, D.; Han, C.; Bo, X.; Liu, J.; Guo, L. Prussian blue analogues derived iron-cobalt alloy embedded in nitrogen-doped porous carbon nanofibers for efficient oxygen reduction reaction in both alkaline and acidic solutions. J. Colloid Interface Sci. 2019, 533, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yao, X.; Zhou, S.; Li, X.; Li, L.; Yu, Z.; Gu, L. ZIF-8/ZIF-67-Derived Co-Nx-Embedded 1D Porous Carbon Nanofibers with Graphitic Carbon-Encased Co Nanoparticles as an Efficient Bifunctional Electrocatalyst. Small 2018, 14, 1800423. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, P.; Li, X.; Zeng, S.; Lan, T.; Huang, H.; Zeng, X.; Zou, J. Nitrogen-doped graphitic hierarchically porous carbon nanofibers obtained: Via bimetallic-coordination organic framework modification and their application in supercapacitors. Dalton Trans. 2018, 47, 7316–7326. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kaneti, Y.V.; Bando, Y.; Lin, J.; Liu, C.; Li, J.; Yamauchi, Y. Metal-organic framework-derived one-dimensional porous or hollow carbon-based nanofibers for energy storage and conversion. Mater. Horiz. 2018, 5, 394–407. [Google Scholar] [CrossRef]
- Zhang, W.; Miao, W.; Liu, X.; Li, L.; Yu, Z.; Zhang, Q. High-rate and ultralong-stable potassium-ion batteries based on antimony-nanoparticles encapsulated in nitrogen and phosphorus co-doped mesoporous carbon nanofibers as an anode material. J. Alloys Compd. 2018, 769, 141–148. [Google Scholar] [CrossRef]
- Jeong, J.; Choun, M.; Lee, J. Tree-Bark-Shaped N-Doped Porous Carbon Anode for Hydrazine Fuel Cells. Angew. Chem. Int. Ed. 2017, 56, 13513–13516. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Xiong, P.; Meng, J.; Liang, Y.; Wang, J.; Xu, Y. High rate and long cycle life porous carbon nanofiber paper anodes for potassium-ion batteries. J. Mater. Chem. A 2017, 5, 19237–19244. [Google Scholar] [CrossRef]
- An, G.-H.; Koo, B.-R.; Ahn, H.-J. Activated mesoporous carbon nanofibers fabricated using water etching-assisted templating for high-performance electrochemical capacitors. Phys. Chem. Chem. Phys. 2016, 18, 6587–6594. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Fu, Y.; Meng, X.; Yu, X.; Wang, X. A novel strategy for the high performance supercapacitor based on polyacrylonitrile-derived porous nanofibers as electrode and separator in ionic liquid electrolyte. Electrochim. Acta 2018, 282, 97–104. [Google Scholar] [CrossRef]
- Yang, D.S.; Chaudhari, S.; Rajesh, K.P.; Yu, J.S. Preparation of nitrogen-doped porous carbon nanofibers and the effect of porosity, electrical conductivity, and nitrogen content on their oxygen reduction performance. ChemCatChem 2014, 6, 1236–1244. [Google Scholar] [CrossRef]
