Electrical Characterization of Carbon Nanotube Reinforced Silver and Copper Composites for Switching Contacts
Abstract
:1. Introduction
2. Materials and Methods
2.1. MMC Production
- CNT dispersion and metallic powder mixture (colloidal mixing process);
- Cold pressing (consolidation of green pellet);
- Sintering (densification via HUP).
2.2. Characterization Techniques
3. Results and Discussions
3.1. Powder Characterization
3.2. Characterization of Sintered MMC
3.3. Electrical Tests
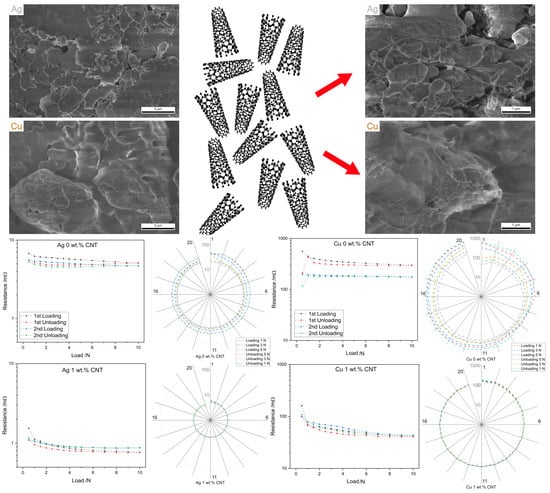
3.3.1. Load-Dependent ECR
3.3.2. Fatigue Cycles
4. Conclusions
- Not only does particle size play an important role in CNT integration but also particle morphology, with the larger-sized copper powder showing better CNT deposition than the smaller-sized silver powder;
- Green pellets formed with silver flakes present an abundance of internal micro-pores. Consequently, a re-pressing post process with prolonged isothermal holding times was required to achieve acceptable silver composite densities;
- The MMC produced did not show the reinforcement effect due to the prolonged sintering process at relatively elevated temperatures. As a consequence, the composites showed low hardness values, which in turn allowed the hard counter electrode to imprint onto the composites’ surfaces—an effect that was more noticeable in the softer silver composites. Nonetheless, the addition of CNT reduced the contact resistance throughout all normal loads measured, with higher concentrations producing the lowest resistance values. Higher CNT concentrations also produced highly reproducible contact surfaces;
- All MMC outperformed the reference material in fatigue tests, rapidly reaching steady-state ECR values and maintaining low resistance throughout the 20 fatigue cycles measured.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saito, R.; Dresselhaus, G.; Dresselhaus, M.S. Physical Properties of Carbon Nanotubes; Imperial College Press: London, UK, 1998; ISBN 978-1-86094-223-5. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R. Physics of Carbon Nanotubes. Carbon 1995, 33, 883–891. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon Nanotubes: Properties and Application. Mater. Sci. Eng. R Rep. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon Nanotubes: A Review on Structure and Their Interaction with Proteins. J. Chem. 2013, 2013, 18. [Google Scholar] [CrossRef] [Green Version]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A. Carbon-Based Nanomaterials/Allotropes: A Glimpse of Their Synthesis, Properties and Some Applications. Materials 2018, 11, 295. [Google Scholar] [CrossRef] [Green Version]
- Ebbesen, T.W. Carbon Nanotubes. Chem. Eng. News 2001, 79, 11. [Google Scholar]
- Maiti, A.; Svizhenko, A.; Anantram, M.P. Electronic Transport through Carbon Nanotubes: Effects of Structural Deformation and Tube Chirality. Phys. Rev. Lett. 2002, 88, 4. [Google Scholar] [CrossRef] [Green Version]
- Yanagi, K.; Udoguchi, H.; Sagitani, S.; Oshima, Y.; Takenobu, T.; Kataura, H.; Ishida, T.; Matsuda, K.; Maniwa, Y. Transport Mechanisms in Metallic and Semiconducting Single-Wall Carbon Nanotube Networks. ACS Nano 2010, 4, 4027–4032. [Google Scholar] [CrossRef]