- Tran, C.; Kalra, V. Fabrication of porous carbon nanofibers with adjustable pore sizes as electrodes for supercapacitors. J. Power Sources 2013, 235, 289–296. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Wang, H.; Jin, X.; Li, J.; Zhu, Z. Electrospinning preparation of a large surface area, hierarchically porous, and interconnected carbon nanofibrous network using polysulfone as a sacrificial polymer for high performance supercapacitors. RSC Adv. 2018, 8, 28480–28486. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, Z.; Wang, S.; Cai, W.; Yang, J.; Zhang, H. Structure and electrochemistry comparison of electrospun porous carbon nanofibers for capacitive deionization. Electrochim. Acta 2016, 210, 171–180. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Fabrication of porous carbon nanofibers and their application as anode materials for rechargeable lithium-ion batteries. Nanotechnology 2009, 20, 155705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Z.; Wang, Y.; Shang, X.; Nie, P.; Liu, J. Electrospun polyacrylonitrile/β-cyclodextrin based porous carbon nanofiber self-supporting electrode for capacitive deionization. RSC Adv. 2017, 7, 55224–55231. [Google Scholar] [CrossRef] [Green Version]
- Le, T.H.; Tian, H.; Cheng, J.; Huang, Z.H.; Kang, F.; Yang, Y. High performance lithium-ion capacitors based on scalable surface carved multi hierarchical construction electrospun carbon fibers. Carbon N. Y. 2018, 138, 325–336. [Google Scholar] [CrossRef]
- Heo, Y.-J.; Lee, H.I.; Lee, J.W.; Park, M.; Rhee, K.Y.; Park, S.-J. Optimization of the pore structure of PAN-based carbon fibers for enhanced supercapacitor performances via electrospinning. Compos. Part B Eng. 2019, 161, 10–17. [Google Scholar] [CrossRef]
- Lee, H.-M.; Kim, H.-G.; Kang, S.-J.; Park, S.-J.; An, K.-H.; Kim, B.-J. Effects of pore structures on electrochemical behaviors of polyacrylonitrile (PAN)-based activated carbon nanofibers. J. Ind. Eng. Chem. 2015, 21, 736–740. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, B.-H. Zinc oxide/activated carbon nanofiber composites for high-performance supercapacitor electrodes. J. Power Sources 2015, 274, 512–520. [Google Scholar] [CrossRef]
- Kim, C.; Yang, K.S. Electrochemical properties of carbon nanofiber web as an electrode for supercapacitor prepared by electrospinning. Appl. Phys. Lett. 2003, 83, 1216–1218. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, B.; Zhou, P.; Xia, L.; Wen, G.; Zhang, H. Porous-carbon-nanotube decorated carbon nanofibers with effective microwave absorption properties. Nanotechnology 2017, 28, 355708. [Google Scholar] [CrossRef] [PubMed]
- Fitzer, E.; Frohs, W.; Heine, M. Optimization of stabilization and carbonization treatment of PAN fibres and structural characterization of the resulting carbon fibres. Carbon N. Y. 1986, 24, 387–395. [Google Scholar] [CrossRef]
- Moon, S.; Farris, R.J. Strong electrospun nanometer-diameter polyacrylonitrile carbon fiber yarns. Carbon N. Y. 2009, 47, 2829–2839. [Google Scholar] [CrossRef]
- Savych, I.; Bernard D’Arbigny, J.; Subianto, S.; Cavaliere, S.; Jones, D.J.; Rozière, J. On the effect of non-carbon nanostructured supports on the stability of Pt nanoparticles during voltage cycling: A study of TiO2 nanofibres. J. Power Sources 2014, 257, 147–155. [Google Scholar] [CrossRef]