- Krupke, R.; Hennrich, F.; Löhneysen, H.V.; Kappes, M.M. Separation of Metallic from Semiconducting Single-Walled Carbon Nanotubes. Science 2003, 301, 344–347. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.; Zhong, G.; Hofmann, S.; Bayer, B.C.; Esconjauregui, C.S.; Telg, H.; Thomsen, C. Use of Carbon Nanotubes for VLSI Interconnects. Diam. Relat. Mater. 2009, 18, 957–962. [Google Scholar] [CrossRef]
- Ando, T.; Matsumura, H.; Nakanishi, T. Theory of Ballistic Transport in Carbon Nanotubes. Physica B 2002, 323, 44–50. [Google Scholar] [CrossRef]
- Svizhenko, A.; Anantram, M.P.; Govindan, T.R. Ballistic Transport and Electrostatics in Metallic Carbon Nanotubes. IEEE Trans. Nanotechnol. 2005, 4, 557–562. [Google Scholar] [CrossRef]
- Li, H.J.; Lu, W.G.; Li, J.J.; Bai, X.D.; Gu, C.Z. Multichannel Ballistic Transport in Multiwall Carbon Nanotubes. Phys. Rev. Lett. 2005, 95, 086601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Veen, M.H.; Barbarin, Y.; Kashiwagi, Y.; Tokei, Z. Electron Mean-Free Path for CNT in Vertical Interconnects Approaches Cu. In Proceedings of the 2014 IEEE International Interconnect Technology Conference/Advanced Metallization Conference, San Jose, CA, USA, 20–23 May 2014; pp. 181–184. [Google Scholar]
- Li, H.; Yin, W.Y.; Banerjee, K.; Mao, J.F. Circuit Modeling and Performance Analysis of Multi-Walled Carbon Nanotube Interconnects. IEEE Trans. Electron. Devices 2008, 55, 1328–1337. [Google Scholar] [CrossRef] [Green Version]
- Che, J.; Tahiŗ, T.; Gın, Ť.; Iii, W.A.G. Thermal Conductivity of Carbon Nanotubes. Nanotechnology 2000, 11, 65–69. [Google Scholar] [CrossRef]
- Osman, M.A.; Srivastava, D. Temperature Dependence of the Thermal Conductivity of Single-Wall Carbon Nanotubes. Nanotechnology 2001, 12, 21–24. [Google Scholar] [CrossRef]
- Kim, P.; Shi, L.; Majumdar, A.; McEuen, P.L. Thermal Transport Measurements of Individual Multiwalled Nanotubes. Phys. Rev. Lett. 2001, 87, 215502. [Google Scholar] [CrossRef] [Green Version]
- Graebner, J.E. Thermal Conductivity of Diamond. In Diamond: Electronic Properties and Applications; Springer: Boston, MA, USA, 1995; pp. 285–318. [Google Scholar]
- Braunovic, M. Effect of Connection Design on the Contact Resistance of High Power Overlapping Bolted Joints. IEEE Trans. Compon. Packag. Technol. 2002, 25, 642–650. [Google Scholar] [CrossRef]
- Suarez, S.; Rosenkranz, A.; Gachot, C.; Mücklich, F. Enhanced Tribological Properties of MWCNT/Ni Bulk Composites—Influence of Processing on Friction and Wear Behaviour. Carbon 2014, 66, 164–171. [Google Scholar] [CrossRef]
- Reinert, L.; Suarez, S.; Rosenkranz, A. Tribo-Mechanisms of Carbon Nanotubes: Friction and Wear Behavior of CNT-Reinforced Nickel Matrix Composites and CNT-Coated Bulk Nickel. Lubricants 2016, 4, 11. [Google Scholar] [CrossRef] [Green Version]
- Suarez, S.; Souza, N.; Lasserre, F.; Mücklich, F. Influence of the Reinforcement Distribution and Interface on the Electronic Transport Properties of MWCNT-Reinforced Metal Matrix Composites. Adv. Eng. Mater. 2016, 18, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Alderete, B.; Mücklich, F.; Suarez, S. Characterization and Electrical Analysis of Carbon-Based Solid Lubricant Coatings. Carbon Trends 2022, 7, 100156. [Google Scholar] [CrossRef]