- Heikkila, P. Electrospinning of polyacrylonitrile (PAN) solution: Effect of conductive additive and filler on the process. eXPRESS Polym. Lett. 2009, 3, 437–445. [Google Scholar] [CrossRef] [Green Version]
- Subianto, S.; Cornu, D.; Cavaliere, S. Fundamentals of Electrospinning. In Electrospinning for Advanced Energy and Environmental Applications; Cavaliere, S., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–27. [Google Scholar]
- Karacan, I.; Erzurumluoğlu, L. The effect of carbonization temperature on the structure and properties of carbon fibers prepared from poly(m-phenylene isophthalamide) precursor. Fibers Polym. 2015, 16, 1629–1645. [Google Scholar] [CrossRef]
- Shi, R.; Han, C.; Xu, X.; Qin, X.; Xu, L.; Li, H.; Li, J.; Wong, C.P.; Li, B. Electrospun N-Doped Hierarchical Porous Carbon Nanofiber with Improved Degree of Graphitization for High-Performance Lithium Ion Capacitor. Chem. A Eur. J. 2018, 24, 10460–10467. [Google Scholar] [CrossRef] [PubMed]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cançado, L.G.; Gomes da Silva, M.; Martins Ferreira, E.H.; Hof, F.; Kampioti, K.; Huang, K.; Pénicaud, A.; Alberto Achete, C.; Capaz, R.B.; Jorio, A. Disentangling contributions of point and line defects in the Raman spectra of graphene-related materials. 2D Mater. 2017, 4, 025039. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, X.; Wang, C.; Fu, S.; Liu, Y.; Shao, C. Polyacrylonitrile and carbon nanofibers with controllable nanoporous structures by electrospinning. Macromol. Mater. Eng. 2009, 294, 673–678. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon nanofibers prepared via electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Atamny, F.; Blöcker, J.; Dübotzky, A.; Kurt, H.; Timpe, O.; Loose, G.; Mahdi, W.; Schlögl, R. Surface chemistry of carbon: Activation of molecular oxygen. Mol. Phys. 1992, 76, 851–886. [Google Scholar] [CrossRef]
- Jaouen, F.; Serventi, A.M.; Lefèvre, M.; Dodelet, J.; Bertrand, P. Non-Noble Electrocatalysts for O 2 Reduction: How Does Heat Treatment Affect Their Activity and Structure? Part II. Structural Changes Observed by Electron Microscopy, Raman, and Mass Spectroscopy. J. Phys. Chem. C 2007, 111, 5971–5976. [Google Scholar] [CrossRef]
- Jaouen, F.; Dodelet, J. Non-Noble Electrocatalysts for O2 Reduction: How Does Heat Treatment Affect Their Activity and Structure? Part I. Model for Carbon Black Gasification by NH 3: Parametric Calibration and Electrochemical Validation. J. Phys. Chem. C 2007, 111, 5963–5970. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A 2013, 1, 999. [Google Scholar] [CrossRef]
- Artyushkova, K.; Kiefer, B.; Halevi, B.; Knop-Gericke, A.; Schlogl, R.; Atanassov, P. Density functional theory calculations of XPS binding energy shift for nitrogen-containing graphene-like structures. Chem. Commun. 2013, 49, 2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, D.; Huang, Z.H.; Lv, R.; Yang, L.; Wang, J.G.; Shen, W.; Lin, Y.; Yu, X.; Ye, L.; Sun, H.; et al. Nitrogen-enriched electrospun porous carbon nanofiber networks as high-performance free-standing electrode materials. J. Mater. Chem. A 2014, 2, 19678–19684. [Google Scholar] [CrossRef]
- Subianto, S.; Cavaliere, S.; Jones, D.J.; Rozière, J. Effect of side-chain length on the electrospinning of perfluorosulfonic acid ionomers. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 118–128. [Google Scholar] [CrossRef]
- Ballengee, J.B.; Pintauro, P.N. Morphological Control of Electrospun Nafion Nanofiber Mats. J. Electrochem. Soc. 2011, 158, B568–B572. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jaouen, F.; Lefèvre, M.; Dodelet, J.-P.; Cai, M. Heat-Treated Fe/N/C Catalysts for O2 Electroreduction: Are Active Sites Hosted in Micropores? J. Phys. Chem. B 2006, 110, 5553–5558. [Google Scholar] [CrossRef] [PubMed]
- Fehse, M.; Cavaliere, S.; Lippens, P.E.; Savych, I.; Iadecola, A.; Monconduit, L.; Jones, D.J.; Rozie, J.; Fischer, F.; Tessier, C.; et al. Nb-Doped TiO2 Nanofibers for Lithium Ion Batteries. J. Phys. Chem. C 2013, 117, 13827–13835. [Google Scholar] [CrossRef]
- Liu, Y.; Murphy, M.W.; Baker, D.R.; Gu, W.; Ji, C.; Jorne, J.; Gasteiger, H.A. Proton Conduction and Oxygen Reduction Kinetics in PEM Fuel Cell Cathodes: Effects of Ionomer-to-Carbon Ratio and Relative Humidity. J. Electrochem. Soc. 2009, 156, B970. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, C.; Gu, W.; Jorne, J.; Gasteiger, H.A. Effects of Catalyst Carbon Support on Proton Conduction and Cathode Performance in PEM Fuel Cells. J. Electrochem. Soc. 2011, 158, B614. [Google Scholar] [CrossRef]
- Baghalha, M.; Eikerling, M.; Stumper, J.; Harvey, D.; Eikerling, M. Modeling the effect of low carbon conductivity of the cathode catalyst layer on PEM fuel cell performance. ECS Trans. 2010, 28, 113–123. [Google Scholar]
- Savych, I.; Subianto, S.; Nabil, Y.; Cavaliere, S.; Jones, D.; Rozière, J. Negligible degradation upon in situ voltage cycling of a PEMFC using an electrospun niobium-doped tin oxide supported Pt cathode. Phys. Chem. Chem. Phys. 2015, 17, 16970–16976. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, C.; Favier, F.; Pinna, N. Electrospun Nanomaterials for Supercapacitor Electrodes: Designed Architectures and Electrochemical Performance. Adv. Energy Mater. 2017, 7, 1601301. [Google Scholar] [CrossRef]






| Porogen (Mw) | Porogen wt % | PAN wt % | CNF Label (after Carbonization in Ar) | CNF Label (after Carbonization in Ar and NH3 Activation) |
|---|---|---|---|---|
| None | 0 | 10 | PAN10-CNF | PAN10-ACNF |
| PMMA (15,000) | 2 | 8 | 15kPMMA2-PAN8-CNF | 15kPMMA2-PAN8-ACNF |
| PMMA (120,000) | 4 | 6 | 120kPMMA4-PAN6-CNF | - |
| PMMA (15,000) | 2 | 8 | 15kPMMA2-PAN8-CNF | - |
| PMMA (120,000) | 4 | 6 | 120kPMMA4-PAN6-CNF | - |
| PVP | 5 | 10 | PVP5-PAN10-CNF | PVP5-PAN10-ACNF |