- Alderete, B.; Suarez, S.; Tejeda, D.B.; Mücklich, F. Fretting and Electrical Contact Resistance Characteristics of Carbon Nanoparticle-Coated Cu Electrical Contacts. In Proceedings of the 2022 IEEE 67th Holm Conference on Electrical Contacts (HLM), Tampa, FL, USA, 23–26 October 2022; pp. 1–8. [Google Scholar]
- Alderete, B.; Mücklich, F.; Suarez, S. Wear Reduction via CNT Coatings in Electrical Contacts Subjected to Fretting. Tribol. Lett. 2023, 71, 54. [Google Scholar] [CrossRef]
- Kozbial, A.; Zhou, F.; Li, Z.; Liu, H.; Li, L. Are Graphitic Surfaces Hydrophobic? Acc. Chem. Res. 2016, 49, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Alderete, B.; Lößlein, S.M.; Bucio Tejeda, D.; Mücklich, F.; Suarez, S. Feasibility of Carbon Nanoparticle Coatings as Protective Barriers for Copper─Wetting Assessment. Langmuir 2022, 38, 15209–15219. [Google Scholar] [CrossRef]
- Alderete, B.; MacLucas, T.; Espin, D.; Brühl, S.P.; Mücklich, F.; Suarez, S. Near Superhydrophobic Carbon Nanotube Coatings Obtained via Electrophoretic Deposition on Low-Alloy Steels. Adv. Eng. Mater. 2021, 23, 2001448. [Google Scholar] [CrossRef]
- Feng, Y.; Yuan, H.L.; Zhang, M. Fabrication and Properties of Silver-Matrix Composites Reinforced by Carbon Nanotubes. Mater. Charact. 2005, 55, 211–218. [Google Scholar] [CrossRef]
- Silvestre, N. State-of-the-Art Review on Carbon Nanotube Reinforced Metal Matrix Composites. Int. J. Compos. Mater. 2013, 2013, 28–44. [Google Scholar] [CrossRef]
- Jamwal, A.; Mittal, P.; Agrawal, R.; Gupta, S.; Kumar, D.; Sadasivuni, K.K.; Gupta, P. Towards Sustainable Copper Matrix Composites: Manufacturing Routes with Structural, Mechanical, Electrical and Corrosion Behaviour. J. Compos. Mater. 2020, 54, 2635–2649. [Google Scholar] [CrossRef]
- Suarez, S.; Lasserre, F.; Mücklich, F. Mechanical Properties of MWNT/Ni Bulk Composites: Influence of the Microstructural Refinement on the Hardness. Mater. Sci. Eng. A 2013, 587, 381–386. [Google Scholar] [CrossRef]
- Pérez, E.M.; Martín, N. π-π Interactions in Carbon Nanostructures. Chem. Soc. Rev. 2015, 44, 6425–6433. [Google Scholar] [CrossRef]
- Klinovaja, J.; Schmidt, M.J.; Braunecker, B.; Loss, D. Carbon Nanotubes in Electric and Magnetic Fields. Phys. Rev. B 2011, 84, 085452. [Google Scholar] [CrossRef] [Green Version]
- Puyol, R.; Suarez, S. A Contact Resistance Measurement Setup for the Study of Novel Contacts. In Proceedings of the IEEE URUCON, Montevideo, Uruguay, 23–25 October 2017; pp. 3–6. [Google Scholar]
- Suarez, S.; Alderete, B.; Puyol, R.; Mücklich, F. Load-Dependent Electrical Contact Resistance of Carbon Nanotube-Reinforced Metal Matrix Composites. In Proceedings of the 2022 IEEE 67th Holm Conference on Electrical Contacts (HLM), Tampa, FL, USA, 23–26 October 2022; pp. 1–6. [Google Scholar]
- Bock, E.M. Low-Level Contact Resistance Characterization. AMP J. Technol. 1993, 3, 64–68. [Google Scholar]
- Guiderdoni, C.; Pavlenko, E.; Turq, V.; Weibel, A.; Puech, P.; Estournes, C.; Peigney, A.; Bacsa, W.; Laurent, C. The preparation of carbon nanotube (CNT)/copper composites and the effect of the number of CNT walls on their hardness, friction and wear properties. Carbon 2013, 58, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Hilding, J.; Grulke, E.A.; George Zhang, Z.; Lockwood, F. Dispersion of Carbon Nanotubes in Liquids. J. Dispers. Sci. Technol. 2003, 24, 1–41. [Google Scholar] [CrossRef]