| Nafion® | 2 | 8 | Nafion2-PAN8-CNF | Nafion2-PAN8-ACNF |
| ZnCl2 | 1 | 10 | Zn1-PAN10-CNF | - |
| ZnCl2 | 3 | 10 | Zn3-PAN10-CNF | - |
| ZnCl2 | 5 | 10 | Zn5-PAN10-CNF | - |
| ZnCl2 | 7 | 10 | Zn7-PAN10-CNF | Zn7-PAN10-ACNF |
| Fiber Precursor | CNFs | ACNFs | ||||
|---|---|---|---|---|---|---|
| SBET, m2·g−1 | SBET, m2·g−1 | CBET | Vmeso, cm³·g−1 | Vmicro, cm³·g−1 | dpore, nm | |
| PAN10 | 20 | n/a* | - | - | - | |
| 15kPMMA2-PAN8 | 35 | 450 | 3034 | 0.0599 | 0.1243 | 2.9 |
| 120kPMMA2-PAN8 | n/a* | 645 | 2410 | 0.0877 | 0.1669 | 1.9 |
| 15kPMMA4-PAN6 | n/a* | 360 | 2927 | - | 0.1006 | 0.5 |
| 120kPMMA4-PAN6 | n/a* | 410 | 3324 | 0.0083 | 0.1177 | 0.8 |
| PVP5-PAN10 | 3 | 325 | 2012 | - | 0.0941 | 1.8 |
| Nafion2-PAN8 | n/a* | 535 | 1993 | 0.1166 | 0.1327 | 2.4 |
| Zn1-PAN10 | n/a* | 680 | 1986 | 0.0791 | 0.1814 | 1.6 |
| Zn3-PAN10 | n/a* | 570 | 1707 | 0.0721 | 0.1614 | 2.5 |
| Zn5-PAN10 | n/a* | 865 | 1791 | - | 0.1982 | 1.5 |
| Zn7-PAN10 | n/a* | 1083 | 3558 | 0.0980 | 0.3098 | 1.4 |
| Fiber Precursor | CNF | ACNF | ||
|---|---|---|---|---|
| AD/G | ID/G | AD/G | ID/G | |
| PAN10 | 3.01 | 1.83 | 2.95 | 1.86 |
| 15kPMMA2-PAN8 | 4.73 | 2.45 | 3.06 | 1.88 |
| 120kPMMA2-PAN8 | 4.59 | 2.02 | 5.00 | 2.39 |
| 15kPMMA4-PAN6 | 3.24 | 2.20 | 3.78 | 2.08 |
| 120kPMMA4-PAN4 | 3.14 | 1.86 | 3.76 | 2.02 |
| PVP5-PAN10 | 4.69 | 2.09 | 3.21 | 2.28 |
| Nafion2-PAN8 | 5.34 | 2.77 | 2.52 | 2.33 |
| Zn1-PAN10 | 4.40 | 2.43 | 4.65 | 2.49 |
| Zn3-PAN10 | 6.34 | 2.40 | 9.61 | 2.56 |
| Zn5-PAN10 | 4.24 | 2.57 | 2.30 | 1.88 |
| Zn7-PAN10 | 6.54 | 2.39 | 3.06 | 1.89 |
| Fiber Precursor | CNF | ACNF | ||||||
|---|---|---|---|---|---|---|---|---|
| Conductivity S·cm−1 | C wt % | N wt % | O Wt % | Conductivity S·cm−1 | C wt % | N wt % | O wt % | |
| PAN10 | 6.6 | 66.8 | 5.6 | 17.5 | 3.8 | 78.4 | 7.0 | 7.8 |
| 15kPMMA2-PAN8 | 8.8 | 75.8 | 5.5 | 11.1 | 1.9 | 77.8 | 5.8 | 6.7 |
| PVP5-PAN10 | 9.7 | 76.2 | 2.2 | 10.9 | 4.8 | 78.8 | 7.0 | 8.2 |
| Nafion2-PAN8 | 7.0 | 74.3 | 5.5 | 16.0 | 2.4 | 70.3 | 5.5 | 9.6 |
| Zn1-PAN10 | 1.1 | 70.3 | 5.2 | 14.6 | - | - | - | - |
| Zn3-PAN10 | 0.8 | 71.5 | 4.6 | 17.1 | - | - | - | - |
| Zn5-PAN10 | 3.3 | 61.2 | 6.8 | 20.9 | - | - | - | - |
| Zn7-PAN10 | 0.8 | 68.1 | 2.5 | 19.2 | 0.3 | 71.3 | 6.1 | 11.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yarova, S.; Jones, D.; Jaouen, F.; Cavaliere, S. Strategies to Hierarchical Porosity in Carbon Nanofiber Webs for Electrochemical Applications. Surfaces 2019, 2, 159-176. https://doi.org/10.3390/surfaces2010013
Yarova S, Jones D, Jaouen F, Cavaliere S. Strategies to Hierarchical Porosity in Carbon Nanofiber Webs for Electrochemical Applications. Surfaces. 2019; 2(1):159-176. https://doi.org/10.3390/surfaces2010013
Chicago/Turabian StyleYarova, Svitlana, Deborah Jones, Frédéric Jaouen, and Sara Cavaliere. 2019. "Strategies to Hierarchical Porosity in Carbon Nanofiber Webs for Electrochemical Applications" Surfaces 2, no. 1: 159-176. https://doi.org/10.3390/surfaces2010013