- Reinert, L.; Zeiger, M.; Suárez, S.; Presser, V.; Mücklich, F. Dispersion Analysis of Carbon Nanotubes, Carbon Onions, and Nanodiamonds for Their Application as Reinforcement Phase in Nickel Metal Matrix Composites. RSC Adv. 2015, 5, 95149–95159. [Google Scholar] [CrossRef] [Green Version]
- Zener, C. Theory of D0 for Atomic Diffusion in Metals. J. Appl. Phys. 1951, 22, 372–375. [Google Scholar] [CrossRef]
- Tomizuka, C.T.; Sonder, E. Self-Diffusion in Silver. Phys. Rev. 1956, 103, 1182–1184. [Google Scholar] [CrossRef]
- Kuper, A.; Letaw, H.; Slifkin, L.; Sonder, E.; Tomizuka, C.T. Self-Diffusion in Copper. Phys. Rev. 1954, 96, 1224–1225. [Google Scholar] [CrossRef]
- Burton, J.J. Analysis of Silver Self-Diffusion Data. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1974, 29, 121–133. [Google Scholar] [CrossRef]
- Slade, P.G. Electrical Contacts Principles and Applications, 2nd ed.; Slade, P.G., Ed.; Taylor & Francis: Boca Raton, FL, USA, 2014; ISBN 13:978-1138077102. [Google Scholar]
- Kang, S.-J. Sintering: Densification, Grain Growth and Microstructure, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2005; ISBN 9780750663854. [Google Scholar]
- Suarez, S. Development of Carbon Nanotube-Reinforced Nickel Matrix Composites: Processing, Microstructure and Physical Properties. Ph.D. Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2014. [Google Scholar]
- García, D.; Suárez, S.; Aristizábal, K.; Mücklich, F. Powder-Metallurgical Fabrication and Electrical Contact Resistance Characterization of Copper–Nickel Composites Reinforced by Multiwalled Carbon Nanotubes. Adv. Eng. Mater. 2022, 24, 2100755. [Google Scholar] [CrossRef]









| Reinforcement Concentration/wt.% | Ag-p * | Ag ** | Cu | |
|---|---|---|---|---|
| Relative density/% | 0.5 | 64 | 99 | 95 |
| 0.75 | 74 | 92 | 99 | |
| 1 | 78 | 99 | 99 | |
| Hardness/MPa | 0 *** | 847 ± 61 | - | 1335 ± 82 |
| 0.5 | - | 515 ± 21 | 582 ± 93 | |
| 0.75 | - | 351 ± 18 | 467 ± 96 | |
| 1 | - | 505 ± 37 | 619 ± 88 |
| Roughness Prior to ECR/nm | Roughness Post-ECR/nm | Imprint Diameter/µm | |
|---|---|---|---|
| Ag 0% | 10 ± 10 | 40 ± 10 | 47.7 ± 4.4 |
| Ag 0.5% | 20 ± 10 | 100 ± 10 | 87.2 ± 1.5 |
| Ag 0.75% | 40 ± 10 | 130 ± 20 | 119.9 ± 5.1 |
| Ag 1% | 60 ± 10 | 110 ± 10 | 98.2 ± 2.1 |
| Cu 0.5% | 10 ± 10 | 110 ± 20 | 95.2 ± 5.9 |
| Cu 0.75% | 10 ± 10 | 120 ± 20 | 101.5 ± 3.6 |
| Cu 1% | 10 ± 10 | 110 ± 20 | 94.2 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alderete, B.; Mücklich, F.; Suarez, S. Electrical Characterization of Carbon Nanotube Reinforced Silver and Copper Composites for Switching Contacts. J. Compos. Sci. 2023, 7, 284. https://doi.org/10.3390/jcs7070284
Alderete B, Mücklich F, Suarez S. Electrical Characterization of Carbon Nanotube Reinforced Silver and Copper Composites for Switching Contacts. Journal of Composites Science. 2023; 7(7):284. https://doi.org/10.3390/jcs7070284
Chicago/Turabian StyleAlderete, Bruno, Frank Mücklich, and Sebastian Suarez. 2023. "Electrical Characterization of Carbon Nanotube Reinforced Silver and Copper Composites for Switching Contacts" Journal of Composites Science 7, no. 7: 284. https://doi.org/10.3390/jcs7070284